Hemoptysis
Authors: Antoine Parrot, M.D., Michel Djibré, M.D., Sébastien Roques, M.D., Antoine Khalil, M.D., Muriel Fartoukh, M.D.
Hemoptysis is the expectoration of blood originating from the lower respiratory tract. It is a common alarming symptom accounting for 10 to 15% of all pulmonary visits (131,146, 170). Even if hemoptysis may result from a wide range of causes (111) and the amount of blood expectorated is scant, a specific diagnosis should be performed to start the treatment before the occurrence of a massive hemoptysis. In less than 5% of the cases, hemoptysis may be a life-threatening event with a mortality rate reaching 50 to 80% in the absence of adequate and early management (30, 34, 137, 146, 166), that may reduce substantially the mortality below 20% (55, 90, 102, 109, 132, 187).
This monograph is designed to help physicians in their diagnostic and therapeutic approach of hemoptysis.
PATHOGENESIS AND MECHANISMS
The lungs are supplied by a dual circulatory system: the pulmonary artery system is a low pressure circuit (systolic pressure 15 to 20 mm Hg) designed to perform the gas exchanges, whereas the bronchial arteries have a high pressure regimen and provide a nutritional supply to the lungs (38). The bronchial arteries mainly arise from the descending portion of aorta between the T5 and T6 vertebrae either directly or from an inter-costal trunk. Several variants have been described (e.g. aberrant arteries may originate from the aortic arch, the internal mammary artery, the inferior phrenic artery…) (32). Moreover, major collaterals such the anterior spinal artery or the inferior esophagus artery could be injured during bronchial artery embolization. The right bronchointercostal trunk (RBICT) is present in up to 80% of patients given the right intercostal artery and the right bronchial arteries. The anterior spinal artery may arise from the intercostal artery of this RBICT, leading to a high-risk embolization. The inferior oesophageal artery could arise from the left inferior bronchial artery rending the embolization of the left inferior artery hazardous. Last, connections between bronchial and pulmonary arteries are described as well as between bronchial and pulmonary veins (38). Vascular remodeling and neo angiogenesis may develop within the systemic bronchial and non-bronchial vessels, conversely to the pulmonary vessels (127). This vessel proliferation may occur in the following clinical settings: (i) after a drop of the proximal pulmonary flow (e.g. congenital pulmonary stenosis, pulmonary embolism, pulmonary vasculitis as Takayasu arteritis or Behcet disease (120, 122, 138); (ii) after a destruction of the cartilage support of the bronchial wall induced by a chronic inflammatory process (e.g. bronchiectasis ![]() ) (12); (iii) in lung cancer (38, 104).
) (12); (iii) in lung cancer (38, 104).
Except for traumatic lesions, the precise mechanisms responsible of hemoptysis are still unknown; persistent airway inflammation, infection and high pressure have been suggested as trigger factors of the vessel rupture. In most cases (90%), hemoptysis is related to the systemic bronchial or non-bronchial arteries involvement. Otherwise, hemoptysis originates from the pulmonary arteries (98). Rarely, hemoptysis originates from the pulmonary micro vessels at the alveolar level. The alveolar-capillary barrier injury may be related to many conditions. The diapedesis of erythrocytes into the alveoli is the primary process producing diffuse alveolar haemorrhage. In a minority of cases, pulmonary veins may be involved (e.g. pulmonary vein stenosis, sclerosing mediastinitis) (194). Exceptionally, hemoptysis is related to the aorta (e.g. aorto bronchial fistula) or to a congenital malformation (e.g. broncho pulmonary sequestration). The diagnosis of the vascular source of hemoptysis should be clarified owing to the different specific treatments to start: bronchial artery embolization in hemoptysis originating from the bronchial arteries; pulmonary artery vaso-occlusion in hemoptysis related to the pulmonary artery involvement. In a minority of cases (e.g. active tuberculosis), hemoptysis may originate from both the systemic and the pulmonary vessels (Rasmussen aneurysm) (141). In summary, different mechanisms of hemoptysis are described (Table 1), the main mechanism being the involvement of the bronchial and the non-bronchial arteries.
ETIOLOGIC DIAGNOSIS
More than 100 causes of hemoptysis have been described (111). The most common of them are listed in the Table 2. Lung cancer, bronchitis, tuberculosis (active and sequels) and pneumonia account for 80% of all causes of hemoptysis. The identification and the distribution of the causes depend on the time of publication, the geographic area, the population of patients studied (i.e. outpatient, inpatient, patient admitted to the intensive care unit) as well as the diagnosis tools used. In the studies published between 1930 and 1960, tuberculosis was the main cause of hemoptysis (2). In the third world countries, where tuberculosis has a high prevalence, this condition still remains the most frequent cause of hemoptysis (1, 192). In western countries, hemoptysis related to tuberculosis has been decreased (<10%) with a shift to chronic inflammatory lung diseases (80, 152). Conversely, in patients requiring admission to the intensive care unit, tuberculosis remains a leading cause of hemoptysis as well as lung cancer, bronchiectasis and mycetoma (54, 118, 132, 155). In outpatients, bronchitis might be a main cause of hemoptysis (152). The use of CT scan in patients with hemoptysis has increased the diagnosis of bronchiectasis whereas bronchitis as well as hemoptysis of unknown origin have been decreased (155).
Pulmonary Causes
Infectious Causes
Tuberculosis
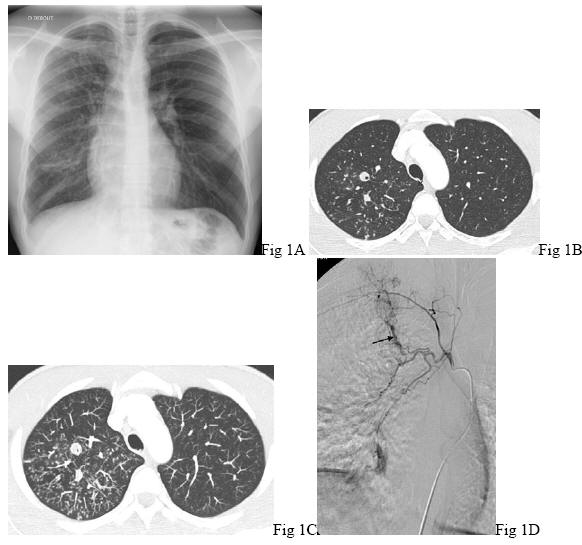
Hemoptysis may reveal active tuberculosis ![]() in up to 10% of the cases (125). Night sweats, fever, weight loss, cavitation on chest-X-ray are suggestive of the diagnosis. Acid-fast bacilli are usually detected in the sputum smears
in up to 10% of the cases (125). Night sweats, fever, weight loss, cavitation on chest-X-ray are suggestive of the diagnosis. Acid-fast bacilli are usually detected in the sputum smears ![]() (Figure 1)
. Hemoptysis may also occur as a late complication of a prior tuberculosis infection and the following diagnoses should therefore be considered. First, hemoptysis may be related to a recurrence of an infection due toMycobacterium tuberculosis or to an infection due to non-tuberculous mycobacteria. Acid-fast-bacilli are found either on sputum and/or identified on culture. Second, scar lesions - bronchiectasis, thin-walled cavities, nodules and/or fibrotic scars - may be responsible of hemoptysis. Sometimes, bronchiectasis are located in an atelectatic lobe secondary to a lymph node enlargement compression. Very occasionally, hemoptysis may be related to the erosion of a calcified lesion into the lumen of a bronchus (broncholithiasis). The broncholith may be expectorated. In smoker patients, a scar cancer should be dreaded. Last, hemoptysis may be secondary to a fungal infection, especially by Aspergillus sp, of a residual cavity (mycetoma).
(Figure 1)
. Hemoptysis may also occur as a late complication of a prior tuberculosis infection and the following diagnoses should therefore be considered. First, hemoptysis may be related to a recurrence of an infection due toMycobacterium tuberculosis or to an infection due to non-tuberculous mycobacteria. Acid-fast-bacilli are found either on sputum and/or identified on culture. Second, scar lesions - bronchiectasis, thin-walled cavities, nodules and/or fibrotic scars - may be responsible of hemoptysis. Sometimes, bronchiectasis are located in an atelectatic lobe secondary to a lymph node enlargement compression. Very occasionally, hemoptysis may be related to the erosion of a calcified lesion into the lumen of a bronchus (broncholithiasis). The broncholith may be expectorated. In smoker patients, a scar cancer should be dreaded. Last, hemoptysis may be secondary to a fungal infection, especially by Aspergillus sp, of a residual cavity (mycetoma).
In most cases, episodes of hemoptysis related to tuberculosis are controlled by the anti-tuberculosis treatment. The source of pulmonary hemorrhage is mainly related to the systemic bronchial or non-bronchial arteries. Nevertheless, in up to 10%, hemoptysis may originate from a pulmonary artery aneurysm (Rasmussen aneurysm) that is described during active as well as inactive tuberculosis (10).
Mycetoma
Mycetoma (fungal ball) is defined as a conglomerate of fungal mycelia, inflammatory cells, fibrin, mucus and tissue debris usually developing in a preformed lung cavity (173). Although other fungi may be at cause (Fusarium,Zygomycetes), Aspergillus spp is by far the most common etiologic agent (171). Tuberculosis is the main cause of cavitary lesions, even if mycetoma has also been reported in cavitary diseases related to sarcoidosis, lung cancer, lung infarction, bullous emphysema, bronchiectasis and fibrobullous disease related to rheumatoid arthritis (25,181). The diagnosis is usually based on the combination of clinical findings and chest radiographic features associated with the serologic evidence of Aspergillus sp. Radiological features may evidence a solid rounded mass that is sometimes mobile within a cavity. This mass is separated from the wall of the cavity by airspace of variable size, which is often in the shape of a crescent. Adjacent thickened pleura is usually described. CT scan may be helpful to visualize the mycetoma. Nevertheless the above radiologic features are not specific of mycetoma and the differential diagnoses include blood clots, a cavitating cancer or infection by other fungi. Therefore, the diagnosis should be confirmed by the sputum examination that may reveal Aspergillus spp in half of the cases and the serum IgG antibodies to Aspergillus spp, which are positive in approximately 90% of the cases (173, 181, 186). Hemoptysis is the cause of death in up to 26% of patients with mycetoma.
Invasive Pulmonary Aspergillosis
Invasive pulmonary aspergillosis is a severe disease that is seen in immunocompromised patients, particularly in those with malignant hematologic diseases during chemotherapy induction or consolidation phases for acute non-lymphocytic leukemia (43, 65, 133, 168). However, few cases of invasive pulmonary aspergillosis have been reported in apparently normal hosts (31, 172). Untreated invasive pulmonary aspergillosis has often a rapid and fatal course. Hemoptysis, chest pain, persistent fever despite antibiotics, prolonged neutropenia, nodular opacities and pleural-based infiltrates on chest-X-ray suggest the diagnosis. Minor hemoptysis has been usually reported in up to 60% of patients with invasive pulmonary aspergillosis. The risk of fatal hemoptysis is particularly high during the phase of marrow recovering (5). Multi-detector row helical CT (MDCT) angiography is essential for both the diagnosis and the treatment. First, MDCT angiography may reveal 2 signs strongly suggestive of invasive pulmonary aspergillosis: the halo sign, a mass-like infiltrate with a surrounding halo of ground glass attenuation which appears early during the course of the disease, and the air crescent sign which represents typical pulmonary cavitations at the later phase. Second, MDCT angiography may indicate if the lesions develop nearby the pulmonary artery or its branches, which may lead to surgery to prevent fatal hemoptysis. Overall, the outcome depends not only on the administration of intensive antifungal therapy but also on the recovery of the underlying host defenses.
Necrotizing Parenchymal Pneumonia
Pneumonia may account for 10% of the causes of hemoptysis. Hemoptysis is usually mild (80, 152). Occasionally, the infectious process is associated with lung necrosis leading to necrotizing pneumonia, lung abscess and lung gangrene. These three conditions represent a parenchymal destruction that is defined by the degree of inflammation, necrosis, time course, degree of sepsis and radiographic patterns (150). Since the advances in antimicrobial therapy, necrotizing parenchymal infections have been rarely reported. In a recent surgical series of 35 patients with acute necrotizing lung infection, hemoptysis was present in 5 patients at the time of management (150). Ayed et al. reported a longitudinal cohort series of 53 consecutive patients undergoing emergency or elective pulmonary resection for massive hemoptysis, 10 of whom had necrotizing pneumonia and lung abscess (11). Hemoptysis related to necrotizing parenchymal infection should be suspected in case of a pulmonary acute illness with cough, sputum and chest pain, fever, although the onset of the disease may be more insidious in a debilitated host (e.g. alcoholic patient with poor dentition) with weight loss, low grade fever and general malaise. Minor hemoptysis is a common symptom, but massive hemoptysis may occur (140). Despite clinical signs of sepsis, sputum and blood cultures may be negative in up to 50% of the cases. Chest-X-ray usually underestimates the parenchymal destruction, emphasizing the usefulness of CT scan in that setting. When isolated, the predominant micro organisms are Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae, other Streptococcus sp. and Actinomyces sp. (71,113, 139, 150). Although antimicrobial treatments have a limited capacity to alter the clinical course of severe infections, early and adequate antibiotics are recommended (96). There are limited data about the efficacy of bronchial artery embolization in that setting and surgery may remain the treatment of choice.
Recently, increasing cases of fatal pneumonia due to Panton Valentine leukocidin (PVL) secreting Staphylococcus aureus have been reported. Pathological examination of lungs frequently demonstrates necrotizing pneumonia with extensive necrotic ulcerations of the tracheal and the bronchial mucosa and massive haemorrhagic necrosis of inter the alveolar septa, probably induced by the PVL toxin (20, 105). In previously healthy children and young adults, symptoms suggestive of the diagnosis include influenza-like syndrome, toxic shock characterized by fever>39°C, tachycardia (usually above 140 bpm), hypotension, moderate to severe hemoptysis, marked leucopenia, multilobar infiltrates on chest-X-ray usually accompanied by effusions and often cavitation, a very high CRP level and a Gram stain of respiratory tract secretions revealing staphylococci (20, 62, 129). An episode of hemoptysis, whatever the amount, revealing or complicating a community-acquired pneumonia (CAP), should alert the physician of the possibility of a PVL secreting strain, since the current recommendations for empirical antimicrobial therapy for CAP may be inadequate.
Miscellaneous Infections
Episodes of hemoptysis related to miscellaneous parasitic infections such paragonimiasis and hydatid cyst have been reported (19, 189). Hemoptysis may also be related to viral infections (17, 112).
Acute and Chronic Bronchitis
Acute and chronic bronchitis are common cause of hemoptysis in western countries (80, 152). Hemoptysis is often mild and associated with purulent sputum, a low grade fever and normal chest-X-ray. In this clinical setting, especially in smokers patients, careful attention should be paid not to misdiagnose a lung cancer. Therefore, we recommend to perform a fiberoptic bronchoscopy and/or a CT scan.
Noninfectious Causes
Lung Cancer
Hemoptysis may reveal a lung cancer ![]() in 7 to 10% of the cases and is usually mild (56, 80, 104, 135). The mechanisms of hemoptysis are generally related to local necrosis and inflammation of blood vessels within the tumor rather than to a direct invasion of the vessels by the tumor (135). A smoker patient, with unexplained weight loss and chest-X-ray opacity suggest the diagnosis. Nevertheless chest-X-ray may be normal (92, 161). Fiberoptic bronchoscopy is essential. In 2 recent epidemiological studies, the primary type of lung cancer was small cell carcinoma (80, 183). Conversely, squamous cell carcinoma is most likely associated with episodes of massive hemoptysis, especially when arising from a main bronchus and presenting as a cavitating lesion (25, 126, 133).
in 7 to 10% of the cases and is usually mild (56, 80, 104, 135). The mechanisms of hemoptysis are generally related to local necrosis and inflammation of blood vessels within the tumor rather than to a direct invasion of the vessels by the tumor (135). A smoker patient, with unexplained weight loss and chest-X-ray opacity suggest the diagnosis. Nevertheless chest-X-ray may be normal (92, 161). Fiberoptic bronchoscopy is essential. In 2 recent epidemiological studies, the primary type of lung cancer was small cell carcinoma (80, 183). Conversely, squamous cell carcinoma is most likely associated with episodes of massive hemoptysis, especially when arising from a main bronchus and presenting as a cavitating lesion (25, 126, 133).
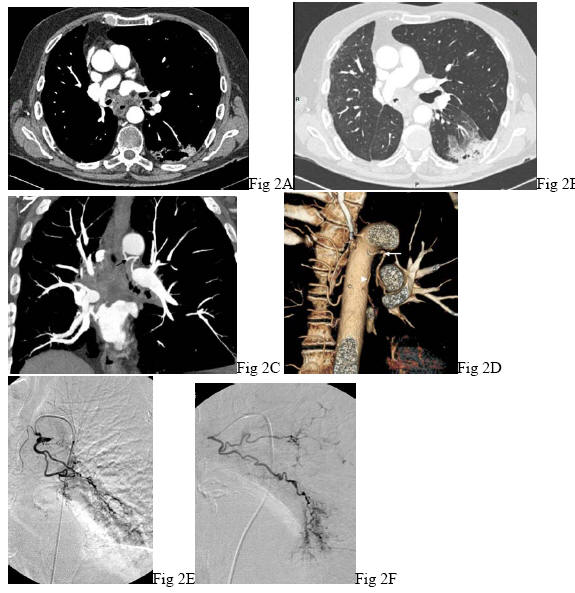
Hemoptysis may also complicate the course of a known lung cancer in the following situations: progression of the tumor which may sometimes involve the pulmonary vessels, pulmonary embolism or tumor necrosis after chemotherapy and/or radiotherapy ![]() (Figure 2). Hemoptysis may result from pulmonary metastases, especially endo bronchial metastases from extra thoracic malignancies (4, 25). Hemoptysis is rarely a revealing symptom. The most common primary tumors of pulmonary metastases include breast, larynx, kidney, colorectal cancer, thyroid cancer and melanoma (4, 80).
(Figure 2). Hemoptysis may result from pulmonary metastases, especially endo bronchial metastases from extra thoracic malignancies (4, 25). Hemoptysis is rarely a revealing symptom. The most common primary tumors of pulmonary metastases include breast, larynx, kidney, colorectal cancer, thyroid cancer and melanoma (4, 80).
Benign tumors such carcinoid tumor or hamartochondrama have been reported to be associated with hemoptysis (69, 144, 190).
Bronchiectasis
Bronchiectasis are a very frequent cause of episodes of mild to severe hemoptysis (13). The diagnosis is often suspected on the association of chronic cough and sputum. High-resolution CT scan is the method of choice for diagnosing bronchiectasis. Additionally, CT scan may determine the location and the extent of the disease. There are numerous causes of bronchiectasis, including congenital conditions (e.g. primary ciliary dyskinesia, cystic fibrosis), immunodeficiency (e.g. IgG subclass deficiency), post-infective conditions (e.g. Mycobacterium tuberculosis, adenovirus, measles), as well as systemic diseases (e.g. rheumatoid arthritis, bowel disease as Crohn's disease) (13). In near half of the patients, no cause is found (136). The trigger factor of hemoptysis might be related to both infection and inflammation. The chronic inflammation results in the development of abnormal bronchial and non-bronchial arteries that become hypertrophic with new vessels formation. The rupture of such vessels with high systemic pressure may result in massive hemoptysis.
Cystic Fibrosis
Episodes of usually mild to moderate hemoptysis may occur in up to 60% of patients over 18 years of age with cystic fibrosis (CF) (39)
![]() . Massive hemoptysis is considered as a late complication of CF and occurs with a prevalence averaging 5%. Most episodes of hemoptysis are related to an acute infective exacerbation of the disease (48). In a recent study, the presence of Staphylococcus aureus in the sputum cultures was predictive of massive hemoptysis (57). Antibiotics are strongly recommended, especially in patients with massive hemoptysis, and empiric antibiotics should be effective against Pseudomonas aeruginosa and Staphylococcus aureus until the culture results are available. Antonelli et al. recommend the use of bronchial artery embolization in CF patients with non massive hemoptysis since that may reduce the number of exacerbations, which is associated with a better quality of life (8).
. Massive hemoptysis is considered as a late complication of CF and occurs with a prevalence averaging 5%. Most episodes of hemoptysis are related to an acute infective exacerbation of the disease (48). In a recent study, the presence of Staphylococcus aureus in the sputum cultures was predictive of massive hemoptysis (57). Antibiotics are strongly recommended, especially in patients with massive hemoptysis, and empiric antibiotics should be effective against Pseudomonas aeruginosa and Staphylococcus aureus until the culture results are available. Antonelli et al. recommend the use of bronchial artery embolization in CF patients with non massive hemoptysis since that may reduce the number of exacerbations, which is associated with a better quality of life (8).
Sarcoidosis
Hemoptysis is a rare event complicating the course of sarcoidosis and reveals this condition in less than 4% of the cases (110,158). Nevertheless it is a dreaded complication since it is the second cause of death among patients with this condition (84). Several causes of hemoptysis have been reported, bronchiectasis and mycetoma at the fibrotic stage being the most common ones. At times, hemoptysis may be related to an irritant cough induced by endobronchial granulomas. Rarely, hemoptysis may occur after the erosion of the pulmonary artery branch into a cavitary sarcoidoisis (47).
Pulmonary Embolism
Hemoptysis is a common symptom of acute pulmonary venous thrombo-embolism disease and result from pulmonary infarction (14,35, 130). Pleuretic chest pain and sudden shortness of breath strongly suggest the diagnosis of pulmonary embolism in the absence of fever.
At an advanced stage of pulmonary embolism, hypertrophy of the bronchial arteries may occur within the first week after the initial episode as collateral compensation and is fully developed at one month (120, 179). Episodes of massive hemoptysis may occur and are facilitated by anticoagulation (26). Transitory discontinuation of anticoagulation may be required and bronchial artery embolization should be considered.
Pulmonary Aneurysm and Pseudo Aneurysm
Pulmonary aneurysms and pseudoaneurysms are rare (138). Pulmonary aneurysms may be associated with congenital heart disease or pulmonary arterial hypertension (160, 188). This alarming symptom is associated with a high mortality rate which may reach 90%, unless promptly treated (145, 167). Pulmonary pseudo aneurysms are mostly related to infectious processes, including bacterial (e.g. tuberculosis, necrotizing pneumonia, lung abscess septicemia, endocarditis) or fungal infection (91, 165, 167). Pulmonary pseudo aneurysms related to vasculitis (e.g. Behçet disease) have also been described. Rarely, pulmonary pseudo aneurysms may complicate lung cancer or invasive investigations (e.g. pulmonary arterial catheter). The evaluation of the pulmonary arteries should be carefully performed by MDCT angiography in hemoptysis related to infectious processes. In pseudo aneurysms related to Behçet disease, hemoptysis is one the leading cause (up to 30%) of death (51). Pulmonary involvement which is present in 5% of the patients occurs at a late stage of the disease (50). In most patients the pulmonary pseudo aneurysms are multiple. At time, hemoptysis may reveal this condition. Recurrent oral and/or genital ulcerations, eyes or skin lesions, a previous peripheral venous thrombosis should suggest the diagnosis.
Pulmonary Arterio-Venous Malformations
Most of the patients with pulmonary arterio-venous malformations have hereditary hemorrhagic telangiectasia (138). Pulmonary complications such hemoptysis occur in 10% of these patients (66). Hemoptysis may be related to the rupture of the arterio-venous malformation itself or to the rupture of endobronchial telangiectasia. Symptoms including epistaxis, telangiectasia, dyspnea, and clubbing suggest the diagnosis.
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension may be associated with episodes of usually mild hemoptysis.
Pulmonary Malformations
Hemoptysis may result from a variety of pulmonary malformations (e.g. unilateral pulmonary artery agenesis, absence of pulmonary artery, pulmonary sequestration) (9, 53, 180).
Cardiovascular Causes
Congenital Heart Diseases
Episodes of hemoptysis related to a variety of congenital heart diseases, especially Eisenmenger syndrome, have been reported (23, 42). Usually, hemoptysis is mild. The source of bleeding is related to the pulmonary or the systemic bronchial or non-bronchial arteries involvement (25).
Pulmonary Venous Hypertension
Mitral stenosis is the most common cause of hemoptysis related to pulmonary venous hypertension (PVH), that results in shunting blood from pulmonary vein to bronchial vein (40). Hemoptysis may result from pulmonary edema, pulmonary infarction as well as the rupture of the bronchial veins. Other causes of PVH include sclerosing mediastinitis, atrial myxoma, congenital pulmonary venous narrowing and lung cancer. Recently, venous pulmonary stenosis after radiofrequency ablation for atrial fibrillation has been reported (159). Infections account for the most common causes of sclerosing mediastinitis, especially fungal infections including histoplasma, aspergillosis, mucormycosis, cryptococcosis and mycobacteria (128, 156). Echocardiogram plays a crucial role in the diagnosis of mitral stenosis. In the absence of this latter, a turbulent flow at the base of the left atrium may suggest the diagnosis of pulmonary venous obstruction (157). Further, MDCT angiography and magnetic resonance imaging should be performed (156).
Aortobronchial Fistula
Aortobronchial fistula may be responsible of massive hemoptysis. Hemoptysis result from a thoracic aneurysm secondary to infection, arterosclerosis as well as aortic surgery complication (27). Chest and back pains suggest the diagnosis. Treatment include emergency surgery or endovascular treatment (103, 142).
Miscellaneous Causes
Coagulation Disorders
Coagulation disorders are rarely primarily responsible of hemoptysis. An underlying disease should be searched.
Endometriosis
Endometriosis is a usual but rare cause of hemoptysis. Periodic hemoptysis occurring during menses suggest the diagnosis (178). Pelvic endometriosis may be unknown in near half of the patients. CT scan and FOB should be performed during menstruation.
Trauma and Iatrogenic Causes
Hemoptysis related to bronchial biopsies, pulmonary artery perforation as well as chest trauma (25, 36, 93) have been reported. A rupture of the innominate artery should be considered in case of massive hemoptysis occurring in a patient with a tracheostomy. The balloon of the tracheostomy should be overflated to avoid a drowning of the airways. Subsequently, emergency surgery should be performed.
Cryptogenic Hemoptysis
Despite complete investigations including clinical examination, FOB and CT scan, no cause of hemoptysis is identified in about 10 to 25% (1, 80, 118, 155, 163). The term of cryptogenic for this unknown etiology is, at times, used (3, 163). Moderate to life-threatening episodes have been reported (163). A follow-up is recommended (78) ![]() (Figure 3)
(Figure 3)
Pulmonary Alveolar Haemorrhage
Rarely, hemoptysis may be related to the injury of the alveolar-capillary barrier. Diffuse alveolar haemorrhage should be suspected in case of hemoptysis, anemia and chest-X-ray diffuse bilateral infiltrates. Alveolar haemorrhage is mainly related to immune disorders and cardiac dysfunction, although infectious diseases may account for a few cases (Table 3) (28, 108, 169).
Leptospirosis is the most frequently reported pathogen associated with alveolar haemorrhage. It should be suspected in case of a flu like syndrome (headache of acute onset, myalgia, fever), liver and renal impairment and major hemorrhagic complications (20, 85, 115). Severe pulmonary haemorrhage may occur in other bacterial conditions (e.g. falciparum malaria, septicaemia with disseminated intravascular coagulation) as well as viral infections (100) such cytomegalovirus in immunocompromised as well as immunocompetent hosts (77, 117).
The main immune causes of alveolar haemorrhage include microscopic polyangiitis (106), Wegener granulomatosis (75, 182), systemic lupus erythematosus (162, 198), anti glomerular basement membrane antibody disease (Goodpasture syndrome) (107). More anecdotic immune alveolar haemorrhage related to antiphospholipid syndrome (37), pauci immune pulmonary capillaritis (87), coeliac disease (119) or drug induces diseases (28) have been reported (169). Alveolar haemorrhage may reveal these latter conditions. Immune alveolar haemorrhage is likely associated with renal involvement (i.e. proteinuria, hematuria, decrease of glomerular filtration rate). Pulmonary-renal syndrome as well as the presence of arthralgias, myalgias, nose-ear-throat symptoms and cutaneous lesions are suggestive of an immune cause. Immune alveolar haemorrhage is a severe condition. Mortality rate, ranging from 20 to 100%, has been reported (15, 60, 198). In patients with ANCA-associated microscopic polyangiitis, the relative risk of death is 9-fold increased when pulmonary haemorrhage is present (81). Additionally the glomerulo-nephritis could lead to irreversible renal failure especially when the initial creatinin value is high (81). Therefore, due to the severity of both alveolar haemorrhage and glomerulonephritis, most authors emphasized the importance of early diagnosis and early etiologic treatment.
MANAGEMENT - OVERVIEW
Physicians should bear in mind that the course of hemoptysis is unpredictable. Therefore, the management should be standardized, including several steps (Table 4). The first step focuses on confirming the diagnosis of hemoptysis. The second step should assess the severity of hemoptysis. The third step should attempt to localize the site and diagnose the cause of hemoptysis. Finally, the last step should focus on the most appropriate treatment to administer. In case of severe hemoptysis, locating the site of bleeding and administering specific treatment including maintain of free airways should be performed simultaneously with determining the cause of bleeding. Therefore, chest-X-ray, CT scan and FOB must be carried out within a few hours in the clinical setting of massive hemoptysis.
Diagnosis Confirmation
Prior launching an extensive diagnostic workup of hemoptysis, it is crucial to determinate that the blood is originating from the respiratory tract (Table 5) (17,111,174). The diagnosis of hemoptysis is usually easy, even if the physician was not present during the episode. Nevertheless, especially when the hemoptysis is active, this frightened symptom may mimic a gastrointestinal or a nasopharyngeal bleeding. The patient describing the event may doubt whether the blood is coughed up, aspirated and then cough up or swallowed and subsequently vomited (41). Therefore, the first step is to distinguish hemoptysis from pseudo-hemoptysis (hematemesis and upper respiratory tract bleeding). Pseudo-hemoptysis rate ranges from 3 to 10% (41, 88, 189). The blood is typically coughed up and usually bright-red, frothy and sometimes mixed with a purulent sputum in episodes of hemoptysis. Conversely, the blood is vomited, usually deep purple and mixed with food particles in hematemesis. Occasionally, a gastrointestinal bleeding should be considered in case of a blood pressure drop contrasting with the absence of respiratory failure. Actually, the most difficult diagnosis to rule out is a nasopharyngeal bleeding, especially when it is abundant. A nasopharyngeal disease should be suspected in a heavy smoker, with a history of oropharyngeal cancer, epistaxis or blood spitting. In mechanically ventilated patients, the absence of active or recurrent bleeding, after having cleared the airways from blood, should lead to suspect a nasopharyngeal rather than a bronchial origin. In doubtful cases, further and prompt investigations may settle this issue, including a thorough examination of the pharynx and/or a neck by CT scan or endoscopy and gastroscopy.
Once the diagnosis of hemoptysis has been established, it remains to determine if the blood originates from the bronchi or from the alveoli because the etiologic and the therapeutic approaches are very different. Alveolar haemorrhage should be suspected in case of haemoptysis, anemia and chest-X-ray diffuse bilateral infiltrates. Broncho alveolar lavage revealing an increasingly bloody lavage fluid return and/or a siderophagic alveolitis is the cornerstone of the diagnosis. Of note, diffuse infiltrates may also result from inhalation of massive hemoptysis. Extra-thoracic clues such renal involvement may be helpful to differentiate alveolar haemorrhage from inhalation related to massive hemoptysis.
Severity Assessment
The severity of hemoptysis is mainly assessed on the amount of bleeding or the magnitude of the respiratory and/or circulatory effects of bleeding (Table 4).
The amount of bleeding is closely correlated to the mortality (34, 80, 166). A mortality of 71% has been reported if the amount of bleeding averaged 600 ml within 4 hours, as compared with a mortality rate of less than 25% for 600 ml within 4 to 16 hours and a mortality rate of 5% for 600 ml during 16 to 48 hours (34). Nevertheless, there are no accepted definitions of the amount of bleeding to define the severity of hemoptysis. Amounts of bleeding from 100 ml/24 hrs to more 1000 ml/48 hrs have been reported (7, 18, 30, 54, 61, 132). Quantifying the amount of bleeding is not always easy. Several graduated containers of a known volume may be presented to the patient to provide an estimate of the amount of bleeding. In Tenon hospital, we use the following scale (Figure 4): a table spoon, a glass and a hospital French spittoon, corresponding to 5 ml, 120-150 and 400-500 ml of blood loss, respectively. In a few cases, the estimate of the expectorated volume is mild and the risk is to underestimate the actual blood loss especially in the following situations: (i) the blood has been swallowed by the patient; (ii) the blood was retained within the lung because of the difficulty to expectorate owing to a decreased muscle strength (elderly patient). In this clinical setting, chest-X-ray may reveal atelectasis; (iii) the blood filled up a pre-existing cavitary lesion. Occasionally, the volume is overestimated owing to a mix of purulent or salivary secretions. The inspection of the sputum may be helpful. The risk of death results mainly from the obstruction of the airways. Since the dead space of the major airways is averaging 200 ml, acute respiratory failure may occur rapidly (174). Respiratory rate and oxygen saturation should be therefore collected in every case.
Hemodynamic failure and anemia should be considered as criteria of severity of hemoptysis. Nevertheless, in our experience, the volume of expectorated blood and the respiratory consequences of bleeding screen most patients with severe hemoptysis (54), who have stable hemodynamic condition. Additionally, shock as well as substantial haemoglobin drop usually occur after respiratory failure and the need for mechanical ventilation and may reflect the underlying disease rather than the blood loss. In doubtful cases, if the above criteria are not relevant, the severity of hemoptysis should be assessed as following: i) the underlying condition: lung cancer and mycetoma are usually associated with poor outcomes (30, 187); ii) clotting disorders expose to massive hemoptysis (6, 80); iii) the use of vasoconstrictive treatment (118) ;iv) and the extent of lobar involvement on high-resolution CT scan, especially if the cough abilities of the patient are decreased (99). 
Topographic and Etiologic Diagnosis
This step aims to provide a side and at best a location of the bleeding as well as the etiology and the mechanism(s) of hemoptysis, especially in massive hemoptysis. Thus, if the lateralization (right or left) of bleeding is known, the patient should be lied on the side of haemorrhage to prevent asphyxia. Unilateral intubation can be performed to protect the non-bleeding lung if needed. Whenever the possibility of surgery exists, the location of hemoptysis is essential. MDCT angiography may modify the initial management if it reveals a pulmonary source of bleeding such a pulmonary aneurysm secondary to tuberculosis (98) that may lead to an endovascular treatment of the pulmonary artery. If the bleeding source originates from the systemic arteries and the bleeding side is known, selective embolization will be performed (22, 82).
History
The side of bleeding may be revealed by the patient‘s symptoms consisting of a gurgling or funny feeling (22). Brinson et al. reported that 47% of CF patients were able to provide the side of bleeding whereas Pursel et al. reported a rate of 10% in unselected patients (22,146). The evaluation of a patient with hemoptysis should include a detailed history. A pre-existing medical condition should be noted. For instance, a history of tuberculosis may reduce the number of possible causes of hemoptysis (infection recurrence, scar lesion, mycetoma). A smoker with weight loss is at risk of lung cancer. The patterns of bleeding may also provide helpful clues for the diagnosis. Recurrent episodes of hemoptysis with purulent sputum or during menses suggest the diagnosis of bronchiectasis or endometriosis. Dark blood might be more associated with a pulmonary artery source of bleeding whereas bright red bleeding suggests a bronchial artery origin of the bleeding. Frothy pink sputum is seen in left ventricular failure. An acute chest pain raises the possibility of a pulmonary embolism. Travels should be noted. For instance, hemoptysis of parasitic origin may develop after a travel to Asia or South America. A history of systemic lupus erythematosus, a history of upper track involvement such as rhinitis or sinusitis, a history of arthralgia or arthritis, as well as a macroscopic hematuria suggest the diagnosis of alveolar haemorrhage.
Physical Examination
The side of bleeding after physical examination is achieved in 43% of the cases (146). A thorough physical examination may provide information on the causes of hemoptysis. Physicians should record vital signs and document fever. A septic condition suggests the diagnosis of pneumonia. Telangiectasia, especially on the lips or buccal mucosa, may raise the diagnosis of hereditary hemorrhagic telangiectasia. Clubbing may be a sign of bronchiectasis or lung cancer. Palpable lymph nodes suggest the diagnosis of lung cancer. Oral or genital ulcerations may be the initial presentation of a Beçhet disease. Calf tenderness may suggest the diagnosis of pulmonary embolism. In patients with alveolar haemorrhage, a careful attention should be paid to extra-pulmonary manifestations. For instance a septal nasal perforation suggests the diagnosis of Wegener granulomatosis. Skin lesions such purpura suggest an inflammatory vasculitis.
Chest-X-Ray
Chest-X-Ray may identify the side of bleeding in 46 to 82% of the cases (82, 132, 146, 155). A large amount of bleeding may decrease the performance of Chest-X-Ray in localizing the bleeding (155). Additionally, several findings on chest radiography may be suggestive of specific diagnoses: a cavitary lesion raises the diagnosis of tuberculosis or lung cancer; an intracavitary mass separated from the surrounding cavity is highly suggestive of aspergilloma; a mass suggests a cancer; tubular or ring opacities suggest bronchiectasis. Chest-X-ray may be normal or non-localizing in 20 to 40% of the cases (121). Further investigations should be performed. Kallenbach et al. reported that bronchoscopy revealed a lung cancer in nearly one quarter of patients with unexplained hemoptysis after physical examination and “normal” chest radiography (92).
Fiberoptic Bronchoscopy
Fiberoptic bronchoscopy (FOB) may be helpful for several reasons. First, FOB may visualize the bleeding and confirm the diagnosis of hemoptysis. Second, FOB may at least lateralize (right vs. left lung) and localize the source of bleeding. The operational value of FOB in identifying the site of bleeding may range from 34 to 93% when performed early, as opposed to 11 to 50% when performed later (64, 146, 170). Conversely, the operational value is decreasing in massive hemoptysis (30, 64). Finally, FOB may identify the cause of hemoptysis: 1) a mucosal erythema and edema as well as mucopurulent secretions suggest the diagnosis of bronchitis; 2) an endobronchial tumor suggest a benign tumor or a lung cancer; 3) a foreign body or a broncholith may be bronchoscopically diagnosed. Subsequently, FOB permits microbiological samples and tissue biopsy, if indicated. FOB and CT scan should be considered as complementary procedures rather than competitive. The advantage of FB or CT scan should be considered in the light of each clinical setting.
Multidetectory Row Computed Tomography (MDCT) Angiography
Currently, MDCT angiography is essential in the initial management of hemoptysis (Table 6). First, CT scan abnormalities reflecting the filling of the airways or the alveolar lumen with blood, namely ground glass opacities, alveolar condensation, atelectasis or unilateral lesion (e.g. tumor or aspergilloma) may be helpful in locating the bleeding. Occasionally, MDCT angiography may show the extravazation of the contrast medium into the bronchial lumen (24, 97). Overall, MDCT angiography may locate the bleeding in 70 to 80% of the cases (72, 99, 155). Interpretation of the MDCT angiography findings should take into account the possible redistribution of blood to dependant portions of the lung in patients with massive hemoptysis. Second, MDCT angiography may diagnose the cause of hemoptysis with high sensitivity (123). The main conditions such lung cancer, bronchiectasis, mycetoma, active tuberculosis are easily diagnosed. Pulmonary embolism should be diagnosed by a careful analysis of the pulmonary arteries. Assessment of the thoracic vasculature should include the pulmonary circulation as well as the systemic bronchial and non-bronchial circulations. Finally, MDCT angiography provides a detailed road map of the thoracic vasculature and the possible(s) mechanism(s) of hemoptysis. The bleeding from a pulmonary arterial origin occurs in 6.9 to 11% of the cases (98, 165). MDCT angiography signs suggesting a pulmonary arterial bleeding may be a pulmonary aneurysm, a pulmonary pseudo aneurysm, a pulmonary branch within the inner aspect of a cavity wall in the same area than the site of bleeding. As MDCT angiography provides a precise depiction of the systemic bronchial and non-bronchial arteries, their non-identification usually leads to the failure of the procedure of embolization.
Before the area of MDCT angiography, it has been demonstrated that CT scan was very useful for locating and diagnosing the cause of bleeding (72). On the other hand, several authors claimed that CT had limited clinical impact on therapeutic decisions (25, 72). However, recent publications suggest an impact on the treatment (74, 98). To our opinion, MDCT should be considered as a primary diagnostic investigation in patients with hemoptysis. In patients with a history of oropharyngeal cancer, the radiological should include the neck and the pharynx areas.
Laboratory Investigations
Further investigations associated with the medical history, the patients’ symptoms as well as the imaging data, should be performed. For instance, if the patient is septic, blood cultures and sputum samples for gram stain and cultures should be performed. Sputum should be sent for microbiological investigation including staining and culture for mycobacteria, if indicated. In cases of anticoagulation, the exploration of the coagulation profile is recommended.
In patients with alveolar haemorrhage, urinary analyses as well as echocardiogram are essential (Figure 5). Serum ANCA (anti neutrophil cytoplasm antibodies), antiglomerular basement membrane, antinuclear and antiphospholipid antibodies should be done. Tissue biopsy directed by the clinical involvement should be performed. Conversely, in patients diagnosed with a known immune disease, the first goal is to rule out an infection before concluding to an exacerbation of the immune disease.
Treatment
The treatment of hemoptysis depends on the underlying disease, the amount of bleeding as well as the facilities of the center and the physicians’ and radiologists’ experience. The advice from a lung pulmonary specialist is required.
Whatever the severity of hemoptysis, the treatment of the underlying disease is essential. Obvious causes should be treated with specific measures. For instance, antituberculous treatment should be initiated in patients suspected of tuberculosis, as well as chemotherapy in cancer patients, and so on. Antibiotics should be administered at least in case of a documented infection. In patients with bronchiectasis, all patients should be treated by antibiotics including antibiotics effective against Pseudomonas aeruginosa as well as Staphylococcus aureus (22, 57). Subsequently, antibiotics should be guided on the respiratory tract specimens’ cultures. The indications of antibiotics remain unclear in bronchitis. Some authors advocate the routine use of antibiotics to patients with hemoptysis in order to limit the infectious complications of aspiration and further bleeding (137). In case of necrotizing pneumonia, adequate antibiotics should be started as soon as possible. Delay in starting appropriate antibiotics for severe infection may be life-threatening. Unfortunately, the current recommendations for empirical antimicrobial treatment for CAP may be inadequate in this clinical setting, especially in pneumonia related to PVL producing Staphylococcus aureus strains. Moreover, some authors recommend the administration of immunoglobulins or antibiotics switching off the toxin production (i.e. clindamycin and linezolid) as adjunctive treatments (44, 124, 129). Additionally, linezolid is effective again all strains of Staphylococcus aureus. Therefore, initial microbiological investigation (i.e. respiratory tract specimens for gram stain and culture, blood culture) is essential. Recently, novel techniques as real time PCR assays have been developed, allowing a rapid identification of PVL producing staphylococcus strains (151, 177) within few hours. Additionally, these techniques provide information on the antimicrobial susceptibility patterns (177).
MANAGEMENT IN ICU OR EMERGENCY ROOM
Massive hemoptysis is a medical emergency and should be initially managed in an intensive care unit or an emergency room before referring the patient to a center able to perform both bronchial arteriography and surgery (25, 54,70, 86, 137, 193). The physician should be able to discuss the therapeutic options with an experienced pneumologist, a radiologist and a thoracic surgeon. Initial medical management includes general measures, bleeding control techniques and airways protection if indicated.
General Measures (Table 7)
Initial medical management includes insertion of wide-bore intravenous canula, bed rest, with the patient placed down on the side of the suspected source of haemorrhage (54, 90). Oxygen should be delivered to obtain arterial saturation above 90%. Treatments associated with an increased risk of bleeding such anticoagulation should be stopped. The correction of coagulation disorders should be discussed. If present, systemic hypertension should be controlled (114). Cough suppression remains controversial (46, 54, 86, 94, 174, 196). If the cough reflex is completely suppressed, blood will be retained within the lung and the severity of hemoptysis may be underestimated as well as atelectasis may occur. Conversely, a violent cough may expose to a massive recurrence of hemoptysis (18, 83,174).
Bleeding Control Techniques
Bronchoscopic Techniques
In case of ongoing bleeding despite the administration of general measures, the patient should be stabilized by endobronchial techniques or systemic agents or both.
If available, flexible fiberoptic bronchoscopy is essential to control the bleeding as well as to perform a suction of the blood and clear the clots that may obstruct the airways. A localized clot should not be removed because it may expose to a massive and lethal hemoptysis. In comparison to flexible fiberoptic bronchoscopy, rigid bronscocopy may provide a more efficient suction to maintain free airways. Nevertheless, it is usually performed under general anaesthesia and requires an experienced bronchoscopist (114). Additionally the ability to identify peripheral lesions is limited. Therefore, the use of rigid bronchoscopy has declined over the past decades (73). Several flexible fiberoptic techniques may be used to stop the bleeding. Cold saline solution lavage or epinephrine instillation are easy and may be successful (29, 45, 191). Iced saline lavage with sequential 50 ml aliquots controlled hemoptysis in a series of 12 patients with massive hemoptysis ( > 600 ml/ 24 hrs) (29). The volume of lavage averaged 500 ml per patient. This technique carried out the rigid bronchoscopy may be adapted to the flexible bronchoscopy. If the bleeding is not controlled, topical vasoconstrictives agents such terlipressin or ornipressin may be used as well as mechanical tamponade (e.g. a forgarty catheter) (21, 164, 185). Other topical hemostatic tamponade have been reported requiring either a rigid bronchoscopy, a flexible bronchoscopy or both (16, 184, 191). Tsukamoto et al. contended that topical treatment had similar efficacy to bronchial artery embolization. (184). Nevertheless, further studies are needed. Moreover, the agents used for topical tamponade are not easily available, except cold saline or epinephrine solutions and topical vasoconstrictive agents.
Systemic Vasoconstrictive Agents
If bronchoscopic techniques are not available or have no efficacy on the bleeding control, systemic vasoconstrictive agents such vasopressin or terlipressin should be administered (90, 149, 174). These pharmacologic agents should be used cautiously in patients with coronary disease and systemic hypertension and may thwart the efficiency of bronchial arteriography owing to the vasoconstriction of the bronchial arteries. It is noteworthy that no controlled trial using these drugs has been conducted in patients with massive hemoptysis.
Airways Protection
In case of active bleeding or airways compromise, the patients should be intubated with a large bore endotracheal tube allowing subsequent adequate suction, using FOB. Several strategies may be used to protect the non-bleeding lung and to stop the bleeding. First, if the bleeding is lateralized, unilateral intubation should be performed. If the bleeding is lateralized to the right, a long endotracheal tube may be advanced into the left main stem. In case of left bleeding, the selective intubation of the right lung may lead to an occlusion of the upper right lobe. An alternative is to place the endotratcheal tube into the trachea and to occlude the left main bronchus by a bronchial blocker. Second, if the bleeding side is unknown, a double-lumen may be inserted. It should be placed by an experienced operator. A misplaced tube may lead to aspiration and to death (68). Additionally, double-lumen tubes are easily obstructed by clots and do not permit the passage of bronchoscopes of adequate size to allow toilet under unobstructed vision. Last, the isolation of the bleeding bronchus may be achieved by a balloon tamponade such Forgarty catheter or endobronchial blockers (59, 67, 79, 89, 94). This latter approach is easier and provides furthermore more lung for gas exchange (59, 191). These bronchial blockers devices may also be used in non-intubated patients.
Recently, the successful use of recombinant factor VII in a patient with massive hemoptysis related to a pneumonia has been reported before the transfer to a tertiary referral center (116). Such medication should be considered as a temporizing measure in patients with massive hemoptysis.
At Referral Center
Once the medical condition has been stabilized, further modalities of treatment should be considered in a referral center.
Interventional Radiology
Interventional radiology has revolutionized the management of massive hemoptysis. Currently, MDCT angiography should be performed prior to endovascular treatment, owing the provided useful data (i.e. location and mechanism of bleeding, visualization of the systemic bronchial and non-bronchial arteries). Nevertheless, if MDCT angiography is not available, bronchial artery embolization should be considered as the first-line temporizing procedure in patients with life-threatening haemoptysis, since the bronchial and non-bronchial systemic arteries are the source of bleeding in more than 90% of the cases (54). Of note, the direct radiological sign of hemoptysis, consisting on the extravasation of the medium contrast into the bronchial lumen, occurs in less of 10% of the cases (82, 148). The location of bleeding suggested by the MDCT angiography and bronchoscopic findings combined with the angiographic findings (i.e., hypertrophic and tortuous bronchial arteries, neovascularity, hypervascularity, shunting into the pulmonary artery or vein ) may identify the vessel to embolize.
Bronchial arteriography with embolization is an effective method. Immediate control of hemoptysis is achieved in 75 to 91% of the cases (33, 54, 132, 147, 153, 175, 195). Immediate success rate may be increased by the use of microcatheter for supra selective embolization. Bleeding recurrence has been reported in 20 to 45% (46) and may be related to the following factors: prior use of vasoconstrictive agents; bleeding from other systemic arteries such the internal mammary artery; the underlying disease (e.g. cystic fibrosis, mycetoma or cancer) (12, 76, 95, 135, 176, 187). Several minor complications (e.g. chest pain, dysphagia, subintimal dissection of the aorta) have been commonly reported (187,197). The most dreaded complication of bronchial artery embolization is the inadvertence embolization of the spinal artery causing a spinal cord ischemic injury. The prevalence of spinal cord ischemia is low (<8%). Other serious adverse events include bronchial infarction, broncho esophageal fistula and ischemic colitis (197). The rate of complications is reduced by the experience of the radiologist and the use of microcatheter that allow supra selective catherization of the bronchial arteries (118).
If the bleeding originates from the pulmonary artery, a pulmonary angiography with vascular occlusion using coils may control the bleeding and avoid the traditional surgical treatment. Therefore, trans-catheter occlusion of the pulmonary arterial circulation may allow definitive and non-surgical treatment of these vascular lesions in selected patients (138, 154). Of note, in patients with pulmonary artery pseudo aneurysms, hemoptysis may also be related to the systemic bronchial arteries which appear in the vicinity of these lesions. Thus, at time, bronchial artery embolization is required. Recently, in cancer patients, stents graft have been used to stop the bleeding (134).
Surgery
Surgery is the treatment of choice of hemoptysis. Unfortunately, near half of the patients with hemoptysis are not candidate for surgery owing to their poor cardio-pulmonary reserve (90, 109). Additionally, a high mortality rate, ranging from 23% to 50%, has been reported for emergency surgery (25, 68, 90, 102). Currently, thoracic surgery for operable patients should be reserved in emergency when bronchial arteriography is unavailable, technically impossible (e.g. instable catheter, presence of the spinal artery) as well as in case of persistent active bleeding after embolization (46, 94). In patients with massive hemoptysis related to focal lesion, interventional radiology should be attempted to control the bleeding and surgery should be postponed. Subsequently, pulmonary resection (i.e. segmentectomy, lobectomy and pneumonectomy) should be considered for focal lesions, especially if those associated with a high likelihood of rebleeding (e.g. mycetoma) (49). Benefits should always be weighted against the risk of operative complications and mortality. Serious postoperative complications, such as haemorrhage, empyema, respiratory infections, wound infections, respiratory failure and broncho pleural fistulae are common (101). Operative mortality is about 6% (90, 102). Exceptionally, bronchial artery ligation have been performed (12, 143).
Other techniques to control hemoptysis Other approaches have been used to control massive hemoptysis such intracavitary administration of antifungal therapy or radiotherapy in mycetoma and laser photocoagulation in lung cancer but there is little experience of those techniques (52, 63, 94).
Pulmonary Alveolar Haemorrhage
The treatment depends on the cause. In immune alveolar haemorrhage, the cornerstone of the treatment is the administration of high-dose of steroids often associated with cyclophosphamide (58). Except in Goodpasture syndrome, the benefit of plasmapheresis remains unclear in immune alveolar haemorrhage. Surgery is mandatory in alveolar haemorrhage related to mitral stenosis.
CONCLUSION
Since the differential diagnoses of hemoptysis are broad, a thorough approach should be performed. An advice from a lung pulmonary specialist is advocated. An early diagnosis of the cause of hemoptysis is the best prevention modality of massive hemoptysis. In case of massive hemoptysis, a multidisciplinary management (pneumologist, intensivist, radiolologist, surgeon) is mandatory. Endovascular treatment is the treatment of choice owing to the lower morbidity and morality rates, as compared with emergency surgery.
REFERENCES
1. Abal AT, Nair PC, Cherian J. Haemoptysis: aetiology, evaluation and outcome--a prospective study in a third-world country. Respir Med 2001;95:548-552. [PubMed]
2. Abbott OA. The clinical significance of pulmonary hemorrhage; a study of 1316 patients with chest disease. Dis Chest 1948;14:824-842. [PubMed]
3. Adelman M, Haponik EF, Bleecker ER, Britt EJ. Cryptogenic hemoptysis. Clinical features, bronchoscopic findings, and natural history in 67 patients. Ann Intern Med 1985;102:829-834. [PubMed]
4. Akoglu S, Ucan ES, Celik G, Sener G, Sevinc C, Kilinc O, Itil, O. Endobronchial metastases from extrathoracic malignancies. Clin Exp Metastasis 2005;22:587-591. [PubMed]
5. Albelda SM, Talbot GH, Gerson S., Miller WT, Cassileth, PA. Pulmonary cavitation and massive hemoptysis in invasive pulmonary aspergillosis. Influence of bone marrow recovery in patients with acute leukemia. Am Rev Respir Dis 1985;131:115-120. [PubMed]
6. Alobeidy ST, Mehth R, Niederman M. Etiology and outcome of hemoptysis in patients on anticoagulation therapy. Am J Respir Crit Care Med 2001:163.
7. Amirana M, Frater R, Tirschwell P, Janis M, Bloomberg A, State D. An aggressive surgical approach to significant hemoptysis in patients with pulmonary tuberculosis. Am Rev Respir Dis 1968;97:187-192. [PubMed]
8. Antonelli M, Midulla F, Tancredi G, Salvatori FM, Bonci E, Cimino G, Flaishman I. Bronchial artery embolization for the management of nonmassive hemoptysis in cystic fibrosis. Chest 2002;121:796-801. [PubMed]
9. Arsalane A, Parrot A, Assouad J, Tchanderli R, Bazelly B. [Spontaneous hemothorax: a rare but serious complication of intralobular pulmonary sequestration]. Rev Pneumol Clin 2006;62: 30-33. [PubMed]
10. Auerbach O. Pathology and pathogenesis of pulmonary arterial aneurysm in tuberculosis cavities. Am Rev Tuberc 1939;39: 99-115.
11. Ayed A. Pulmonary resection for massive hemoptysis of benign etiology. Eur J Cardiothorac Surg 2003;24: 689-693. [PubMed]
12. Barben J, Robertson D, Olinsky A, Ditchfield M. Bronchial artery embolization for hemoptysis in young patients with cystic fibrosis. Radiology 2002;224:124-130. [PubMed]
13. Barker AF. Bronchiectasis. N Engl J Med 2002;346:1383-1393. [PubMed]
14. Bell WR, Simon TL, DeMets DL. The clinical features of submassive and massive pulmonary emboli. Am J Med 1977;22:355-360. [PubMed]
15. Benoit FL, Rulon DB, Theil GB, Doolan PD, Watten RH. Goodpasture's Syndrome: a Clinicopathologic Entity. Am J Med 1964;47:424-444. [PubMed]
16. Bhattacharyya P, Dutta A, Samanta AN, Chowdhury SR. New procedure: bronchoscopic endobronchial sealing; a new mode of managing hemoptysis. Chest 2002;121: 2066-2069. [PubMed]
17. Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Physician 2005;72:1253-1260. [PubMed]
18. Bobrowitz ID, Ramakrishna S, Shim YS. Comparison of medical v surgical treatment of major hemoptysis. Arch Intern Med 1983;143:1343-1346. [PubMed]
19. Boe DM, Schwarz MI. A 31-year-old man with chronic cough and hemoptysis. Chest 2007;132:721-726. [PubMed]
20. Boussaud V, Parrot A, Mayaud C, Wislez M, Antoine M, Picard C, Delisle F, Etienne, J, Cadranel J. Life-threatening hemoptysis in adults with community-acquired pneumonia due to Panton-Valentine leukocidin-secreting Staphylococcus aureus. Intensive Care Med 2003; 29:1840-1843. [PubMed]
21. Breuer HW, Charchut S, Worth H, Trampisch HJ, Glanzer K. Endobronchial versus intravenous application of the vasopressin derivative glypressin during diagnostic bronchoscopy. Eur Respir J 1989;2:225-228. [PubMed]
22. Brinson GM, Noone PG, Mauro MA, Knowles MR, Yankaskas JR, Sandhu JS, Jaques PF. Bronchial artery embolization for the treatment of hemoptysis in patients with cystic fibrosis. Am J Respir Crit Care Med 1998;157:1951-1958.[PubMed]
23. Broberg C, Ujita M, Babu-Narayan S, Rubens M, Prasad, SK, Gibbs JS, Gatzoulis MA. Massive pulmonary artery thrombosis with haemoptysis in adults with Eisenmenger's syndrome: a clinical dilemma. Heart 2004; 90:e63.[PubMed]
24. Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006;26:3-22. [PubMed]
25. Cahill BC, Ingbar DH. Massive hemoptysis. Assessment and management. Clin Chest Med 1994;15:147-167. [PubMed]
26. Chang JC, Cregler LL. Hemoptysis in a patient with congestive heart failure and pulmonary emboli. J Natl Med Assoc 1994;86:383-386. [PubMed]
27. Clarot C, Leleu O, Touati G, Reix T, Jounieaux V. [Aortobronchial fistulas.]. Rev Mal Respir 2004;21:943-949. [PubMed]
28. Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583-592, vii. [PubMed]
29. Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980;35:901-904. [PubMed]
30. Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci 1987;294:301-309. [PubMed]
31. Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, Chevrier S, Meunier C, Lebert C, Aupee M, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis 2006;43:577-584. [PubMed]
32. Cowling MG, Belli AM. A potential pitfall in bronchial artery embolization. Clin Radiol 1995;50:105-107. [PubMed]
33. Cremaschi P, Nascimbene C, Vitulo P, Catanese C, Rota L, Barazzoni GC, Cornalba GP. Therapeutic embolization of bronchial artery: a successful treatment in 209 cases of relapse hemoptysis. Angiology 1993;44:295-299. [PubMed]
34. Crocco JA, Rooney JJ, Fankushen DS, DiBenedetto RJ, Lyons HA. Massive hemoptysis. Arch Intern Med 1968;121:495-498. [PubMed]
35. Dalen JE, Haffajee CI, Alpert J, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977;296:1431-1435. [PubMed]
36. Davis SD, Neithamer CD, Schreiber TS, Sos TA. False pulmonary artery aneurysm induced by Swan-Ganz catheter: diagnosis and embolotherapy. Radiology 1987;164:741-742. [PubMed]
37. Deane KD, West SG. Antiphospholipid antibodies as a cause of pulmonary capillaritis and diffuse alveolar hemorrhage: a case series and literature review. Semin Arthritis Rheum 2005;35:154-165. [PubMed]
38. Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987;135:463-481. [PubMed]
39. di Sant'agnese PA, Davis PB. Cystic fibrosis in adults. 75 cases and a review of 232 cases in the literature. Am J Med 1979;66:121-132. [PubMed]
40. Diamond, M. A., and Genovese, P. Life-threatening hemoptysi in mitral stenosis. Emergency mitral valve replacement resulting in rapid, sustained cessation of pulmonary bleeding. JAMA 1971; 215:441-444. [PubMed]
41. DiLeo MD, Amedee RG, Butcher RB. Hemoptysis and pseudohemoptysis: the patient expectorating blood. Ear Nose Throat J 1995;74:822-824, 826, 828, passim. [PubMed]
42. Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007;115:1039-1050. [PubMed]
43. Dohen-Becue F, Salez F, Ramon P, Leblond-Tillie I, Wallaert B, Bauters A, Tonnel AB. [Management of hemoptysis in invasive pulmonary aspergillosis]. Rev Mal Respir 1998;15:791-796. [PubMed]
44. Dumitrescu O, Boisset S, Badiou C, Bes M, Benito Y, Reverdy ME, Vandenesch F, Etienne J, Lina G. Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob Agents Chemother 2007;51:1515-1519. [PubMed]
45. Dupree HJ, Lewejohann JC, Gleiss J, Muhl E, Bruch HP. Fiberoptic bronchoscopy of intubated patients with life-threatening hemoptysis. World J Surg 2001;25:104-107. [PubMed]
46. Dweik RA, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest Med 1999;20:89-105. [PubMed]
47. Edelman RR, Johnson TS, Jhaveri HS, Kim D, Kasdon E, Frank HA, Simon M. Fatal hemoptysis resulting from erosion of a pulmonary artery in cavitary sarcoidosis. AJR Am J Roentgenol 1985;145:37-38. [PubMed]
48. Efrati O, Harash O, Rivlin J, Bibi H, Meir MZ, Blau H, Mussaffi H, Barak A, Levy I, Vilozni D, et al. Hemoptysis in Israeli CF patients - Prevalence, treatment, and clinical characteristics. J Cyst Fibros. 2008. [PubMed]
49. Endo S, Otani S, Saito N, Hasegawa T, Kanai Y, Sato Y, Sohara Y. Management of massive hemoptysis in a thoracic surgical unit. Eur J Cardiothorac Surg 2003;23:467-472. [PubMed]
50. Erkan F, Cavdar T. Pulmonary vasculitis in Behcet's disease. Am Rev Respir Dis 1992;146:232-239. [PubMed]
51. Erkan F, Gul A, Tasali E. Pulmonary manifestations of Behcet's disease. Thorax 2001;56:572-578. [PubMed]
52. Falkson C, Sur R, Pacella J. External beam radiotherapy: a treatment option for massive haemoptysis caused by mycetoma. Clin Oncol (R Coll Radiol) 2002;14:233-235. [PubMed]
53. Farghly E, Bousamra M. Hemoptysis resulting from unilateral pulmonary artery agenesis. Ann Thorac Surg 2002;74: 255-257. [PubMed]
54. Fartoukh M, Khalil A, Louis L, Carette MF, Bazelly B, Cadranel J, Mayaud C, Parrot A. An integrated approach to diagnosis and management of severe haemoptysis in patients admitted to the intensive care unit: a case series from a referral centre. Respir Res2007;8:11. [PubMed]
55. Fernando HC, Stein M, Benfield JR, Link DP. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg 1998;133:862-866. [PubMed]
56. Fidan A, Ozdogan S, Oruc O, Salepci B, Ocal Z, Caglayan B. (2002). Hemoptysis: a retrospective analysis of 108 cases. Respir Med 2002;96:677-680. [PubMed]
57. Flume PA, Yankaskas JR, Ebeling M, Hulsey T, Clark LL. Massive hemoptysis in cystic fibrosis. Chest 2005;128:729-738. [PubMed]
58. Frankel SK, Cosgrove GP, Fischer A, Meehan, R. T., and Brown, K. K. (2006). Update in the diagnosis and management of pulmonary vasculitis. Chest 129, 452-465. [PubMed]
59. Freitag L, Tekolf E, Stamatis G, Montag M, Greschuchna D. Three years experience with a new balloon catheter for the management of haemoptysis. Eur Respir J 1994;7:2033-2037. [PubMed]
60. Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002;39:42-47. [PubMed]
61. Garzon AA, Cerruti MM, Golding ME. Exsanguinating hemoptysis. J Thorac Cardiovasc Surg 1982;84:829-833. [PubMed]
62. Gillet Y, Vanhems P, Lina G, Bes M, Vandenesch F, Floret D, Etienne J. (2007). Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin Infect Dis 2007;45:315-321. [PubMed]
63. Giron J, Poey C, Fajadet P, Sans N, Fourcade D, Senac JP, Railhac JJ. CT-guided percutaneous treatment of inoperable pulmonary aspergillomas: a study of 40 cases. Eur J Radiol 1998;28:235-242. [PubMed]
64. Gong H, Salvatierra C. Clinical efficacy of early and delayed fiberoptic bronchoscopy in patients with hemoptysis. Am Rev Respir Dis 1981;124:221-225. [PubMed]
65. Gorelik O, Cohen N, Shpirer I, Almoznino-Sarafian D, Alon I, Koopfer M, Yona R, Modai D. (2000). Fatal haemoptysis induced by invasive pulmonary aspergillosis in patients with acute leukaemia during bone marrow and clinical remission: report of two cases and review of the literature. J Infect 2000;41:277-282. [PubMed]
66. Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med 1998;158:643-661. [PubMed]
67. Gottlieb LS, Hillberg R. (1975). Endobronchial tamponade therapy for intractable hemoptysis. Chest 1975;67:482-483. [PubMed]
68. Gourin A, Garzon AA. Operative treatment of massive hemoptysis. Ann Thorac Surg 1974;18:52-60. [PubMed]
69. Gungor S, Damadoglu E, Aybatli A, Yilmaz A, Kir A, Akkaya E. Typical pulmonary carcinoid tumors: presentation and outcome of 24 cases. Med Sci Monit 2006;12:CR315-318. [PubMed]
70. Hakanson E, Konstantinov IE, Fransson SG, Svedjeholm R. Management of life-threatening haemoptysis. Br J Anaesth 2002;88:291-295. [PubMed]
71. Hamer DH, Schwab LE, Gray R. Massive hemoptysis from thoracic actinomycosis successfully treated by embolization. Chest 1992;101:1442-1443. [PubMed]
72. Haponik EF, Britt EJ, Smith PL, Bleecker ER. Computed chest tomography in the evaluation of hemoptysis. Impact on diagnosis and treatment. Chest 1987;91:80-85. [PubMed]
73. Haponik EF, Fein A, Chin R. (2000). Managing life-threatening hemoptysis: has anything really changed? Chest 2000;118:1431-1435. [PubMed]
74. Hartmann IJ, Remy-Jardin M, Menchini L, Teisseire A, Khalil C, Remy J. Ectopic origin of bronchial arteries: assessment with multidetector helical CT angiography. Eur Radiol 2007;17:1943-1953. [PubMed]
75. Haworth SJ, Savage CO, Carr D, Hughes JM, Rees AJ. Pulmonary haemorrhage complicating Wegener's granulomatosis and microscopic polyarteritis. Br Med J (Clin Res Ed) 1985;290:1775-1778. [PubMed]
76. Hayakawa K, Tanaka F, Torizuka T, Mitsumori M, Okuno Y, Matsui A, Satoh Y, Fujiwara K, Misaki T. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol 1992;15:154-158; discussion 158-159. [PubMed]
77. Herry I, Cadranel J, Antoine M, Meharzi J, Michelson S, Parrot A, Rozenbaum W, Mayaud C. Cytomegalovirus-induced alveolar hemorrhage in patients with AIDS: a new clinical entity? Clin Infect Dis 1996;22:616-620. [PubMed]
78. Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001;120:1592-1594. [PubMed]
79. Hiebert CA. Balloon catheter control of life-threatening hemoptysis. Chest 1974;66: 308-309. [PubMed]
80. Hirshberg B, Biran I, Glazer M, Kramer MR. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest 1997;112:440-444. [PubMed]
81. Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996;7:23-32.[PubMed]
82. Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001;177:861-867. [PubMed]
83. Imgrund SP, Goldberg SK, Walkenstein MD, Fischer R, Lippmann ML. Clinical diagnosis of massive hemoptysis using the fiberoptic bronchoscope. Crit Care Med 1985;13: 438-443. [PubMed]
84. Israel HL, Lenchner GS, Atkinson GW. Sarcoidosis and aspergilloma. The role of surgery. Chest 1982;82:430-432. [PubMed]
85. Jaureguiberry S, Roussel M, Brinchault-Rabin G, Gacouin A, Le Meur A, Arvieux C, Michelet C, Tattevin P. Clinical presentation of leptospirosis: a retrospective study of 34 patients admitted to a single institution in metropolitan France. Clin Microbiol Infect 2005; 11:391-394. [PubMed]
86. Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000;28:1642-1647. [PubMed]
87. Jennings CA, King TE, Tuder R, Cherniack RM, Schwarz MI. Diffuse alveolar hemorrhage with underlying isolated, pauciimmune pulmonary capillaritis. Am J Respir Crit Care Med 1997;155: 1101-1109. [PubMed]
88. Johnston H, Reisz G. Changing spectrum of hemoptysis. Underlying causes in 148 patients undergoing diagnostic flexible fiberoptic bronchoscopy. Arch Intern Med 1989; 149:1666-1668. [PubMed]
89. Jolliet P, Soccal P, Chevrolet JC. Control of massive hemoptysis by endobronchial tamponade with a pulmonary artery balloon catheter. Crit Care Med 1992;20:1730-1732. [PubMed]
90. Jougon J, Ballester M, Delcambre F, Mac Bride T, Valat P, Gomez F, Laurent F, Velly JF. Massive hemoptysis: what place for medical and surgical treatment. Eur J Cardiothorac Surg 2002;22:345-351. [PubMed]
91. Kalina M, Giberson F. Hemoptysis secondary to pulmonary artery pseudoaneurysm after necrotizing pneumonia. Ann Thorac Surg 2007;84:1386-1387. [PubMed]
92. Kallenbach J, Song E, Zwi S. Haemoptysis with no radiological evidence of tumour - the value of early bronchoscopy. S Afr Med J 1981;59:556-558. [PubMed]
93. Karak P, Dimick R, Hamrick KM, Schwartzberg M, Saddekni S. Immediate transcatheter embolization of Swan-Ganz catheter-induced pulmonary artery pseudoaneurysm. Chest 1997;111:1450-1452. [PubMed]
94. Karmy-Jones R, Cuschieri J, Vallieres E. Role of bronchoscopy in massive hemoptysis. Chest Surg Clin N Am 2001;11:873-906. [PubMed]
95. Katoh O, Kishikawa T, Yamada H, Matsumoto S, Kudo S. Recurrent bleeding after arterial embolization in patients with hemoptysis. Chest 1990;97:541-546. [PubMed]
96. Kerem E, Bar Ziv Y, Rudenski B, Katz S, Kleid D, Branski D. Bacteremic necrotizing pneumococcal pneumonia in children. Am J Respir Crit Care Med 1994;149:242-244. [PubMed]
97. Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007;188:W117-125. [PubMed]
98. Khalil A, Parrot A, Nedelcu C, Fartoukh M, Marsault C, Carette MF. Severe hemoptysis of pulmonary arterial origin: signs and role of multidetector row CT angiography. Chest 2008;133:212-219. [PubMed]
99. Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007;80:21-25. [PubMed]
100. Kim EA, Lee KS, Primack SL, Yoon HK, Byun HS, Kim TS, Suh GY, Kwon OJ, Han J. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics 2002;22:Spec No, S137-149. [PubMed]
101. Kim YT, Kang MC, Sung SW, Kim JH. Good long-term outcomes after surgical treatment of simple and complex pulmonary aspergilloma. Ann Thorac Surg 2005;79:294-298. [PubMed]
102. Knott-Craig CJ, Oostuizen JG, Rossouw G, Joubert JR, Barnard PM. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J Thorac Cardiovasc Surg 1993;105:394-397. [PubMed]
103. Kokotsakis J, Misthos P, Athanasiou T, Romana C, Skouteli E, Lioulias A, Kaskarelis I. Endovascular stenting for primary aortobronchial fistula in association with massive hemoptysis. Tex Heart Inst J 2007;34:369-372. [PubMed]
104. Kvale PA, Selecky PA, Prakash UB. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:368S-403S. [PubMed]
105. Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 2007;315:1130-1133. [PubMed]
106. Lauque D, Cadranel J, Lazor R, Pourrat J, Ronco P, Guillevin L, Cordier JF. Microscopic polyangiitis with alveolar hemorrhage. A study of 29 cases and review of the literature. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P). Medicine (Baltimore) 2000;79:222-233. [PubMed]
107. Lazor R, Bigay-Game L, Cottin V, Cadranel J, Decaux O, Fellrath JM, Cordier JF. Alveolar hemorrhage in anti-basement membrane antibody disease: a series of 28 cases. Medicine (Baltimore) 2007;86:181-193. [PubMed]
108. Leatherman JW, Davies SF, Hoidal JR. Alveolar hemorrhage syndromes: diffuse microvascular lung hemorrhage in immune and idiopathic disorders. Medicine (Baltimore) 1984;63:343-361. [PubMed]
109. Lee TW, Wan S, Choy DK, Chan M, Arifi A, Yim AP. Management of massive hemoptysis: a single institution experience. Ann Thorac Cardiovasc Surg 2000;6: 232-235. [PubMed]
110. Lemay V, Carette MF, Parrot A, Bazelly B, Grivaux M, Milleron B. (1995). [Hemoptysis in sarcoidosis. Apropos of 6 cases including 4 with fatal outcome]. Rev Pneumol Clin 1995;51:61-70. [PubMed]
111. Lenner R, Schilero GJ, Lesser M. Hemoptysis: diagnosis and management. Compr Ther 2002;28:7-14. [PubMed]
112. Lewis M, Kallenbach J, Kark P, Zaltzman M, Hockman M, Zwi S. Severe haemoptysis associated with viral tracheitis. Thorax 1982;37:869. [PubMed]
113. Lopez Alvarez JM, Valeron Lemaur ME, Consuegra Llapur E, Urquia Marti L, Moron Saen de Casas A, Gonzalez Jorge R. [Lethal streptococcal toxic shock syndrome in pediatrics. Presentation of 3 cases]. Med Intensiva 2007;31:100-103. [PubMed]
114. Lordan JL, Gascoigne A, Corris PA. The pulmonary physician in critical care *Illustrative case 7: Assessment and management of massive haemoptysis. Thorax 2003;58: 814-819. [PubMed]
115. Luks AM, Lakshminarayanan S, Hirschmann JV. Leptospirosis presenting as diffuse alveolar hemorrhage: case report and literature review. Chest 2003;123:639-643. [PubMed]
116. Macdonald JA, Fraser JF, Foot CL, Tran K. Successful use of recombinant factor VII in massive hemoptysis due to community-acquired pneumonia. Chest 2006;130:577-579. [PubMed]
117. Magro C, Ali N, Williams JD, Allen JN, Ross P. Cytomegalovirus-associated pulmonary septal capillary injury sine inclusion body change: a distinctive cause of occult or macroscopic pulmonary hemorrhage in the immunocompetent host. Appl Immunohistochem Mol Morphol 2005;13:268-272. [PubMed]
118. Mal H, Rullon I, Mellot F, Brugiere O, Sleiman C, Menu Y, Fournier M. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 1999;115:996-1001. [PubMed]
119. Malhotra P, Aggarwal R, Aggarwal AN, Jindal SK, Awasthi A, Radotra BD. Coeliac disease as a cause of unusually severe anaemia in a young man with idiopathic pulmonary haemosiderosis. Respir Med 2005;99:451-453. [PubMed]
120. Malik AB, Tracy SE. Bronchovascular adjustments after pulmonary embolism. J Appl Physiol 1980;49:476-481. [PubMed]
121. Marshall TJ, Flower CD, Jackson JE. The role of radiology in the investigation and management of patients with haemoptysis. Clin Radiol 1996;51:391-400. [PubMed]
122. Matsuda A. Bronchial arteriography in patients with pulmonary embolism. Chest 1984;85:767-773. [PubMed]
123. McGuinness G, Beacher J, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994;105:1155-1162. [PubMed]
124. Micek ST, Dunne M, Kollef MH. Pleuropulmonary complications of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus: importance of treatment with antimicrobials inhibiting exotoxin production. Chest 2005;128:2732-2738. [PubMed]
125. Middleton JR, Sen P, Lange M, Salaki J, Kapila R, Louria DB. Death-producing hemoptysis in tuberculosis. Chest 1977;72:601-604. [PubMed]
126. Miller RR, McGregor DH. Hemorrhage from carcinoma of the lung. Cancer 1980;46:200-205. [PubMed]
127. Mitzner W, Wagner EM. Vascular remodeling in the circulations of the lung. J Appl Physiol 2004;97:1999-2004. [PubMed]
128. Mole TM, Glover J, Sheppard MN. Sclerosing mediastinitis: a report on 18 cases. Thorax 1995;50:280-283. [PubMed]
129. Morgan MS. Diagnosis and treatment of Panton-Valentine leukocidin (PVL)-associated staphylococcal pneumonia. Int J Antimicrob Agents 2007;30:289-296. [PubMed]
130. Moser KM. Venous thromboembolism. Am Rev Respir Dis 1990:141:235-249. [PubMed]
131. O'Neil KM, Lazarus AA. Hemoptysis. Indications for bronchoscopy. Arch Intern Med 1991;151:171-174. [PubMed]
132. Ong TH, Eng P. Massive hemoptysis requiring intensive care. Intensive Care Med 2003;29:317-320. [PubMed]
133. Panos RJ, Barr LF, Walsh TJ, Silverman HJ. Factors associated with fatal hemoptysis in cancer patients. Chest 1988;94:1008-1013. [PubMed]
134. Park A, Cwikiel W. Endovascular treatment of a pulmonary artery pseudoaneurysm with a stent graft: report of two cases. Acta Radiol 2007;48:45-47. [PubMed]
135. Park HS, Kim YI, Kim HY, Zo JI, Lee JH, Lee JS. Bronchial artery and systemic artery embolization in the management of primary lung cancer patients with hemoptysis. Cardiovasc Intervent Radiol 2007;30:638-643. [PubMed]
136. Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, Flower CD, Bilton D, Keogan MT. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000;162:1277-1284. [PubMed]
137. Patel U, Pattison CW, Raphael M. Management of massive haemoptysis. Br J Hosp Med 1994;52:74-78. [PubMed]
138. Pelage JP, El Hajjam M, Lagrange C, Chinet T, Vieillard-Baron A, Chagnon S, Lacombe P. Pulmonary artery interventions: an overview. Radiographics 2005;25:1653-1667. [PubMed]
139. Pfitzner J, Peacock MJ, Tsirgiotis E, Walkley IH. Lobectomy for cavitating lung abscess with haemoptysis: strategy for protecting the contralateral lung and also the non-involved lobe of the ipsilateral lung. Br J Anaesth 2000;85:791-794. [PubMed]
140. Philpott NJ, Woodhead MA, Wilson AG, Millard FJ. Lung abscess: a neglected cause of life threatening haemoptysis. Thorax 1993;48:674-675. [PubMed]
141. Picard C, Parrot A, Boussaud V, Lavole A, Saidi F, Mayaud C, Carette MF. Massive hemoptysis due to Rasmussen aneurysm: detection with helicoidal CT angiography and successful steel coil embolization. Intensive Care Med 2003;29:1837-1839. [PubMed]
142. Pirrelli S, Bozzani A, Arici V, Odero A. Endovascular treatment of acute haemoptysis secondary to aortobronchial fistula. Eur J Vasc Endovasc Surg 2006;32:366-368. [PubMed]
143. Planquette B, Bagan P, Cox A, Valcke J, Riquet M. Massive hemoptysis: Successful treatment with surgical ligation of the thyrocervical artery. J Thorac Cardiovasc Surg 2007;133:1106-1108. [PubMed]
144. Plantier L, Saidi F, Choukroun G, Boussaud V, Parrot A, Bazelly B, Mayaud C. [Moderately abundant hemoptysis revealing endobronchial hamartochondroma]. Rev Pneumol Clin 2003;59:49-51. [PubMed]
145. Plessinger VA, Jolly PN. Rasmussen's aneurysms and fatal hemorrhage in pulmonary tuberculosis. Am Rev Tuberc 1949;60:589-603, illust. [PubMed]
146. Pursel SE, Lindskog GE. Hemoptysis. A clinical evaluation of 105 patients examined consecutively on a thoracic surgical service. Am Rev Respir Dis 1961;84:329-336. [PubMed]
147. Rabkin JE, Astafjev VI, Gothman LN, Grigorjev YG. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology 1987;163:361-365. [PubMed]
148. Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology 1996;200:691-694. [PubMed]
149. Ramon P, Wallaert B, Derollez M, D'Odemont JP, Tonnel AB. [Treatment of severe hemoptysis with terlipressin. Study of the efficacy and tolerance of this product]. Rev Mal Respir 1989;6:365-368. [PubMed]
150. Reimel BA, Krishnadasen B, Cuschieri J, Klein MB, Gross J, Karmy-Jones R. Surgical management of acute necrotizing lung infections. Can Respir J 2006;13:369-373. [PubMed]
151. Reischl U, Tuohy MJ, Hall GS, Procop GW, Lehn N, Linde H. Rapid detection of Panton-Valentine leukocidin-positive Staphylococcus aureus by real-time PCR targeting the lukS-PV gene. Eur J Clin Microbiol Infect Dis 2007;26:131-135. [PubMed]
152. Reisz G, Stevens D, Boutwell C, Nair V. The causes of hemoptysis revisited. A review of the etiologies of hemoptysis between 1986 and 1995. Mo Med 1997;94:633-635. [PubMed]
153. Remy J, Arnaud A, Fardou H, Giraud R, Voisin C. Treatment of hemoptysis by embolization of bronchial arteries. Radiology 1977;122:33-37. [PubMed]
154. Remy J, Lemaitre L, Lafitte JJ, Vilain MO, Saint Michel J, Steenhouwer F. Massive hemoptysis of pulmonary arterial origin: diagnosis and treatment. AJR Am J Roentgenol 1984;143:963-969. [PubMed]
155. Revel MP, Fournier LS, Hennebicque AS, Cuenod CA, Meyer G, Reynaud P, Frija G. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002;179:1217-1224. [PubMed]
156. Rossi SE, McAdams HP, Rosado-de-Christenson ML, Franks TJ, Galvin JR. Fibrosing mediastinitis. Radiographics 2001;21:737-757. [PubMed]
157. Routsi C, Charitos C, Rontogianni D, Daniil Z, Zakynthinos E. Unilateral pulmonary edema due to pulmonary venous obstruction from fibrosing mediastinitis. Int J Cardiol 2006;108:418-421. [PubMed]
158. Rubinstein I, Baum GL, Hiss Y, Solomon A. Hemoptysis in sarcoidosis. Eur J Respir Dis 1985;66, 302-305. [PubMed]
159. Saad EB, Rossillo A, Saad CP, Martin DO, Bhargava M, Erciyes D, Bash D, Williams-Andrews M, Beheiry S, Marrouche NF, et al. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: functional characterization, evolution, and influence of the ablation strategy. Circulation 2003;108, 3102-3107. [PubMed]
160. Sakuma M, Demachi J, Suzuki J, Nawata J, Takahashi T, Sugimura K, Oikawa M, Takase K, Hoshino K, Souma S, Shirato K. Peripheral pulmonary artery aneurysms in patients with pulmonary artery hypertension. Intern Med 2007;46:979-984. [PubMed]
161. Santiago SM, Lehrman S, Williams AJ. Bronchoscopy in patients with haemoptysis and normal chest roentgenograms. Br J Dis Chest 1987;81:186-188. [PubMed]
162. Santos-Ocampo AS, Mandell BF, Fessler BJ. Alveolar hemorrhage in systemic lupus erythematosus: presentation and management. Chest 2000;118:1083-1090. [PubMed]
163. Savale L, Parrot A, Khalil A, Antoine M, Theodore J, Carette MF, Mayaud C, Fartoukh M. Cryptogenic hemoptysis: from a benign to a life-threatening pathologic vascular condition. Am J Respir Crit Care Med 2007;175, 1181-1185.[PubMed]
164. Saw EC, Gottlieb LS, Yokoyama T, Lee BC. Flexible fiberoptic bronchoscopy and endobronchial tamponade in the management of massive hemoptysis. Chest 1976;70: 589-591. [PubMed]
165. Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005;184:1253-1259. [PubMed]
166. Sehhat S, Oreizie M, Moinedine K. Massive pulmonary hemorrhage: surgical approach as choice of treatment. Ann Thorac Surg 1978;25:12-15. [PubMed]
167. Shin TB, Yoon SK, Lee KN, Choi JS, Kim YH, Sung CG, Kim YJ, Kim CW. The role of pulmonary CT angiography and selective pulmonary angiography in endovascular management of pulmonary artery pseudoaneurysms associated with infectious lung diseases. J Vasc Interv Radiol 2007;18:882-887. [PubMed]
168. Silveira F, Paterson DL. Pulmonary fungal infections. Curr Opin Pulm Med 2005;11:242-246. [PubMed]
169. Silverman ES, Mark EJ. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 36-2002. A 32-year-old man with hemoptysis of nearly three decades' duration. N Engl J Med 2002;347:1693-1701. [PubMed]
170. Smiddy JF, Elliott RC. (1973). The evaluation of hemoptysis with fiberoptic bronchoscopy. Chest 1973;64:158-162. [PubMed]
171. Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002;121:1988-1999. [PubMed]
172. Stergiopoulou T, Meletiadis J, Roilides E, Kleiner DE, Schaufele R, Roden M, Harrington S, Dad L, Segal B, Walsh TJ. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol 2007;127:349-355. [PubMed]
173. Stevens DA, Kan VL, Judson MA, Morrison VA, Dummer S, Denning DW, Bennett JE, Walsh TJ, Patterson TF, Pankey GA. Practice guidelines for diseases caused by Aspergillus. Infectious Diseases Society of America. Clin Infect Dis 2000;30:696-709. [PubMed]
174. Stoller JK. Diagnosis and Management of Massive Hemoptysis. Respiratory care 1992;37:564-581.
175. Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002;121:789-795. [PubMed]
176. Tamura S, Kodama T, Otsuka N, Kihara Y, Nisikawa K, Yuki Y, Samejima M, Uwada O, Watanabe K, Minoda S. Embolotherapy for persistent hemoptysis: the significance of pleural thickening. Cardiovasc Intervent Radiol 1993;16:85-88. [PubMed]
177. Tang, Y. W., Kilic, A., Yang, Q., McAllister, S. K., Li, H., Miller, R. S., McCormac, M., Tracy, K. D., Stratton, C. W., Han, J., and Limbago, B. (2007). StaphPlex system for rapid and simultaneous identification of antibiotic resistance determinants and Panton-Valentine leukocidin detection of staphylococci from positive blood cultures. J Clin Microbiol 45, 1867-1873. [PubMed]
178. Terada Y, Chen F, Shoji T, Itoh H, Wada H, Hitomi S. A case of endobronchial endometriosis treated by subsegmentectomy. Chest 1999;115:1475-1478. [PubMed]
179. Thomas CS, Endrys J, Abul A, Cherian G. Late massive haemoptyses from bronchopulmonary collaterals in infarcted segments following pulmonary embolism. Eur Respir J 1999;13:463-464. [PubMed]
180. Thomas P, Reynaud-Gaubert M, Bartoli JM, Auge A, Garbe L, Giudicelli R, Fuentes P. Exsanguinating hemoptysis revealing the absence of left pulmonary artery in an adult. Ann Thorac Surg 2001;72:1748-1750. [PubMed]
181. Tomlinson JR, Sahn SA. Aspergilloma in sarcoid and tuberculosis. Chest 1987;92:505-508. [PubMed]
182. Travis WD, Carpenter HA, Lie JT. Diffuse pulmonary hemorrhage. An uncommon manifestation of Wegener's granulomatosis. Am J Surg Pathol 1987;11:702-708. [PubMed]
183. Tsoumakidou M, Chrysofakis G, Tsiligianni I, Maltezakis G, Siafakas NM, Tzanakis N. A prospective analysis of 184 hemoptysis cases: diagnostic impact of chest X-ray, computed tomography, bronchoscopy. Respiration 2006;73: 808-814. [PubMed]
184. Tsukamoto T, Sasaki H, Nakamura H. Treatment of hemoptysis patients by thrombin and fibrinogen-thrombin infusion therapy using a fiberoptic bronchoscope. Chest 1989;96, 473-476. [PubMed]
185. Tuller C, Tuller D, Tamm M, Brutsche M. Hemodynamic effects of endobronchial application of ornipressin versus terlipressin. Respiration 2004;71:397-401. [PubMed]
186. Uffredi ML, Mangiapan G, Cadranel J, Kac G. Significance of Aspergillus fumigatus isolation from respiratory specimens of nongranulocytopenic patients. Eur J Clin Microbiol Infect Dis 2003;22:457-462. [PubMed]
187. Uflacker R, Kaemmerer A, Neves C, Picon PD. Management of massive hemoptysis by bronchial artery embolization. Radiology 1983;146:627-634. [PubMed]
188. Ungaro R, Saab S, Almond CH, Kumar S. Solitary peripheral pulmonary artery aneurysms. Pathogenesis and surgical treatment. J Thorac Cardiovasc Surg 1976;71:566-571. [PubMed]
189. Unsal E, Koksal D, Cimen F, Taci Hoca N, Sipit T. Analysis of patients with hemoptysis in a reference hospital for chest diseases. Tuberk Toraks 2006;54:34-42. [PubMed]
190. Vadasz P, Kotsis L, Egervary M, Palffy G. Radicality and prognosis of surgical treatment of thoracal carcinoid tumors: a review of 152 operated cases. Thorac Cardiovasc Surg 1999;47:235-239. [PubMed]
191. Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005;127:2113-2118. [PubMed]
192. van Kralingen KW, van Kralingen-Heijboer AC, Zimmerman M, Postmus PE. Management of hemoptysis in a Third World city hospital: a retrospective study. Tuber Lung Dis 1995;76:344-348. [PubMed]
193. Wedzicha JA, Pearson MC. Management of massive haemoptysis. Respir Med 1990;84:9-12. [PubMed]
194. Williamson WA, Tronic BS, Levitan N, Webb-Johnson DC, Shahian DM, Ellis FH. Pulmonary venous infarction. Chest 1992;102:937-940. [PubMed]
195. Wong, M. L., Szkup, P., and Hopley, M. J. (2002). Percutaneous embolotherapy for life-threatening hemoptysis. Chest 2002;121:95-102. [PubMed]
196. Yeoh CB, Hubaytar RT, Ford JM, Wylie RH. Treatment of massive hemorrhage in pulmonary tuberculosis. J Thorac Cardiovasc Surg 1967;54:503-510. [PubMed]
197. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395-1409. [PubMed]
198. Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997;76:192-202. [PubMed]
Tables
Table 1: Mechanisms of Hemoptysis
Mechanism |
Frequency |
Treatment |
|---|---|---|
Non-bronchial and Bronchial Arteries |
+++++ |
Bronchial artery embolization Treatment of the cause |
Pulmonary Arteries |
++ |
Pulmonary Artery Vaso occlusion Treatment of the cause |
Pulmonary veins |
+ |
Surgery Treatment of the cause |
Alveolar Capillary Barrier |
+ |
Treatment of the cause |
Table 2: Main Causes of Hemoptysis
| NEOPLASMS | PULMONARY CAUSES |
|---|---|
Bronchial cancer
Metastatic lung cancer |
Pulmonary embolism
Pulmonary aneurysm and pulmonary artery pseudo aneurysmPulmonary arterio-venous malformationsPulmonary arterial hypertensionPulmonary malformations |
| BENIGN TUMORS | |
| Carcinoid tumor | |
| INFECTIONS | CARDIO-VASCULAR CAUSES |
Tuberculosis
Mycetoma; invasive pulmonary aspergillosisNecrotizing parenchymal pneumoniaParasitic infection |
Congenital heart diseases
Pulmonary venous hypertensionAorto bronchial fistula |
| BRONCHIAL CAUSES | TRAUMA/ FOREIGN BODY |
Bronchiectasis
Cystic FibrosisAcute and Chronic Bronchitis |
Aspirated foreign body
Tracheo vascular fistulaPulmonary arterial catheter induced pulmonary pseudo aneurysm |
| MISCELLANEOUS |
|
Sarcoidoisis
EndometriosisCoagulation Disorders |
CRYPTOGENIC HEMOPTYSIS |
Table 3: Main Causes of Alveolar Haemorrhage
| IMMUNOLOGIC | CARDIAC DISEASES |
|---|---|
Vasculitides
Microscopic PolyangiitisWegener GranulomatosisMixed CryoglobulinemiaHenoch-Schönlein PurpuraPauciimmune Pulmonary CapillaritisSystemic Lupus ErythematosusGoodpasture Syndrome |
Mitral Stenosis
Left Ventricular FailurePulmonary Veno-Occlusive Disease |
| COAGULATION DISORDERS | PULMONARY METASTASES |
Anticoagulants, Antiplatelet Agents, Thrombolytic Agents
Thrombotic Thrombocytopenic Purpura |
Angiosarcoma |
| INFECTIONS | DRUGS OR TOXIC AGENTS |
Leptospirosis
Angioinvasive AspergillosisCytomegalovirus Infection |
Penicillamine
CordaronePropylthiouracilCocaine |
| MISCELLANEOUS | |
Coeliac Disease
Idiopathic Pulmonary FibrosisNegative Pressure Edema |
ANTIPHOSPHOLIPID SYNDROME |
Table 4: Diagnosis and Therapeutic Approaches
1- Does the blood originate from the respiratory tract ?
No = Pseudo hemoptysisYes = where?O BronchiO Alveoli |
2- Is the hemoptysis life-threatening ? Volume/rate of bleeding ++++Respiratory rate, SaO2, PaO2, mechanical ventilation++++Hb levelBlood pressureUnderlying disease: Lung cancer, mycetoma, chronic respiratory disease (FEV1 < 30%)Coagulopathy (anticoagulants)Use of systemic vasoconstrictive agents (terlipressine; vasopressine) |
3- Where is the bleeding located and what is the cause ? |
4- What is the most appropriate treatment ? |
Table 5: Differentiating Features Between Hemoptysis and Pseudohemoptysis
| Features | Hemoptysis | Hematemesis | Nasal / pharynx bleeding |
|---|---|---|---|
| Clinical clues | History of lung disease
Chest gurgling sensation |
History of cirrhosis
Abdominal discomfortNausea |
History of oropharyngeal carcinoma
EpistaxisLaryngeal dyspnea |
| Clinical presentation | Cough | Emesis | Spitting before or without cough |
| Sputum examination | Bright-red
Purulent sputum |
Deep purple
Food particles |
Bright-red |
Table 6: Multidetector Row Computed Tomography Angiography Objectives
1. Location of the hemoptysis |
2. Diagnosis of the cause of the hemoptysis |
3. Assessment of the mechanism of hemoptysis |
4. Depiction of the bronchial and the non-bronchial arteries |
5. Assessment of the severity of hemoptysis |
Table 7: Massive Hemoptysis Treatments
Emergency room or ICU |
|---|
General measures O Wide-bore intravenous canula O Bed rest O Placement on the side of the suspected source of haemorrhage O Oxygen (SaO2 > 90%) O Withdrawal anticoagulation therapy O Cough suppression ? O Antibiotics |
If bleeding ongoing O Fiberoptic Techniques § Ice saline lavage § Epinephrine solution § topical vasoconstrictives agents (terlipressin) O Systemic vasoconstrictive agents (terlipressin, vasopressin) |
If bleeding ongoing or acute respiratory failure O Selective intubation O Endobronchial blockers |
| Referral center |
Interventional radiology+++ Surgery |
Figure 1: A 24-year-old patient with active tuberculosis was admitted for minor hemoptysis.
1A- Chest x ray showed a right lung parenchyma infiltration with micro nodules. 1B- The MDCTA confirmed the micro nodules and right upper lobe cavitations. 1C- 5 mm thin MIP showed the centrolobular distribution of these micronudules as a bud on tree in relation with aeration dissemination. Two days later, the patient presented a massive hemoptysis of 200 ml in one time. Furthermore, the bronchoscopy clearly showed an active bleeding from the right upper lobe. 1D- Right broncho-intercostal trunk showed an abnormal right upper hypervascularization with contrast media extravasation (arrow) within the bronchial tree. During this injection, the patient presented an episode of hemoptysis.
|
Figure 2. A 72-year-old patient was admitted for mild hemoptysis with the diagnosis of left lower lobe excavated cancer treated with chemotherapy.
2A- MDCTA at the level of lower lobes showed the necrotic tumor on mediastinal window of the left lower lobe and the mediastinal necrotic adenopathies. 2B- On parenchyma window, the MDCTA showed the ground glace opacities related to hemoptysis. 2C- Coronal thin MIP (6mm) image clearly showed a left bronchial artery arising from the lower aspect of the aortic arch (arrow). 2D- 3D volume rendering images showed the left upper lobe bronchial artery (arrow) and the left lower lobe bronchial artery (arrowhead) arising from the anterior aspect of the descending thoracic aorta. 2E, 2F- Selective angiograms of the left lung bronchial arteries. The left upper lobe artery arising from the aortic arch (E) and the left lower lobe bronchial artery arising from the anterior aspect of the descendant aorta (F).
|
Figure 3: A 28-year-old patient was admitted for recurrent hemoptysis each morning of 30mL for 5 days. Physical examination and biological investigations were unremarkable. Chest-X-ray did not evidence any abnormalities (A). The bronchoscopy was normal. The MDCT angiography clearly showed the site of bleeding in the lower left lobe as a parenchyma condensation surrounded by ground glass opacities (B, C). The MDCT performed one month later was normal (D).
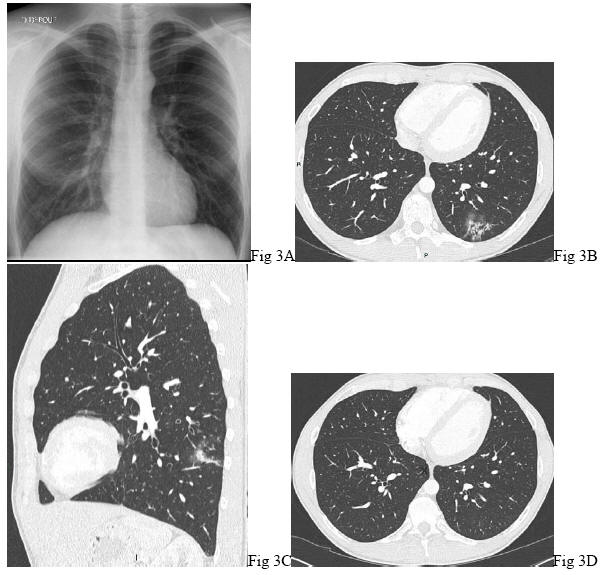
Figure 4: Scale for Assessment of the Volume of Hemoptysis.