Mycobacterium tuberculosis in Organ Transplant Recipients
Authors: Patricia Muñoz, MD, PhD, Marta Lado, MD, Emilio Bouza, MD, PhD
ETIOLOGIC AGENT
Human tuberculosis is caused by microorganisms of the M. tuberculosis complex which includes seven species in the genus Mycobacterium. Mycobacterium tuberculosis causes the vast majority of human tuberculosis. M. bovis (the bovine tubercle bacillus) is transmitted to humans by unpasteurized milk and animal contact; M. africanum and M. canetti are rare causes of tuberculosis in Africa, M. microti, M. caprae and M. pinnipedii have been reported to cause zoonotic TB in humans.
PATHOGENESIS
Most TB cases among solid organ transplant recipients are presumably due to reactivation of pre-transplant infection following transplantation and initiation of immunosuppressive therapy. However, in solid organ transplant patients three main sources of TB disease are possible (15).
- Reactivation of pre-transplant latent infection of the host (85-90%)
- Primary infection resulting from post-transplant exposure (5-10%)
- Transmission through an infected allograft (<5%).
EPIDEMIOLOGY
Incidence rates of TB in solid organ transplant recipients vary widely depending on factors such as prevalence in the general population, type of transplantation, immunosuppression, underlying condition, etc (Table 1). In the US and Western Europe incidence is 0.35-6.5%, but may reach 15% in areas of high endemic rates. In general, solid organ transplant recipients have a much higher probability of TB than the general population of their country (20-74 folds higher) (25, 27, 38). Risk of active TB is higher in lung and kidney transplant recipients and most cases occur during the first post-transplantation year (6, 25, 26, 28).
CLINICAL MANIFESTATIONS
Prompt recognition of TB in solid organ transplant patients is critical for successful outcome (28, 33, 34). Clinical manifestations may be atypical since the proportion of disease due to extrapulmonary TB is much higher than in the general population and 30-49% of the patients will have disseminated disease (2, 31). The main clinical manifestations of tuberculosis are summarized in Table 2. Patients may be initially asymptomatic and pulmonary TB can be discovered on a chest X-ray or may present with nonspecific constitutional symptoms (anorexia, fatigue, weight loss, fever and night sweats. Pulmonary involvement should be always excluded in all patients with TB (3 sputum samples), even in patients with normal chest X ray. In the majority of cases cough develops, initially non-productive and subsequently accompanied by purulent sputum. In solid organ transplant recipients primary pulmonary TB may progress rapidly to clinical illness and even to hematogenous dissemination into various organs. Extensive disease may produce dyspnea and occasionally adult respiratory distress syndrome (ARDS). Suggestive radiological findings include a nodular infiltrate in the apical areas of the upper lobes or the superior segment of the lower lobes, non-resolving pneumonia, mediastinal adenopathies and pleural effusion. Cavities are uncommon in transplant patients.
Extrapulmonary tuberculosis is more common in solid organ transplant patients than in the general population. The extrapulmonary sites most commonly involved are the lymph nodes, pleura, genitourinary tract, bones and joints, meninges, peritoneum and pericardium. The most common clinical manifestations are summarized in Table 2. Highly suggestive findings include pleural effusion, non-resolving pneumonia with hilar adenopathy, sterile pyuria, osteomyelitis of the weight-bearing joints or the vertebrae with disc involvement and paraspinal cold abscess, lymphocytic meningitis or pericarditis.
Disseminated tuberculosis should always be excluded in all transplant patients with suspicion or confirmation of TB (25, 26, 31). Clinical manifestations are non specific and protean. They include liver and spleen enlargement, lymphadenopathy and in up to 30% cases, choroidal tubercles. More than 50% of the patients may have a negative PPD and in 80% the sputum smear is negative. Chest X-ray may be normal or reveal a miliary reticulonodular pattern. Hematologic abnormalities may be seen, such as anemia with leukopenia, neutrophilic leukocytosis, leukemoid reactions or polycythemia. Elevation of alkaline phosphatase levels and other abnormal values in liver function tests are detected in patients with severe hepatic involvement. Bronchoalveolar lavage and transbronchial biopsy, bone marrow biopsy and blood cultures are more likely to yield a bacteriologic diagnosis.
Less common extrapulmonary forms. TB can affect the eye causing chorioretinitis, uveitis, panophthalmitis, or conjunctivitis (10). Tuberculous otitis may cause hearing loss, otorrhea and tympanic membrane perforation. In the nasopharynx TB may simulate Wegener´s granulomatosis. Cutaneous manifestations include abscesses and chronic ulcers, scrofuloderma, lupus vulgaris, military lesions and erythema nodosum.
DIAGNOSIS
When To Suspect TB
Tuberculosis should be suspected in all patients with compatible symptoms and negative bacterial cultures (25, 26). A high index of suspicion is required to achieve a rapid diagnosis of TB in solid organ transplant patients. Manifestations may be subtle or absent, so it is a safe and effective practice to ask for TB isolation in most cultures obtained in this population, at least in areas of high endemicity (Figure 1).
Samples For Diagnosis
TB can be diagnosed in practically all clinical samples. A summary of the samples that can be used for TB diagnosis and the available microbiological techniques is shown in Table 3. An invasive procedure is frequently required to obtain an adequate respiratory (bronchoscopy with bronchial brushing or transbronchial biopsy, bronchoalveolar lavage, early morning gastric lavage in children, CSF, pleural fluid and biopsy samples, bone marrow or liver biopsy). These techniques should be performed promptly in immunocompromised hosts with no immediate results, since empirical therapy has to be started.
As mentioned in Table 3, different techniques may help in the diagnosis of TB in this population.
Tuberculin (purified protein derivative) skin test. It should be ordered in all patients with clinical suspicion of TB if not known to be previously positive. A conversion from negative to positive is highly suggestive of active disease in the presence of compatible manifestations, but a negative result does not exclude TB. A positive result is defined as an induration of 5 mm or more in diameter 48-72 hours after the administration of 2 UI of strain RT-23 (equivalent to 5 UI of tuberculin PPD). If negative, the test should be repeated 7-10 days after the first one (Booster effect) in the other arm. The same criteria for positivity are applicable (5 mm).
Ex vivo Interferon-γ release assays such as QuantiFERON-TB Gold. If available, these tests should be ordered in patients with a negative PPD.
Acid-fast bacillus (AFB) smears of clinical specimens such as expectorated sputum, pleural or peritoneal fluid, tissue (for example a lymph node biopsy) etc. For suspected pulmonary TB, three sputum specimens preferably collected early in the morning should be analyzed. The use of AFB microscopy in urine and gastric lavage fluid is limited by the presence of mycobacterial commensals which can cause false positive results. The sensitivity of the test depends on the experience of the observer and on the bacterial load.
M. tuberculosis culture. It should be required for all samples, including blood cultures. New liquid culture media have substantially shortened the growth period to approximately 7-21 days. Biopsies should not be put in formaldehyde before culture. Culture is the gold standard or the diagnosis and it is required for speciation, strain identification and susceptibility testing.
Nucleic acid amplification for M. tuberculosis may provide a rapid diagnosis (same-day). Although it may have false positive and negative results its sensitivity is higher than AFB. Identifies organisms as members of M. tuberculosis complex.
Molecular methods are available for prompt identification of M. tuberculosis complex, and for detection of antimicrobial resistance to the first line drugs.
THERAPY
General Principles
TB causes significant morbidity and mortality in solid-organ-transplant recipients partially due to the fact that treatment of TB in this population poses unique difficulties due to the interactions with immunosuppressive drugs of some antituberculous drugs, the lack of clear cut indications for treatment of latent TB infection and the risk of toxicity of antituberculous drugs, particularly in liver recipients(3, 23, 33, 34).
When To Initiate Empirical Therapy
Prompt empirical anti-TB therapy should be given to patients with proven or probable TB (Table 4) and to patients with possible TB if clinically unstable.
What Drugs Should I Prescribe
The recommendations for treatment of TB in transplant recipients differ to those of the general population in two aspects: the interaction between rifampicin and calcineurin inhibitor immunosuppressive agents (cyclosporine and tacrolimus), rapamycin and corticosteroids and the recommended duration of therapy, that is generally longer than in the general population (3, 5, 9, 17). The duration of treatment and type of drugs to be used after the first 2 months are very controversial areas, especially if rifampicin is not used in the first 2 months, or must be suspended due to intolerance. Table 5 and 6 show the recommended doses and some possible therapeutic regimens according to the severity of the disease and the suspicion of resistance. Rifabutin could be an alternative to rifampicin since it is a less potent inducer of cytochrome p450 (12, 21).
Clinically stable patients with localized, non-severe forms of TB (excluding CNS and pericardial disease) and no suspicion or evidence of resistance to isoniazid may be treated without rifamycins. Isoniazid, ethambutol and pyrazinamide are recommended for 12-18 months (Table 6).
In patients with severe forms TB (CNS, pericarditis, and probably spinal TB) or with suspicion or evidence of resistance to isoniazid adding rifampicin or rifabutin to the regimen can be considered. If rifamycins are used, rejection due to low levels of calcineurin inhibitors is a risk, so levels should be carefully monitored and doses of cyclosporine or tacrolimus increased (3-5 fold) (Table 7). If Isoniazid cannot be used, induction and maintenance treatment that includes 4 drugs for at least 18 months is recommended. One study demonstrated no recurrence in patients who received more than 12 months of treatment, irrespective of whether this included rifampicin (35).
Special Situations
HIV Infected Transplant Recipients
The risk of TB does not seem to be significantly greater in these patients compared to non-HIV infected transplant recipients however, drug interactions between the antiretroviral agents and immunosuppressives can be challenging and recurrence of hepatitis C virus infection may increase the risk of toxicity (24). Rifamycins may lead to greater hepatotoxicity in HIV-infected patients and jeopardize antiretroviral therapy because of their interaction with protease inhibitors and non nucleoside reverse-transcriptase inhibitors. The recommended regimen in this population is isoniazid, pyrazinamide and ethambutol with a moxifloxacin or levofloxacin.
Liver transplantations initial treatment with isoniazid, rifampicin and pyrazinamide in liver recipients with TB has been associated with hepatotoxicity in a high proportion of the cases cases. Close monitoring of liver enzyme values is necessary (19).
Immune Reconstitution Syndrome can occur in solid organ transplant recipients with TB under treatment. A paradoxical worsening of symptoms with fever, cough, lymph node enlargement or roentgenographic abnormalities has been described.
Alternative Therapy
Second line drugs are usually needed for treatment of multidrug-resistant TB or when there is some limitation for the use of the first line agents (20). This therapy should always be supervised by an experienced consultant (7, 39).
Fluoroquinolones are an alternative in solid organ transplant patients, given the disadvantages associated with rifamycins and aminoglycosides, and they can sometimes become first-line agents (14, 27). Combined and prolonged use of levofloxacin and pyrazinamide has been associated with poor gastrointestinal tolerance (22).
When drug resistance is suspected, the treatment regimen should include INH, rifamycin, pyrazinamide, ethambutol, a fluoroquinolone, and an injectable agent (STM, capreomycin or amikacin), pending susceptibility results. The combination of injectable drugs is not recommended because of their intolerance and the association of adverse effects.
If isoniazid and rifamycins cannot be used (i.e., MDR-TB), tuberculosis should be treated with ethambutol, pyrazinamide, a fluoroquinolone, an injectable drug and probable an additional agent. Surgery may be required and the total treatment duration is 18 to 24 months.
Tuberculosis resistant to INH, rifamycin, fluoroquinolones, and injectable drugs (i.e., XDR-TB) should be treated with at least 5 drugs to which the organisms is susceptible. Optimal duration of therapy is not established and surgery may be needed.
In rifamycin-sparing regimens resistance is more frequent. Resistance to rifampin is almost systematically associated with cross-resistance to rifabutin and rifapentine. In cases of resistance to streptomycin, there is no cross-resistance with other injectable drugs (e.g., amikacin, kanamycin, and capreomycin); however, cross-resistance between amikacin and kanamycin is universal.
In special cases of resistance or toxicity, linezolid has proven to be effective in patients with tuberculosis (4, 13). However, prolonged use of this drug is associated with thrombopenia and anemia, and, in some cases, irreversible neuropathy, especially in patients with other associated conditions such as diabetes or kidney disease.
A summary of our personal opinion on the Do’s and Don’ts of the anti TB treatment in solid organ transplant patients is provided in Table 8.
ADJUNCTIVE THERAPY
Surgery
Surgical drainage of tuberculous empyema is usually required. Ventricular shunting may be necessary for symptomatic hydrocephalus. Pericardiectomy is indicated in hemodynamic compromise persists for 6 to 8 weeks. Complete excision of tuberculous peripheral lymphadenitis is recommended if a biopsy has been necessary for diagnosis, to avoid fistula formation.
Corticosteroids Are Recommended For
- Tuberculous meningitis (especially in patients with objective neurologic findings or with stupor-coma): prednisone, 60 to 80 mg daily. This may be gradually reduced after 1 to 2 weeks and discontinued by 4 to 6 weeks, as guided by symptoms.
- Tuberculous pericarditis: prednisone, 60 mg/day for 4 weeks, 30 mg/d for 4 weeks and 15 mg/d for two weeks.
- Tuberculous pleurisy: corticosteroids therapy hastens symptomatic improvement, but no long-term benefits have been proven.
ENDPOINTS FOR MONITORING THERAPY
Bacteriologic evaluation is the preferred method of monitoring the response to tuberculosis treatment.
Patients with pulmonary disease should have their sputum examined monthly until cultures become negative. After the second month of treatment, >80% of patients will have negative sputum cultures. By the end of the third month, all patients must be culture-negative and/ or AFB smears should be negative at the fifth month. When a patients sputum culture remain positive at >3 months, treatment failure and drug resistance should be suspected. Other possibilities include noncompliance with therapy and malabsorption of antituberculosis drugs. Sensitivity testing should be performed and adding at least two new drugs should be considered. A sputum specimen can be collected by the end of treatment to document cure.
Bacteriologic monitoring of patients with extrapulmonary tuberculosis is more difficult and often not feasible. In these cases, the response to treatment must be assessed clinically. Monitoring of the response to treatment during chemotherapy by serial chest radiography is not recommended.
PROPHYLAXIS
A summary of the subsequent steps that should be followed in the management of latent TB infection is provided in Table 9.
All candidates to transplantation and all living donors should undergo PPD testing, even patients who have received the Bacille Calmette-Guérin vaccine (11, 16, 36, 37). Negative PPDs should be repeated 7-10 days after the first test (Booster effect). If available, interferon-γ release assays should be performed in patients with negative PPD. Positivity in any of the tests should be considered an indication of prophylaxis (3, 29). The only reasons for not performing PPD testing are previous positive PPD or history of TB.
Cellular immune testing (multitest or specific testing for selected antigens, such as Candida albicans or tetanous toxoid) can be performed in patients with negative PPD skin test to determine the presence of anergy.
An induration of >5mm in any of the tests (initial or Booster) indicates a positive result. In this case, active TB should be ruled out with chest X-ray, and with cultures and PCR for mycobacterium in blood, sputum and urine samples even in asymptomatic patients. Additional clinical guided examinations may be necessary.
Indications for treatment of latent TB infection Patients on the waiting list for transplantation or transplant recipients must be provided treatment of latent TB infection when they have one or more of these conditions:
- Positive PPD skin test (initial or after booster effect >5mm).
- History of untreated TB
- History of contact with a patient with active TB
- Chest Radiography with findings compatible with untreated TB such as apical fibronodular lesions, calcified solitary nodules, calcified lymph nodes or pleural thickening.
Treatment Of Latent TB Infection
Isoniazid 5 mg/kg in adults and 10-15 mg/kg in children (maximum 300mg) daily supplemented with vitamin B6 (25-50 mg daily) for 9 months (1, 18, 40). The ideal approach is to treat latent TB infection before transplantation. Patients who have completed therapy before transplantation do not need to repeat it after the procedure.
Other alternatives include rifampicin (10 mg/kg in adults, 10-20 mg/kg in children – maximum 600 mg/daily) for 4 months or rifampicin and pyrazinamide for 2 months. This last regimen is not recommended for patients with previous liver disease, alcohol consumers or patients who have developed isoniazid-induced hepatotoxicity. The regimens that include rifampicin are only recommended for pre-transplantation treatment (due to drug interactions).
When withdrawal of treatment is necessary because of toxicity, the patient must be monitored and treatment should be completed with drugs other than isoniazid in recent converters. For patients at high risk of TB, we recommend levofloxacin or moxifloxacin with ethambutol for at least 6 months.
Exclusions And Precautions
Patients with properly treated previous TB do not require treatment for latent TB infection.
When active TB cannot be ruled out in a transplantation recipient, treatment with 3 drugs (isoniazid, ethambutol and pyrazinamide) must be initiated. Treatment can be completed only with isoniazid if, after 8 weeks of samples incubation, cultures are negative for M. tuberculosis and the chest radiograph findings remains normal.
The administration of treatment for latent TB infection is usually delayed in liver recipients until after transplantation, when liver function is stable. Some authors do not provide prophylaxis even then due to the risk of toxicity. However, isoniazid is usually well tolerated in liver transplant recipients with stable liver function (32). Prophylaxis should be strongly considered in patients with recent PPD status conversion (from negative into positive), a history of incorrectly treated TB, residual TB lesions on the chest radiograph, contact with an untreated person and added immunosuppression. Alterations in liver function tests should not be ascribed only to isoniazid and other potential causes must be excluded as well (8, 30).
Monitoring
Baseline hepatic measurements: serum aspartate aminotransferase, alanine aminotransferase and bilirrubin levels must be measured at 2 weeks interval for the first two months and then monthly. Treatment of latent TB must be suspended if aspartate aminotransferase or alanine aminotransferase values increase 3 folds in patients with symptoms and 5 fold in patients with no accompanying symptoms.
INFECTION CONTROL MEASURES
To decrease the risk of transmission of TB, patients should be placed in individual rooms with negative pressure (compared with the corridor). The doors and windows must be closed, except when people are entering or leaving the room. Health care providers entering the room must use a mask until the disease has been excluded or the patient has negative smear results.
Recommendations For Isolation Include
- For suspicious cases, Isolation must be maintained until the disease has been excluded.
- For TB patients who have been receiving treatment. The isolation can be suspended when they improve clinically and have 3 consecutive negative sputum smear results.
- Patients can be discharged from the hospital if contact with especially susceptible people, such as children or immunosuppressed patients can be avoided.
REFERENCES
1. Agarwal SK, Gupta S, Dash SC, Bhowmik D, Tiwari SC. Prospective randomised trial of isoniazid prophylaxis in renal transplant recipient. Int Urol Nephrol. 2004;36(3):425-31. [PubMed]
2. Aguado JM, Herrero JA, Gavalda J, Torre-Cisneros J, Blanes M, Rufi G, et al. Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain. Spanish Transplantation Infection Study Group, GESITRA. Transplantation. 1997;63(9):1278-86. [PubMed]
3. Aguado JM, Torre-Cisneros J, Fortun J, Benito N, Meije Y, Doblas A, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48(9):1276-84. [PubMed]
4. Alcala L, Ruiz-Serrano MJ, Perez-Fernandez Turegano C, Garcia De Viedma D, Diaz-Infantes M, Marin-Arriaza M, et al. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob Agents Chemother. 2003;47(1):416-7. [PubMed]
5. al-Sulaiman MH, Dhar JM, al-Khader AA. Successful use of rifampicin in the treatment of tuberculosis in renal transplant patients immunosuppressed with cyclosporine. Transplantation. 1990;50(4):597-8. [PubMed]
6. Atasever A, Bacakoglu F, Toz H, Basoglu OK, Duman S, Basak K, et al. Tuberculosis in renal transplant recipients on various immunosuppressive regimens. Nephrol Dial Transplant. 2005;20(4):797-802. [PubMed]
7. Baciewicz AM, Chrisman CR, Finch CK, Self TH. Update on rifampin and rifabutin drug interactions. Am J Med Sci. 2008;335(2):126-36. [PubMed]
8. Benito N, Sued O, Moreno A, Horcajada JP, Gonzalez J, Navasa M, et al. Diagnosis and treatment of latent tuberculosis infection in liver transplant recipients in an endemic area. Transplantation. 2002;74(10):1381-6. [PubMed]
9. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603-62. [PubMed]
10. Bouza E, Merino P, Muñoz P, Sanchez-Carrillo C, Yanez J, Cortes C. Ocular tuberculosis. A prospective study in a general hospital. Medicine (Baltimore). 1997;76(1):53-61. [PubMed]
11. Cohn DL. Treatment of latent tuberculosis infection. Semin Respir Infect. 2003;18(4):249-62. [PubMed]
12. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.7.2. Late infections. Tuberculosis. Nephrol Dial Transplant. 2002;17 Suppl 4:39-43. [PubMed]
13. Fortun J, Martin-Davila P, Navas E, Perez-Elias MJ, Cobo J, Tato M, et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother. 2005;56(1):180-5. [PubMed]
14. Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect Dis. 2003;3(7):432-42. [PubMed]
15. Graham JC, Kearns AM, Magee JG, El-Sheikh MF, Hudson M, Manas D, et al. Tuberculosis transmitted through transplantation. J Infect. 2001;43(4):251-4. [PubMed]
16. Hernandez-Hernandez E, Alberu J, Gonzalez-Michaca L, Bobadilla-del Valle M, Correa-Rotter R, Sifuentes-Osornio J. Screening for tuberculosis in the study of the living renal donor in a developing country. Transplantation. 2006;81(2):290-2. [PubMed]
17. Horsburgh CR, Jr., Feldman S, Ridzon R. Practice guidelines for the treatment of tuberculosis. Clin Infect Dis. 2000;31(3):633-9. [PubMed]
18. John GT, Thomas PP, Thomas M, Jeyaseelan L, Jacob CK, Shastry JC. A double-blind randomized controlled trial of primary isoniazid prophylaxis in dialysis and transplant patients. Transplantation. 1994;57(11):1683-4. [PubMed]
19. Kunimoto D, Warman A, Beckon A, Doering D, Melenka L. Severe hepatotoxicity associated with rifampicin-pyrazinamide preventative therapy requiring transplantation in an individual at low risk for hepatotoxicity. Clin Infect Dis. 2003;36:e158-61. [PubMed]
20. Lee J, Yew WW, Wong CF, Wong PC, Chiu CS. Multidrug-resistant tuberculosis in a lung transplant recipient. J Heart Lung Transplant. 2003;22(10):1168-73. [PubMed]
21. Lopez-Montes A, Gallego E, Lopez E, Perez J, Lorenzo I, Llamas F, et al. Treatment of tuberculosis with rifabutin in a renal transplant recipient. Am J Kidney Dis. 2004;44(4):e59-63. [PubMed]
22. Lou HX, Shullo MA, McKaveney TP. Limited tolerability of levofloxacin and pyrazinamide for multidrug-resistant tuberculosis prophylaxis in a solid organ transplant population. Pharmacotherapy. 2002;22(6):701-4. [PubMed]
23. Meyers BR, Papanicolaou GA, Sheiner P, Emre S, Miller C. Tuberculosis in orthotopic liver transplant patients: increased toxicity of recommended agents; cure of disseminated infection with nonconventional regimens. Transplantation. 2000;69(1):64-9. [PubMed]
24. Miro JM, Agüero F, Laguna M, et al. Liver transplantation in HIV/hepatitis co-infection. J HIV Ther. 2007;12:24-35. [PubMed]
25. Muñoz P, Rodriguez M, Giannella M, Vega A, Anaya F, Serrano MJ, et al. A painful hand in a kidney transplant recipient. Nephrol Dial Transplant. 2007;22(3):971-2. [PubMed]
26. Muñoz P, Valerio M, Palomo J, Fernandez-Yanez J, Fernandez-Cruz A, Guinea J, et al. Infectious and non-infectious neurologic complications in heart transplant recipients. Medicine (Baltimore). 2010;89(3):166-75. [PubMed]
27. O'Brien RJ. Development of fluoroquinolones as first-line drugs for tuberculosis--at long last! Am J Respir Crit Care Med. 2003;168(11):1266-8. [PubMed]
28. Park SB, Joo I, Park YI, Suk J, Cho WH, Park CH, et al. Clinical manifestations of tuberculosis in renal transplant patients. Transplant Proc. 1996;28(3):1520-2. [PubMed]
29. Roman A, Bravo C, Levy G, Monforte V, Vidal R, Sole J, et al. Isoniazid prophylaxis in lung transplantation. J Heart Lung Transplant. 2000;19(9):903-6. [PubMed]
30. Schluger LK, Sheiner PA, Jonas M, Guarrera JV, Fiel IM, Meyers B, et al. Isoniazid hepatotoxicity after orthotopic liver transplantation. Mt Sinai J Med. 1996;63(5-6):364-9. [PubMed]
31. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27(5):1266-77. [PubMed]
32. Singh N, Wagener MM, Gayowski T. Safety and efficacy of isoniazid chemoprophylaxis administered during liver transplant candidacy for the prevention of posttransplant tuberculosis. Transplantation. 2002;74(6):892-5. [PubMed]
33. Subramanian A, Dorman S. Mycobacterium tuberculosis in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S57-62. [PubMed]
34. Subramanian AK, Nuermberger EL. Tuberculosis in transplant recipients: diagnostic and therapeutic dilemmas. Transpl Infect Dis. 2008 Jul;10(4):229-30. [PubMed]
35. Sud K, Muthukumar T, Singh B, Garg SK, Kohli HS, Jha V, et al. Isoniazid does not affect bioavailability of cyclosporine in renal transplant recipients. Methods Find Exp Clin Pharmacol. 2000;22(8):647-9. [PubMed]
36. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49(RR-6):1-51. [PubMed]
37. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54(RR-12):1-81. [PubMed]
38. Torre-Cisneros J, Doblas A, Aguado JM, San Juan R, Blanes M, Montejo M, et al. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis. 2009;48(12):1657-65. [PubMed]
39. Vandevelde C, Chang A, Andrews D, Riggs W, Jewesson P. Rifampin and ansamycin interactions with cyclosporine after renal transplantation. Pharmacotherapy. 1991;11(1):88-9. [PubMed]
40. Vikrant S, Agarwal SK, Gupta S, Bhowmik D, Tiwari SC, Dash SC, et al. Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transpl Infect Dis. 2005;7(3-4):99-108. [PubMed]
Figure 1. Approach to the diagnosis of tuberculosis (TB) in patients undergoing non urgent transplantation and in patients with normal chest radiograph findings.
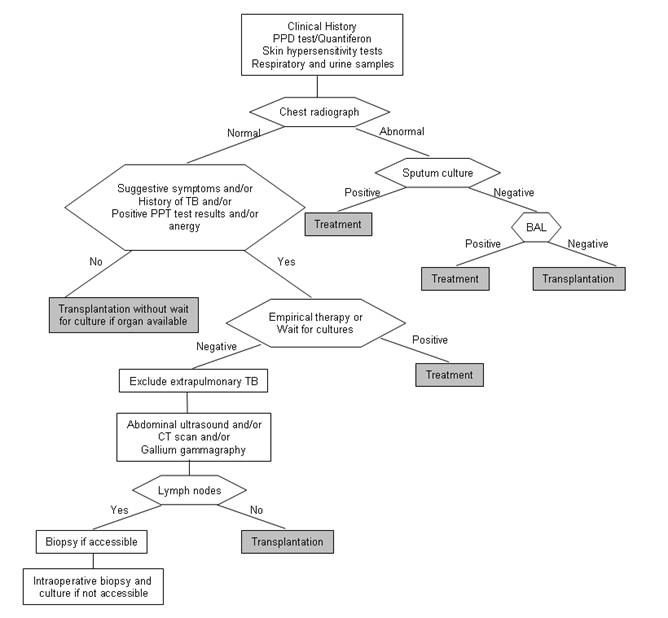
Table 1. Risk factors for tuberculosis (TB) after transplantation.
| Risk factor |
|---|
Therapy
|
History of exposure to Mycobacterium tuberculosis
|
Clinical condition
|
Other coexisting infections: invasive mycoses, cytomegalovirus disease, or Pneumocystis jirovecii or Nocardia pneumonia |
a Not enough information is available on recently introduced immunosuppressors, such as sirolimus, everolimus, or monoclonal antibodies (daclizumab and basiliximab).
Table 2. Most common clinical findings and radiological manifestations of tuberculosis
| Site of infection | Signs and symptoms. |
|---|---|
| Pulmonary (50-65%) | Fever, chills, night sweats, weight loss, anorexia, fatigue, malaise, cough, sputum production (sometimes with blood streaking or hemoptysis), chest pain. Extensive disease may produce dyspnea and occasionally adult respiratory distress syndrome. Painful pharyngeal ulcers, non-healing ulcers in mouth.
Chest X- ray: Lung nodules or infiltrates (cavitation uncommon); Hilar or mediastinal adenopathy; Pleural effusion; Non resolving pneumonia |
| Extrapulmonary (10-15%) 1,2 | |
| Lymphadenitis (>25%) | Painless swelling of the lymph nodes, most commonly at cervical and supraclavicular sites (scrofula). |
| Genitourinary (15%) | Genitourinary symptoms, infertility, epididymitis, orchitis, chronic prostatitis. Pathologic urinalysis (hematuria, pyuria) with negative bacterial culture. Abnormal intravenous pyelogram. |
| Skeletal (10%) | Pain in the spine, hips and knees. Psoas abscess, tenosynovitis. CT: two or more adjacent vertebral bodies or with disc involvement; paraspinal cold abscess; weight-bearing joints osteomyelitis, |
| CNS (5%) | Subacute/acute lymphocytic meningitis, cranial nerves paralysis (ocular nerves), focal ischemia. CT: meningitis, tuberculomas, basilar arachnoiditis, cerebral infarction, or hydrocephalus |
| Gastrointestinal | Abdominal pain, palpable mass, fever, diarrhea, obstruction of the biliary tract or bowel, hematochezia, weight loss, ascites, and night sweats. CT: low density nodes, abscesses in liver, spleen, pancreas or kidney, local ileal thickening, fistula formation. |
| Pleural | Fever, pleuritic chest pain, dyspnea, chronic constrictive pericarditis. Chest X- ray: Pleural effusion, parenchymal lesions (<30%) |
| Pericarditis | Chest pain, cardiovascular symptoms, cardiac tamponade |
2 Less common sites of TB include chorioretinitis, uveitis, panophtalmitis, otitis, nasopharynx TB, skin (abscesses, chronic ulcers, scrofuloderma, lupus vulgaris, miliary lesions and erythema nodosum)
Table 3. Diagnostic approach to the microbiological diagnosis of TB
| PPD | To all patients with previous negative or unknown test | 2 UI of strain RT-23. Positive if induration ≥5 mm after 48-72 h |
| Second PPD | To patients with the previous PPD negative (Booster effect) | 2 UI of strain RT-23 in the other arm, one week later. Positive if induration ≥5 mm after 48-72 h |
| Interferon-γ release assays | To patients with a negative PPD | |
| Respiratory samples | Sputum (X 3) Bronchoscopy obtained samples | AFS. If negative ask for PCR if available. Culture (3-8 wks on solid media and 2-3 wks on liquid media) |
| Blood cultures | To all patients in conventional or special bottles | If conventional bottles, contact the laboratory |
| Urine (if pathologic urinalysis) | Culture of three morning urine specimens | AFS. If negative ask for PCR if available. Culture + 90% |
| Other fluids | Pleural effusion, peritoneal fluid | Cytology, glucose, proteins, pH; adenosine deaminase; AFB, PCR if AFB negative, culture (+ in < 30%) |
| CSF | Perform lumbar puncture if any neurological manifestation is present | Cytology, glucose, proteins, pH; adenosine deaminase; AFB, PCR if AFB negative, Culture (+ in 80%) |
| Tissue | Needle biopsy of the pleura, peritoneum, lymph nodes, intraoperative specimens, etc | AFB, PCR if AFB negative, Culture (+ in < 30%) |
| Bone marrow | Recommended in fever of unknown origin | AFB, PCR if AFB negative, Culture (+ in 80%) |
Table 4. Therapeutic recommendations according to the level of certainty of the diagnosis and to the clinical situation
|
Available information |
Action |
|---|---|---|
| Proven case | Isolation of M. tuberculosis from any clinical sample | Administer therapy |
| Probable case | Visualization of a single AFB in a clinical sample different from feces with compatible clinical findings Compatible clinical findings with a positive PPD or Quantiferon test (mainly if recent conversion) | In patients with presumed TB, initiate therapy and withdraw later if MTB excluded. In very stable patients with low suspicion consider waiting 24 hours for M. tuberculosis confirmation with molecular methods. If cultures are negative are there are no alternative diagnosis, clinical and radiographic response to 2 months of therapy is consistent with a diagnosis of TB. |
| Possible case | Compatible clinical signs or symptoms, epidemiological risk factors 1 and no other confirmed etiology | In unstable patient, initiate therapy and withdraw later if MTB excluded |
Table 5. Doses of antituberculous drugs
| Drug | Daily dose (adults) | Daily dose (pediatrics) | Dose alteration for renal dysfunction |
|---|---|---|---|
| First line drugs | |||
Isoniazid (I) Rifampicin (R) Pyrazinamide (P) Ethambutol (E) Streptomycin (S) Levofloxacin 3 |
5 mg/kg PO or IV (max 300 mg) 10 mg/kg PO or IV (max 600 mg) 1 25-30 mg/kg PO (max 2 g) 15-25mg/kg PO (max 1.6 g) 15 mg/kg (max 1 g) IM or IV 2 1000 mg/kg PO or IV |
10-15mg/kg (max 300 mg) 10-20 mg/kg (max 600 mg) 20-30 mg/kg (max 2 g) 15-20 mg/kg (max 1.0 g) 20-30 mg/kg (max 1 g) Not applicable |
Minimal None Minimal Mild Major Moderate |
| Second line drugs | |||
Kanamycin Amikacin Rifabutin EthionamideCycloserineCapreomycin |
15 mg/kg IM or IV (max 1 g) 15 mg/kg IM or IV (max 1 g) 5 mg/kg PO (max 300 mg) 15-20 mg/kg PO (max 1g/d; usually 500-750 mg/d)10-15 mg/kg PO (max 1g/d, usually 500-750 mg/d in 2 doses)15 mg/kg IM or IV (max 1 g) |
15-30 mg/kg (max 1 g) 15-30 mg/kg (max 1 g) Unknown 15-20 mg/kg (max 1g/d)10-15 mg/kg (max 1g/d in 2 doses)15-30 mg/kg (max 1 g) |
Major Major None MildModerateMajor |
2 Smaller doses (10 mg/kg) are generally used in adults over the age of 50. Streptomycin is usually not given more than 5 times a week and frequency may be reduced as infection is cleared.
3 Moxifloxacin can also be used, since has excellent antituberculous activity
Table 6. Interactions between first line antituberculous drugs and immunosuppressive agents most commonly used in Solid Organ Transplant
| Isoniazid | Rifampicin | Pyrazinamide | Ethambutol | Streptomycin | |
|---|---|---|---|---|---|
| Corticosteroids | Combo may increase corticosteroid levels, risk of adverse effects. (Hepatic metabolism inhibited) | Combo may decrease corticosteroid levels and efficacy (hepatic metabolism induced) | None | None | None |
| Cyclosporine A | None | Monitor levels of CyA. Combo may decrease CyA levels, reducing efficacy. (Hepatic metabolism induced) | None | None | Avoid combo. Monitor renal function. Combo may increase risk of nephrotoxicity (Additive toxicity) |
| Tacrolimus | None | Avoid combo or use alternative. Combo may increase risk of nephrotoxicity, diabetes mellitus, impaired wound healing, and other adverse effects (Overlapping toxicity, additive effects) | None | None | Monitor renal function. Combo may increase risk of nephrotoxicity (Overlapping toxicity) |
| Sirolimus | None | Use alternative or monitor levels. Combo may decrease sirolimus levels and efficacy. (Hepatic metabolism induced). | None | None | Avoid combo or monitor renal function. Combo may increase risk of nephrotoxicity (Additive toxicity) |
| Mycophenolate | None | Weight risk/benefit. Combo may decrease MMF levels and efficacy (Enterohepatic recirculation decrease). | None | None | None |
Table 7. Summary of some therapeutic options considering the clinical situation of the patient and the risk of resistance
| Possible initial therapy (2 months) | Maintenance therapy | |
|---|---|---|
| Clinically unstable patient 1 or meningitis or tuberculous spondylitis | I + E + P + quinolone I + E + P + rifampicin or rifabutin2 | I + E + P 10 months or I+E 16 months I + rifampicin or rifabutin 7-10 months in CNS, 4-6 months in spondylitis and 4 months in the rest. |
| Clinically stable patient 2 , no CNS involvement, no suspicion or evidence of resistance to isoniazid | I + E + P or I+E + quinolone | I + E + P or I + E+ quinolone3 10 months I+E or I+P16 months |
| Isoniazid resistance or intolerance | Rifampicin or rifabutin2+ E + P + quinolone | Rifampicin or rifabutin2+ E + P 10 months E + P + quinolone3 at least 16 months |
| Suspicion or confirmed multidrug-resistant TB or limitation for the use of the aforementioned drugs 1 | CONSULTATION WITH AN EXPERT REQUIRED.
Induction treatment should include 4-6 drugs, including injectable antimicrobials (streptomycin, amikacin, kanamycin or capreomycin), linezolid, a fluoroquinolone or other second line drugs |
The absence of isoniazid and rifamycins in the initial treatment makes it difficult to calculate the duration of treatment (18-24 months) and the types of drugs to be used: therapy should be individualized. Surgery may be required. |
2 If rifamycins are used, a close monitoring of the levels of immunosuppressors is necessary. Cyclosporine or tacrolimus dose must be increased (3-5 folds);
3 Prolonged use of fluoroquinoles may cause arthralgias and its combination with pyrazinamide may be poorly tolerated (GI toxicity)
Table 8. Do’s and Dont's of the anti TB treatment in Solid Organ Transplant patients
|
|
|
|
|
|
|
|
|
|
Table 9. Suggested steps for the management of latent TB infection in Solid Organ Transplant candidates
| PPD skin test | To all Tx candidates and all living donors 1 | 5 mm positive.
If negative: repeat PPD test (Booster) |
| Positive PPD | Exclude active tuberculosis | Clinical history, chest X-ray, cultures and TB PCR in blood, sputum and urine samples (additional examinations if clinically indicated) |
| Indications for treatment of latent TB infection |
|
|
| Preferred regimen | INH 9 months 2 Patients who have completed therapy before transplantation do not need to repeat it after the procedure. | |
Alternatives |
Before Tx: rifampicin 4 months or rifampicin and pyrazinamide for 2 months (not recommended if previous liver disease)
After Tx or if intolerance to other drugs: levofloxacin or moxifloxacin with ethambutol for at least 6 months |
|
Monitoring |
Baseline hepatic measurements at 2 weeks interval for the first two months and then monthly.
Treatment of latent TB must be suspended if aspartate aminotransferase or alanine aminotransferase values increase 3 folds in patients with symptoms and 5 fold in patients with no accompanying symptoms. |
|
2 When active TB cannot be ruled out in a transplantation recipient, treatment with 3 drugs (isoniazid, ethambutol and pyrazinamide) must be initiated.
Chang KC, et al. Can Intermittent Dosing Optimize Prolonged Linezolid Treatment of Difficult Multidrug-Resistant Tuberculosis. Antimicrob Agents Chemother 2013;57:3445-3449.
Ha YE, Joo EJ, et al. Tacrolimus as a risk factor for tuberculosis and outcome of treatment with rifampicin in solid organ transplant recipients. Transpl Infect Dis. 2012 Feb 29.
Martinson NA, et al. New regimens to prevent Tuberculosis in adults with HIV infection. NEJM. 2011 July 7 ;365:11-20.
Karim SS. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010;362;(8):697-706.
McGrath EE, et al. Diagnostic Tests for Tuberculosis pleural effusion. Eur J Clin Microbiol Infect Dis 2010;29:1187-1193.
Cain KP, et al. An Algorithm for Tuberculosis Screening and Diagnosis in People with HIV. N Engl J Med 2010;362(8):707-716.
Boehme CC, et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. New Engl J Med 2010:Epub ahead of publication.
Small PM, et al. Tuberculosis Diagnosis - Time for a Game Change. New Engl J Med 2010:Epub ahead of publication.
Low DE. Fluoroquinolones for Treatment of Community-Acquired Pneumonia and Tuberculosis: Putting the Risk of Resistance into Perspective. Clin Infect Dis 2009;48:1361-1363.
Bakir M et al. Use of T Cell-based Diagnosis of Tuberculosis Infection to Optimize Interpretation of Tuberculin Skin Testing for Child Tuberculosis Contacts.Clin Infect Dis. 2009 Feb 1;48(3):302-12.
Finnell SM et al. Latent Tuberculosis Infection in Children: A Call for Revised Treatment Guidelines. Pediatrics. 2009 Mar;123(3):816-22.
Forgacs P, Wengenack NL, et al. Tuberculosis and trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother. 2009 Nov;53:4789-93.
Gao XF, et al. Rifapentine vs. rifampicin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2009 Jul;13:810-9.
Holland DP, Sanders GD, et al. Costs and Cost-Effectiveness of Four Treatment Regimens for Latent Tuberculosis Infection. Am J Respir Crit Care Med. 2009 Jun 1;179:1055-60.
Cattamanchi A et al. Clinical Characteristics and Treatment Outcomes of Patients with Isoniazid-monoresistant Tuberculosis.Clin Infect Dis. 2009 Jan 15;48(2):179-85.
Lighter, J. et al. Latent Tuberculosis Diagnosis in Children by Using the QuantiFERON-TB Gold In-Tube Test. PEDIATRICS Vol. 123 No. 1 January 2009, pp. 30-37 (doi:10.1542/peds.2007-3618)
McIlleron H, Willemse M, et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009 Jun 1;48:1547-53.
Park SH, Yang SK, et al. Prospective Randomized Trial of Six-Month Versus Nine-Month Therapy for Intestinal Tuberculosis. Antimicrob Agents Chemother. 2009 Oct;53:4167-71.
Thee S, et al. Rifampicin Serum Levels in Childhood Tuberculosis. Int J Tuberc Lung Dis. 2009 Sep;13:1106-11.
Ziakas PD, Mylonakis E. 4 months of rifampin compared with 9 months of isoniazid for the management of latent tuberculosis infection: a meta-analysis and cost-effectiveness study that focuses on compliance and liver toxicity. Clin Infect Dis. 2009 Dec 15;49:1883-9.
Alffenaar JWC, et al. Pharmacokinetics of Moxifloxacin in Cerebrospinal Fluid and Plasma in Patients with Tuberculous Meningitis. Clin Infect Dis 2009;49:1080-1082.
Karim SSA, et al. Concurrent Antiretroviral/TB Treatment Decreases Mortality in HIV Patients. CAPRISA Newsletter September 2008.
Tovar M, et al. Improved diagnosis of pleural tuberculosis using the microscope-observation drug-susceptibility technique. Clin Infect Dis. 2008;46:909-12.
Mitnick CD, et al. Comprehensive Treatment of Extensively Drug-Resistant Tuberculosis. N Engl J Med 2008;359:563-574.
Achkar et al. Differences in Clinical Presentation among Persons with Pulmonary Tuberculosis: A Comparison of Documented and Undocumented Foreign-Born versus US-Born Persons. Clin Infect Dis. 2008 Nov 15;47(10):1277-83.
Jacob JT, Nguyen TM, Ray SM. Male genital tuberculosis. Lancet Infect Dis. 2008 May;8:335-42.
Pai M, et al. Systematic Review: T-Cell-based assays for the Diagnosis of Latent Tuberculosis Infection: An Update. Ann Intern Med 2008;149:177-184.
Pai, M, Ramsay, O'Brien R. Table: Evidence-Based Tuberculosis Diagnosis. PLoS Medicine | www.plosmedicine.org 2008; 5:e156.
Kwon et al. Treatment Outcomes for HIV-uninfected Patients with Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis. Clin Infect Dis. 2008 Aug 15;47(4):496-502
Boulle A. Outcomes of Nevirapine- and Efavirenz-Based Antiretroviral Therapy When Coadministered With Rifampicin-Based Antitubercular Therapy. JAMA2008;300(5):530-539.
Christie LJ, et al. Diagnostic Challenges of Central Nervous System Tuberculosis. Emerg Infect Dis 2008;14:1473-1475.Danchaivijitr N, et al. Diagnostic Accuracy of MR Imaging in Tuberculosis Spondylitis. Journal of the Medical Association of Thailand. 2007:90(8):1581-1589.
Thee S, et al. Ethambutol in paediatric tuberculosis: aspects of ethambutol serum concentration, efficacy and toxicity in children. Int J Tuberc Lung Dis 2007;11:965-971.
Guided Medline Search for
Sun HY, et al. Mycobacterium tuberculosis-associated immune reconstitution syndrome in solid-organ transplant recipients. Transplantation 2013;95:1173-1181.
Leung CC, Chang KC, Sun HY, Daley CL. Mycobacterium tuberculosis Complex
Sun HY, et al. Tuberculosis in solid-organ transplant recipients: disease characteristics and outcomes in the current era. Prog Transplant 2014;24:37-43.
Sun, HY. Efavirenz-based antiretroviral therapy is a better choice for HIV-positive patients treated with rifampicin-based antituberculous therapy. 2009.
Meintjes G, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008;8(8):516-523.
Mitnick CD, et.al. Comprehensive Treatment of Extensively Drug-Resistant Tuberculosis. N Engl J Med. 2008 Aug 7;359(6):563-74.
Jain SK, et al. Why is duration of Tuberculosis therapy so long?: Sterilization or "persisters" Microbe 2008;3(6):285-292.
CDC. Managing Interactions in the Treatment of HIV Related Tuberculosis. 2007. Available from URL: http://www.cdc.gov.tb/TB-HIV_Drugs/default.htm.
International Standards for Tuberculosis Care (Endorsed by IDSA) World Health Organization, 2006.
American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Controlling Tuberculosis in the United States. Am J Respir Crit Care Med 2005;172:1169-1227.
Pillay T, Khan M, Moodley J, Adhikari M, Coovadia H. Perinatal tuberculosis and HIV-1: considerations for resource-limited settings. Lancet Infect Dis. 2004 Mar;4(3):155-65.
Drobniewski FA, Caws M, Gibson A, Young D. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis. 2003 Mar;3:141-7.
ATS/CDC/IDSA. Practice Guidelines for the Treatment of Tuberculosis. MMWR 2003;52 (RR-11).
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
Iseman MD. Extensively Drug-Resistant Mycobacterium tuberculosis: Charles Darwin Would Understand. Clin Infect Dis. 2007;45:1415–6.
Ullman A. Pasteur-Koch: Distinctive Ways of Thinking about Infectious Diseases. Microbe 2007;2(8):383-387.
Mackowiak PA, et al. On the Origin of American Tuberculosis. Clin Infect Dis 2005;41:515-518.
Schultz, M. Theobald Smith. Emerg Infect Dis. 2008. Dec; 14:1940-2.
Wirth, T et al. Mycobacterium tuberculosis and Homo sapiens: Microbiological and Anthropological Coevolution. Clin Infect Dis. 2009;48:v–vi.
