Hospital-Acquired and ICU Pneumonia
Authors: Joan R. Badia, Mauricio Valencia, Miquel Ferrer, Silvia Blanco, Joseph Mensa, Jose Antonio Martinez, Antoni Torres
DEFINITION OF HOSPITAL-ACQUIRED PNEUMONIA
Hospital-Acquired Pneumonia
(HAP) is an inflammatory process due to the infection of the pulmonary parenchyma by pathogenic microorganisms and may develop in a patient admitted to the hospital for more than 48 hours or the incubation period of this infection is no longer than 2 days (3).
Ventilator-Associated Pneumonia
(VAP) is hospital-acquired pneumonia that develops in patients who have been intubated and have received mechanical ventilation for at least 48 hours (1). Ventilator-associated tracheobronchitits (8) has not been as widely studied as ventilator-associated pneumonia and is characterized by the presence of signs of respiratory infection such as an increase in the volume and purulence of respiratory secretions, fever and leukocytosis in patients undergoing mechanical ventilation (7). However, in contrast to ventilator-associated pneumonia (see section on diagnosis of hospital-acquired pneumonia) radiologic infiltrates suggestive of consolidation on chest x-ray are not observed.
Healthcare-Associated Pneumonia
Healthcare-associated pneumonia is a clinical entity which has been defined in the latest guidelines of the ATS for the diagnosis and treatment of hospital-acquired pneumonia. This pneumonia is found in patients who are not hospitalized at the time the infection develops. These patients present different epidemiological characteristics that make them susceptible to colonization by bacteria, similar to hospitalized patients and consequently are at risk of infection by potentially multiresistant microorganisms (6). Risk factors for healthcare-associated pneumonia include the following: hospitalization for 2 days or more within the preceding 90 days, residence in a nursing home or extended care facility (5), home infusion therapy (including antibiotics, chronic dialysis within 30 days, home wound care and a family member(s) with multidrug resistant pathogen colonization or infection).
Another classification used is based on the presence of microorganisms isolated in cultures of epidemiologic surveillance samples and includes the following categories (4):
Endogenous Pneumonia
Primary endogenous pneumonia: the causative pathogenic microorganisms of pneumonia are isolated in the surveillance cultures on admission. Secondary endogenous pneumonia: these are caused by nosocomial pathogens, not present in the patients on admission, which colonize the oropharnyx, the stomach and/or the intestine where they multiply and thereafter invade the lower respiratory tract.
Exogenous Pneumonia
This is caused by microorganisms which are not isolated in the surveillance cultures, that is, the patients are not previously carriers. Colonization of the material of the artificial airway (ventilatory tubes, humidifiers), infection by invasive devices such as bronchoscopes, infection by the nebulization procedure or inhalation play an important role in this category (2).
Early-Onset vs. Late-Onset
The distinction of early-onset (presented within the first 4 days of hospital admission) or late-onset (5 days of more) hospital-acquired pneumonia has important implications in terms of possible etiology, empiric antimicrobial treatment and outcome. However, there are no well-designed trials supporting these time cut-offs. An interesting trial performed by Trouillet et al (9) showed that according to logistic regression analysis, three variables among potential factors remained significant for predicting infection with multidrug resistant ventilator-associated pneumonia: duration of mechanical ventilation (MV) > or = 7 days (odds ratio [OR] = 6.0), prior antibiotic use (OR = 13.5) and prior use of broad-spectrum drugs (third-generation cephalosporin, fluoroquinolone and/or imipenem) (OR = 4.1).
DIAGNOSIS OF HOSPITAL-ACQUIRED AND ICU PNEUMONIA
Hospital acquired pneumonia (HAP) is the most frequent hospital-acquired infection in critically ill patients (3,19). HAP accounts for as much as 27% of all nosocomial infections acquired in medical intensive care units (ICU). Mechanical ventilation is the most important risk factor. The incidence of HAP increases 6 to 21 times in mechanically ventilated patients compared to those breathing spontaneously and the incidence ranges between 1-10 cases for every 1000 days of mechanical ventilation (10, 43). The diagnosis of hospital-acquired pneumonia is complex. Multiple efforts have been directed to identify the most appropriate management strategies.
Clinical Diagnosis
Radiological findings are the hallmark of HAP. The diagnosis of HAP is suspected if the patient has new and persistent pulmonary infiltrates in the standard chest radiography along with new clinical signs and symptoms that may be explained by this diagnosis. The three main clinical findings suggesting infection include the new onset of fever or hypothermia (>38.3 ºC), leukocytosis (>12,000mm3) or leukopenia (<4000/mm3), and purulent respiratory secretions. These criteria are less reliable for the diagnosis of pneumonia in mechanically ventilated patients. The differential diagnosis of pulmonary infiltrates in the ICU is varied and extensive. Also changes in white blood cell count and fever are largely non-specific findings in the critically-ill patient.
The accuracy of the clinical diagnosis of HAP has been studied on the basis of autopsy findings or quantitative cultures samples as comparison standards (48,24). The diagnostic criteria of a radiographic infiltrate, plus the presence of at least one of the three clinical data of infection mentioned above, provides a high sensitivity but with low specificity. In a study in which the gold standard was histology plus positive microbiologic cultures of immediate postmortem lung samples, the presence of chest infiltrates, plus two of three clinical criteria resulted in a 69% sensitivity and 75% specificity (24). As a recommended rule, the presence of pulmonary infiltrates plus two of the other three criteria (fever, leukocytosis, or purulent tracheal secretions) establishes the suspicion for HAP.
Patients with acute respiratory distress syndrome and acute lung injury have a high incidence of lower airway infections and a chest radiography itself is of limited value for the diagnosis of HAP in this condition (12, 20). Thus, one of the three clinical criteria should be enough to start additional diagnostic tests. A high index of suspicion should also be present in patients with hemodynamic instability not explained by other causes or worsening blood gases during mechanical ventilation.
Several strategies have been proposed to overcome these diagnostic difficulties on clinical grounds. The use of integrative scoring systems that take into account multiple clinical data and microbiological findings has been extensively investigated. The clinical pulmonary infection score (CPIS) has been used to establish the likelihood of HAP in ICU patients (41). This instrument takes into account 6 easily obtained variables including, body temperature, white blood cell count, quantity and purulence of tracheal secretions, chest radiograph, oxygenation, and, bacterial growth in lower respiratory tracheal secretions (Table 1). The final score ranges from 0 to 12 and a score above 6 would correlate with the diagnosis of microbiologically confirmed HAP. The CPIS has been used as an operational criteria to select patients that can be treated safely with short-course antibiotic (37, Singh AJRCCM 2000;162:505-511). However, some authors have shown that the score may lack sensitivity and specificity to establish the diagnosis of HAP by itself (26). The application of this score has some pitfalls, which include significant variability between observers and availability of microbiological results. Additionally, most of the research involving CPIS has been carried out with different modifications and variations of the original score, and has applied for very different purposes.
The main practical problem has to do with the delay in obtaining microbiological results. Standard cultures of respiratory samples will take at least two to three days for processing and bacterial growth. Thus, microbiological data are rarely available to obtain the CPIS when HAP is being initially considered as a diagnosis and it has to be decided if empiric antibiotic treatment is started. Two possibilities have been investigated to overcome this limitation. A first approach may be to consider only the results of Gram stain which can be obtained the same day (26). A second even more pragmatic approach is to apply the score using the same cut-off point for high probability of HAP, but eliminating microbiology (30,33). Our proposed algorithm for the management of HAP applies this strategy, and described in the next section. Nevertheless, the diagnosis of HAP cannot be sustained on clinical data alone, specially in mechanically ventilated patients. Diagnostic testing is necessary for two reasons: to define whether pneumonia is present and to identify the etiologic pathogen. Our current diagnostic tools have limitations and do not always reliably achieve this end.
APPROACH TO PULMONARY INFILTRATES IN THE ICU - METHOD OF ANTONI TORRES
In our intensive care unit, we have developed an approach to diagnosis (61) and treatment of ICU pneumonia ( Figure 1). The major differential diagnosis of pulmonary infiltrates includes only two entities in which antibiotics are clearly indicated: pneumonia and aspiration (Table 2). The presence of an infiltrate on simple chest x-ray raises the possibility that a patient may have pneumonia (54). Our suspicion of hospital-acquired pneumonia occurs when two out of three clinical criteria are noted in a patient with pulmonary infiltrate: fever or hypothermia, leukocytosis or leukopenia and purulent respiratory secretions (50).
Approach
We calculate the Clinical Pulmonary Infection Score (CPIS) (56) which allows objective analysis of the varying clinical and radiologic variables (Table 1) (Singh AJRCCM 2000;162:505-511). Cultures of the lower respiratory secretions are immediately taken (53) (Table 3). We emphasize the use of the Gram stain of the sample of respiratory secretions; this improves diagnostic value of the score (52). Culturing should be performed prior to initiation of antibiotic treatment, but it should not delay the administration of antibiotics (57-59).
Collection may be performed by sputum, tracheobronchial aspirate (55, 56, 57, 58, 59, 60, 61), bronchoalveolar lavage or protected specimen brush (1,51). Blood cultures should be obtained. Pleural fluid cultures should be obtained, if the effusion is large and the cause is uncertain or if etiologic diagnosis of pneumonia has eluded identification. Legionella urinary antigen is performed routinely given its occurrence as an occult cause of hospital-acquired pneumonia. If the patient has been only recently hospitalized within 5 days, a urinary antigen for Streptococcus pneumoniae is performed.
We obtain C-reactive protein (CRP) and procalcitonin (PCT). Elevated levels suggest more severe pneumonia.
Assessment of CPIS, Clinical Status, Gram Stain
If the CPIS < 6, systemic inflammatory response syndrome (SIRS) (Table 8) is not present and the Gram stain of respiratory secretions is negative or intracellular organisms < 2%, then pneumonia is not likely. Patients fulfilling all of these criteria need not receive antibiotic treatment but be strictly monitored. This is our unique addition to the protocol designed by Singh et al.; we have found it to be reliable and clinically-plausible. These patients do not fulfill classic criteria for pneumonia and they are clinically stable. Monitoring such patients does not pose a substantial risk for poor outcome, since antibiotics can be added if deterioration is observed. Keep in mind, it is probable that noninfectious causes of pulmonary infiltrates will be diagnosed in such patients within 3 days.
At the other extreme, if any of the following are present: CPIS > 6, presence of SIRS, and Gram stain of respiratory secretions shows a predominant bacterium or intracellular bacteria, these patients most likely have pneumonia. Broad spectrum antibiotic treatment should be initiated (Figure 2).
If the CPIS is > 6 and SIRS is not present, pneumonia is still likely, and broad spectrum antibiotic therapy is initiated immediately.
If the CPIS is < 6 and the Gram stain of the repiratory tract sample is difficult to interpret, then we administer monotherapy. In the original protocol by Singh, et al., a quinolone was used for monotherapy, and in an NIH proposed protocol, a carbapenem was used.
Re-Evaluation on Day 3
On the third day, response to empiric antibiotics is observed using clinical, laboratory and radiologic parameters and the re-calculated CPIS (Figure 3). The criteria of treatment failure are: 1) absence of improvement in the PaO2/FiO2 or the need for mechanical ventilation, 2) persistence of fever or hypothermia, 3) 50 % progression in the pulmonary infiltrates, and 4) development of septic shock or multiple organ dysfunction syndrome. For patients whose original CPIS < 6, if the patient appears stable and the cultures are negative, all antibiotic treatment should be discontinued on day 3. For those patients in whom cultures are revealing, antibiotic therapy should be focused on the specific pathogen and the spectrum narrowed. In patients with increasing level of biomarkers (RCP and procalcitonin) on day 3-5, an extensive microbiological and radiological re-evaluation is warranted and broadening the spectrum of the antimicrobial therapy is done.
Antibiotic Selection
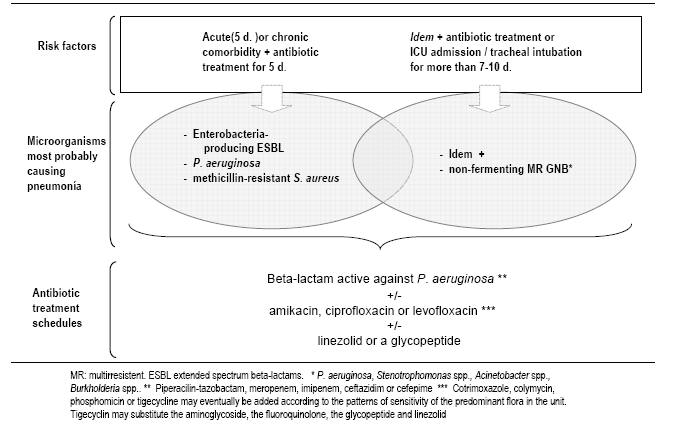
Empiric antibiotic therapy should be based on local in vitro susceptibility patterns and patient risk factors for infection by multiresistant microorganisms (Table 4, Figure 4 and 5). For patients with CPIS > 6, and risk factors for infection by multiresistant microorganisms or duration of mechanical ventilation more than 5 days, we use a combination of an anti-pseudomonal beta-lactam antibiotic with the addition of a quinolone or an aminoglycoside (Table 5, Figure 5 and 6). This is a reasonable choice for both Pseudomonas aeruginosa or multi-drug resistant microorganisms.
Empiric therapy for methicillin-resistant Staphylococcus aureus (MRSA) is implemented only in patients who demonstrate colonization or previous infection by MRSA or who have received mechanical ventilation for more than 6 days. In many hospitals, linezolid is preferred to vancomycin.
In the absence of risk factors for infection by multiresistant microorganisms or in patients who have been hospitalized or have received mechanical ventilation for less than 5 days, pathogens of community-acquired pneumonia should be covered; Ceftriaxone or levofloxacin can be added for these pathogens (Streptococcus pneumoniae, Legionella pneumophila) (Table 6, Figure 5, 6).
Table 7 shows the antibiotics, the doses, treatment schedule and the length of infusion. If beta-lactam agents are selected, administration by continuous infusion should be considered.
PREVENTION OF HOSPITAL-ACQUIRED PNEUMONIA
Attempts at prevention have focused on reducing cross transmission, the likelihood of aspiration and reducing the bacterial load in the oropharynx. Microorganisms can reach the lung by other routes, such as direct spread to the lungs from the pleura or the mediastinum, hematogenous spread from distal foci (including the possibility of bacterial translocation from an ischemic gut in critically ill patients), and inoculation of aerosols. The modifiable risk factors for HAP are:
-
General prophylactic measures
-
Intubation and mechanical ventilation
-
Aspiration, body position, and enteral feeding
-
Modulation of colonization by oral antiseptics and antiobiotics
-
Stress ulcer prophylaxis, transfusion, and hyperglycemia
General Prophylactic Measures
Maintaining adequate staffing levels in the ICU can reduce length of stay, improve infection control practices, and reduce duration of mechanical ventilation (121). Other effective infection control measures include staff education, compliance with alcohol-based hand disinfection, and isolation to reduce cross-infection with multi-drug resistant pathogens should be used routinely (97, 99, 125, 137). The surveillance of ICU infections to identify and quantify endemic and new multi-drug resistant pathogens is recommended (82, 99, 117, 125, 137).
Intubation and Mechanical Ventilation
Intubation and mechanical ventilation is associated to increased risk of HAP and therefore should be avoided whenever possible (75, 125, 137). Non-invasive positive-pressure ventilation (NPPV) is an attractive alternative for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) or acute hypoxemic respiratory failure, and for some immunosuppressed patients with pulmonary infiltrates and respiratory failure (67, 69, 89, 93, 114).
Reduced duration of intubation and mechanical ventilation may prevent VAP. Specific strategies have been recommended in order to reduce the duration of mechanical ventilation, such as improved methods of sedation and the use of protocols to facilitate and accelerate weaning (68, 101, 107, 112, 126).
Attention to the specific type of endotracheal tube, its maintenance, and the site of insertion may also be valuable. Orotracheal intubation and orogastric tubes are preferred over nasotracheal intubation and nasogastric tubes in order to prevent nosocomial sinusitis and to reduce the risk of VAP, although direct causality has not been proved (90, 123, 125).
Measures to reduce the likelihood of aspiration of oropharyngeal bacteria around the endotracheal tube cuff and into the lower respiratory tract include limiting the use of sedative and paralytic agents that depress cough and other protective mechanisms of the host, and maintaining an adequate inflation of the endotracheal cuff pressure (70, 121). Continuous aspiration of sub-glottic secretions, through the use of a specially designed endotracheal tube, has significantly reduced the incidence of early-onset VAP in several studies, and should be used, if available (100, 106, 131). Moreover, the endotracheal tube cuff pressure should be maintained higher than 20 cm H2O in order to prevent leakage of contaminated secretions around the cuff into the lower respiratory tract (70, 121).
VAP may also be related to colonization of the ventilator circuit (63). The frequency of ventilator circuit change does not affect the incidence of HAP, but condensate collecting in the ventilator circuit can become contaminated from patients secretions (70, 74, 80, 87, 95). Therefore, the inadvertent flushing of the contaminated condensate into the lower airway or nebulizers should be avoided by careful emptying from ventilator circuits (70, 73, 74). Although passive humidifiers or heat moisture exchangers decrease ventilator circuit colonization, there are no consistent data showing reduction of the incidence of VAP, and thus they cannot be recommended as a pneumonia prevention tool (64, 87, 94, 98, 108).
Aspiration, Body Position, and Enteral Feeding
Supine patient positioning may also facilitate aspiration, which may be decreased by a semirecumbent positioning. Using radioactive labeled technetium instilled into the stomach, cumulative radioactive counts of endotracheal secretions were higher when patients were placed in the supine position (115, 128). One randomized trial demonstrated a reduction in the incidence of ICU-acquired HAP in patients treated in the semirecumbent position compared with patients treated completely supine (79). Infection in patients in the supine position was strongly associated with the simultaneous administration of enteral nutrition. Thus, intubated patients should be kept in the semirecumbent position rather than supine to prevent aspiration, especially when receiving enteral feeding.
Enteral nutrition has been considered a risk factor for the development of HAP, mainly because of an increased risk of aspiration of gastric contents (116, 125). However, its alternative, parenteral nutrition, is associated with higher risks for catheter-related infections, complications of line insertions, higher costs, and loss of intestinal villous architecture, which may facilitate enteral microbial translocation. Although some have advised feeding critically ill patients enterally as early as possible, a strategy of early enteral feeding was, when compared with late administration, associated with a higher risk for VAP (91). Seven studies have evaluated the risks for ICU-acquired pneumonia in patients randomized to either gastric or postpyloric feeding. Although significant differences were not demonstrated in any individual study, postpyloric feeding was associated with a 24% significant reduction in ICU-acquired HAP in meta-analysis (88). Overall, enteral nutrition is preferred over parenteral nutrition to reduce the risk of complications related to central intravenous catheters and to prevent reflux villous atrophy of the intestinal mucosa that may increase the risk of bacterial translocation (91, 97, 125).
Modulation of Colonization: Oral Antiseptics and Antibiotics
Oropharyngeal colonization, either present on admission or acquired during ICU stay, has been identified as an independent risk factor for the development of ICU-acquired pneumonia caused by enteric gram-negative bacteria and P. aeruginosa (65). In a randomized trial, the use of the oral antiseptic chlorhexidine significantly reduced the rates of nosocomial infection in patients undergoing coronary artery bypass surgery (78). Modulation of oropharyngeal colonization, by combinations of oral antibiotics, with or without systemic therapy, or by selective decontamination of the digestive tract (SDD), is also effective in significantly reducing the frequency of HAP, although methodologic study quality appeared to be inversely related to the magnitude of the preventive effects (62, 63, 76, 97, 119, 122, 135).
In two prospective randomized trials SDD was associated with higher ICU survival among patients receiving SDD (67, 102), although the benefits of SDD in one study appeared restricted to patients with a midrange APACHE II score on admission only (102). In the other larger study, SDD administered to 466 patients in one unit was associated with a relative risk for ICU and hospital mortality of 0.65 and 0.78, respectively, when compared with 472 patients admitted in a control ward (67). In addition, infections due to antibiotic-resistant microorganisms occurred more frequently in the control ward. Importantly, levels of antibiotic-resistant pathogens were low in both wards, with complete absence of MRSA. Moreover, a small preexisting difference in outcome between two wards and the absence of a cross-over design warrant confirmation of these beneficial effects of SDD. The preventive effects of SDD for HAP have also been considerably lower in ICUs with high endemic levels of antibiotic resistance. In such a setting, SDD may increase the selective pressure for antibiotic-resistant microorganisms (84, 85, 105, 109, 110, 136, 138). Although SDD reduces HAP, routine prophylactic use of antibiotics should be discouraged, especially in hospital settings where there are high levels of antibiotic resistance.
Routine prophylaxis of HAP with SDD, with or without systemic antibiotics, reduces the incidence of ICU-acquired pneumonia, has helped contain outbreaks of multi-drug resistant bacteria), but is not recommended for routine use, especially in patients who may be colonized with multi-drug resistant pathogens.
The role of systemic antibiotics in the development of HAP is less clear. In one study, prior administration of antibiotics was associated with a three-fold increase in the risk for development of late-onset ICU-acquired pneumonia (96). Moreover, antibiotics clearly predispose patients to subsequent colonization and infection with antibiotic-resistant pathogens (129). In contrast, prior antibiotic exposure conferred protection for ICU-acquired pneumonia in another study (62). In addition, antibiotic use at the time of emergent intubation may prevent pneumonia within the first 48 hours of intubation (120). Preventive effects of intravenous antibiotics were evaluated in only one randomized trial: administration of cefuroxime for 24 hours, at the time of intubation reduced the incidence of early-onset, ICU-acquired pneumonia in patients with closed head injury (124); however, its routine use is not recommended until more data become available. However, circumstantial evidence of the efficacy of systemic antibiotics also follows from the results of meta-analyses of SDD, which have suggested that the intravenous component of the regimens was largely responsible for improved survival (76).
In summary, prior administration of systemic antibiotics has reduced the risk of nosocomial pneumonia in some patient groups, but if a history of prior administration is present at the time of onset of infection, there should be increased suspicion of infection with MDR pathogens (77, 84, 85, 105, 109, 110, 136).
Stress Bleeding Prophylaxis, Transfusion, and Glucose Control
Histamine type 2 (H2) antagonists and antacids have been identified as independent risk factors for ICU-acquired pneumonia. Sucralfate has been used for stress bleeding prophylaxis, as it does not decrease intragastric acidity or significantly increase gastric volume. Comparative data from randomized trials suggest a trend toward reduced VAP with sucralfate, but there is a slightly higher rate of clinically significant gastric bleeding, compared with H2 antagonists. If needed, stress bleeding prophylaxis with either H2 antagonists or sucralfate is acceptable (65, 66, 71, 81, 113, 118, 130).
A prospective randomized trial comparing liberal and conservative "triggers" to transfusion in ICU patients not exhibiting active bleeding and without underlying cardiac disease demonstrated that awaiting a hemoglobin level of 7.0 g/dl as opposed to a level of 9.0 g/dl before initiating transfusion resulted in less transfusion and no adverse effects on outcome (86). In fact, in those patients less severely ill, as judged by low APACHE II scores, mortality was improved in the "restricted transfusion" group, a result thought to result from immunosuppressive effects of non-leukocyte-depleted red blood cell units with consequent increased risk for infection. Multiple studies have identified exposure to allogeneic blood products as a risk factor for postoperative infection and postoperative pneumonia, and the length of time of blood storage as another factor modulating risk (92, 103, 104, 132, 133). In one prospective randomized control trial the use of leukocyte-depleted red blood cell transfusions resulted in a reduced incidence of postoperative infections, and specifically a reduced incidence of pneumonia in patients undergoing colorectal surgery (92). Routine red blood cell transfusion should be conducted with a restricted transfusion trigger policy. Whether leukocyte-depleted red blood cell transfusions will further reduce the incidence of pneumonia in broad populations of patients at risk remains to be determined.
Hyperglycemia, relative insulin deficiency, or both may directly or indirectly increase the risk of complications and poor outcomes in critically ill patients. A randomized clinical trial in surgical ICU patients assessed a strategy based to receive either intensive insulin therapy to maintain blood glucose levels between 80 and 110 mg/dl or to receive conventional treatment (134). The group receiving intensive insulin therapy had reduced mortality and the difference was greater in patients who remained in the intensive care unit more than 5 days. When compared with the control group, those treated with intensive insulin therapy had a 46% reduction of bloodstream infections, decreased frequency of acute renal failure requiring dialysis by 41%, fewer antibiotic treatment days, and significantly shorter length of mechanical ventilation and ICU stay. Intensive insulin therapy is recommended to maintain serum glucose levels between 80 and 110 mg/dl in ICU patients to reduce nosocomial blood stream infections, duration of mechanical ventilation, ICU stay, morbidity, and mortality.
REFERENCES
1. Chastre J, Fagon JY. Invasive diagnostic testing should be routinely used to manage ventilated patients with suspected pneumonia. Am J Respir Crit Care Med 1994; 150:570-574.[PubMed]
2. Craven DE, Goularte TA, Make BJ. Contaminated condensate in mechanical ventilator circuits. A risk factor for nosocomial pneumonia? Am Rev Respir Dis 1984; 129:625-628.[PubMed]
3. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am J Respir Crit Care Med 2005; 171:388-416.[PubMed]
4. Murray AE, Chambers JJ, van Saene HK. Infections in patients requiring ventilation in intensive care: application of a new classification. Clin Microbiol Infect 1998; 4(2):94-99.[PubMed]
5. Mylotte JM. Nursing-home-acquired pneumonia. Clin Infect Dis 2002;35:1205-1211. [PubMed]
6. Naughton BJ, Mylotte JM. Treatment guideline for nursing home-acquired pneumonia based on community practice. J Am Geriatr Soc 2000;48:82-88. [PubMed]
7. Nseir S, Di Pompeo C, Pronnier P, Beague S, Onimus T, Saulnier F et al. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respir J 2002; 20:1483-1489. [PubMed]
8. Torres A, Valencia M. Does ventilator-associated tracheobronchitis need antibiotic treatment? Crit Care 2005; 9(3):255-256. [PubMed]
9. Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am Rev Respir Dis 1998; 157(2):531-539.[PubMed]
10. CDC Guidelines for Prevention of Healthcare Associated Pneumonias 2003. [PubMed]
11. Barreiro B, Dorca J, Manresa F. Protected alveolar lavage in the diagnosis of ventilator-associated pneumonia. Eur Respir J 1996; 9:1500-1507. [PubMed]
12. Bauer TT, Valencia M, Badia JR, et al. Respiratory Microbiology Patterns Within the First 24 h of ARDS Diagnosis: Influence on Outcome. Chest 2005; 128: 273-279. [PubMed]
13. Baughman RP, Tapson V, McIvor A. The diagnosis and treatment challenges in nosocomial pneumonia. Diagn Microbiol Infect Dis 1999;33:131-9. [PubMed]
14. Boussekey N, Leroy O, Georges H, et al. Diagnostic and prognostic values of admission procalcitonin levels in community-acquired pneumonia in an intensive care unit. Infection 2005;33:257-263. [PubMed]
15. Bowton DL. Nosocomial pneumonia in the ICU--year 2000 and beyond. Chest 1999;115:28s-33s. [PubMed]
16. Castelli GP, Pognani C, Meisner M, et al. Procalcitonin and C-reactive protein during systemic inflamatory response syndrome, sepsis and organ dysfunction. Crit Care Med 2004; 8:234-240. [PubMed]
17. Christ-Crain M, Jaccard-Stoltz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections. The Lancet 2004.[PubMed]
18. Clec'h C, Ferriere F, Karoubi P, et al. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med 2004; 32: 1166-1169. [PubMed]
19. Cook DJ, Walter SD, Cook RJ, et al. Incidence of and Risk Factors for Ventilator-Associated Pneumonia in Critically Ill Patients. Ann Intern Med 1998 ;129:433-440. [PubMed]
20. Delclaux C, Roupie E, Blot F, et al. Lower respiratory tract colonization and infection during severe acute respiratory distress syndrome: incidence and diagnosis. Am JRespir Crit Care Med 1997;156:1092-1098 [PubMed]
21. Detemann RM, Millo JL, Gibot S, et al. Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during development of ventilator-associated pneumonia. Intensive Care Med 2005; 31:1495-500. [PubMed]
22. Dev D, Wallace E, Sankaran R, Cunniffe J, et al. Value of C-reactive protein measurements in exacerbations of chronic obstructive pulmonary disease. Respir Med 1998; 92: 664-667. [PubMed]
23. El-Ebiary M, Torres A, Gonzalez J, et al. Quantitative cultures of endotracheal aspirates for the diagnosis of ventilator-associated pneumonia. Am Rev Respir Dis 1993; 148: 1552-1557. [PubMed]
24. Fabregas N, Ewig S, Torres A, et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate postmortem lung biopsies. Thorax 1999;54:867-873. [PubMed]
25. Fagon JY, Chastre J, Wolff M, et al. A Tenaillon. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. Ann Intern Med 2000; 132; 621-630. [PubMed]
26. Fartoukh M, Maitre B, Honore S, et al. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168(2):173-9. [PubMed]
27. Gibot S, Cravoisy A, Levy B, et al. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med 2004; 350: 451-8. [PubMed]
28. Gibot S, Kolopp-sarda MN, Bene MC, et al. Plasma levels of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med 2004; 141: 9-15 [PubMed]
29. Gibot S, Le Renard PE, Bollaert PE, et al. Surface triggering receptor expressed on myeloid cells 1 expression patterns in septic shock. Intensive Care Med 2005; 31: 594-597. [PubMed]
30. Iregui M, Ward S, Sherman G, et al. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 2002; 122: 262-268. [PubMed]
31. Jourdain B, Novara A, Joly-Guillou ML, et al. Role of quantitative cultures of endotracheal aspirates in the diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med 1995; 152: 241-246. [PubMed]
32. Kirtland SH, Corley DE, Winterbauer RH, et al. The diagnosis of ventilator-associated pneumonia: a comparison of histologic, microbiologic, and clinical criteria. Chest 1997;112:445-457. [PubMed]
33. Luna CM, Aruj P, Niederman MS, et al for the Grupo Argentino de Estudio de la Neumon Asociada al Respirador (GANAR) group. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J 2005; 27: 158-164 [PubMed]
34. Luna CM, Videla A, Mattera J, et al. Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator-associated pneumonia. Chest 1999;116:1075-1084. [PubMed]
35. Luyt CE, Guerin V, Combes A, et al. Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med 2005; 171: 48-53. [PubMed]
36. Marquete CH, Copin MC, Wallet F, et al. Diagnostic tests for pneumonia in ventilated patients: prospective evaluation of diagnostic accuracy using histology as a diagnostic gold standard. Am J Respir Crit Care Med 1995; 151: 1878-1888. [PubMed]
37. Niederman M, Torres A, Summer W. Invasive diagnostic testing is not needed routinely to manage suspected ventilator-associated pneumonia. Am J Respir Crit Care Med 1994;150:565-9. [PubMed]
38. Povoa P, Almeida E, Moreira P, et al. C-reactive protein as an indicator of sepsis. Intensive Care Med 1998; 24: 1052-6. [PubMed]
39. Povoa P, Coelho L, Almeida E, et al. C-reactive protein as a marker of ventilator-associated pneumonia resolution: a pilot study. Eur Respir J; 2005; 25: 804 -812. [PubMed]
40. Prats E, Dorca J, Pujol M. Effect of antibiotics on protected specimen brush sampling in ventilator associated pneumonia. Eur Respir J 2003; 19:944-951. [PubMed]
41. Pugin J, Auckenthaler R, Mili N, et al. Diagnosis of ventilator associated pneumonia by bacteriologic analysis of bronchoscopic and non-bronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respir Dis 1991;143:1121-9. [PubMed]
42. Ruiz M, Torres A, Ewig S, et al. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med. 2000; 162:119-25. [PubMed]
43. Safdar N, Dezfulian C, Collard HR, et al. Clinical and economic consequences of ventilator-associated pneumonia: A systematic review. Crit Care Med 2005; 33: 2184-2193[PubMed]
44. Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004; 39: 206-217. [PubMed]
45. Singh N, Rogers P, Atwood CHW, et al. Short course empiric therapy for patients with pulmonary infiltrates in the intensive care unit. Am J Respir Crit Care Med 2000; 162:505-11. [PubMed]
46. Sole-Violan J, Fern AB, et al. Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia. Crit Care Med 2000;28 :2737-2741. [PubMed]
47. Torres A, De la Bellacasa JP, Xaubet A, et al. Diagnostic value of quantitative cultures of bronchoalveolar lavage and telescoping plugged catheters in mechanically ventilated patients with bacterial pneumonia. Am Rev respir Dis 1989; 140:306-310. [PubMed]
48. Torres A, El-Ebiary M. Bronchoscopic BAL in the diagnosis of ventilator-associated pneumonia. Chest 2000;117:198S-202S. [PubMed]
49. Torres A, Ewig S. Diagnosing ventilator-associated pneumonia. N Engl J Med 2004; 350: 433-435.[PubMed]
50. Fabregas N, Ewig S, Torres A, El-Ebiary M, Ramirez J, Puig de la Bellacasa J et al. Clinical diagnosis of ventilator associated pneumonia revisited: Comparative validation using immediate postmortem lung biopsies . Thorax. 1999 ;54:867-73.[PubMed]
51. Fagon JY, Chastre J, Wolff M. Invasive and non-invasive strategies for management of suspected ventilator-associated pnemonia. Ann Intern Med 2000; 132:612-630.[PubMed]
52. Fartoukh M, Maitre B, Honore S, Cerf C, Zahar JR, Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med 2003; 168:173-179.[PubMed]
53. Ioanas M, Ferrer R, Angrill J, Ferrer M, Torres A. Microbial investigation in ventilator-associated pneumonia. Eur Respir J 2001;17:791-801.[PubMed]
54. Kirtland SH, Corley DE, Winterbauer RH, Springmeyer SC, Casey KR, Hampson NB et al. The diagnosis of ventilator-associated pneumonia. A comparison of histologic, microbiologic, and clinical criteria. Chest 1997; 112:445-457.[PubMed]
55. Niederman MS, Torres A, Summer W. Invasive diagnostic testing is not needed routinely to manage suspected ventilator-associated pneumonia. Am J Respir Crit Care Med 1994; 150:565-569.[PubMed]
56. Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter P. Diagnosis of ventilator associated pneumonia by bacteriologic analysis of bronchoscopic and non-bronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respir Dis 1991;143:1121-1129.[PubMed]
57. Ruiz M, Torres A, Ewig S, Marcos MA, et al. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med 2000; 162:119-125.[PubMed]
58. Sanchez-Nieto JM, Torres A, Garcia-Cordoba F, El-Ebiary M, Carrillo A, Ruiz J et al. Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator-associated pneumonia: a pilot study. Am J Respir Crit Care Med 1998;157:371-376.[PubMed]
59. Sole-Violan J, Arroyo Fernandez J, Bordes Benitez A, Cardenosa Cendrero JA, Rodriguez de Castro F. Impact of quantitative invasive diagnostic techniques in the management of outcome of mechanically ventilated patients with suspected pneumonia. Crit Care Med 2000;28:2737-41.[PubMed]
60. Torres A, Ewig S. Diagnosing ventilator-associated pneumonia. N Engl J Med 2004; 350:433-435.[PubMed]
61. Valencia M, Torres A, Insausti J, Alvarez-Lerma F, Carrasco N, Herranz M, et al. Diagnostic value of quantitative cultures of endotracheal aspirate in ventilator-associated pneumonia: a multicenter study. Arch Bronconeumol 2003;39:394-399.[PubMed]
62. Abele-Horn M, Dauber A, Bauernfeind A, Russwurm W, Seyfarh-Metzger I, Gleich P, Ruckdeschel G. Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination. Intensive Care Med 1997;23:187-95. [PubMed]
63. Bergmans DC, Bonten M, Gaillard CA, et al. Prevention of ventilator-associated pneumonia by oral decontamination. Am J Respir Crit Care Med 2001;164:382-8. [PubMed]
64. Boisson C, Viviand X, Arnaud S, Thomachot L, Miliani Y, Martin C. Changing a hydrophobic heat and moisture exchanger after 48 hours rather than 24 hours: a clinical and microbiological evaluation. Intensive Care Med 1999 November;25:1237-43. [PubMed]
65. Bonten MJ, Bergmans DC, Ambergen AW, de Leeuw PW, van der Geest S, Stobberingh EE et al. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am J Respir Crit Care Med 1996;154:1339-46.[PubMed]
66. Bonten MJ, Gaillard CA, van der Geest S, van Thiel FH, Beysens AJ, Smeets HG et al. The role of intragastric acidity and stress ulcer prophylaxis on colonization and infection in mechanically ventilated ICU patients. A stratified, randomized, double-blind study of sucralfate versus antacids. Am J Respir Crit Care Med 1995;152:1825-34. [PubMed]
67. Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995;333:817-22. [PubMed]
68. Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 1999;27:2609-15. [PubMed]
69. Carlucci A, Richard JC, Wysocki M, Lepage E, Brochard L. Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med 2001;163:874-80. [PubMed]
70. Cook D, De Jonghe B, Brochard L, Brun-Buisson C. Influence of airway management on ventilator-associated pneumonia: evidence from randomized trials. JAMA 1998;279:781-7. [PubMed]
71. Cook DJ, Guyatt GH, Marshall J, Leasa D, Fuller H, Hall R et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med 1998;338:791-7. [PubMed]
72. Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med 1998;129:433-40. [PubMed]
73. Craven DE, Goularte TA, Make BJ. Contaminated condensate in mechanical ventilator circuits. A risk factor for nosocomial pneumonia? Am Rev Respir Dis 1984;129:625-8. [PubMed]
74. Craven DE, Lichtenberg DA, Goularte TA, Make BJ, McCabe WR. Contaminated medication nebulizers in mechanical ventilator circuits. Am J Med 1984;77:834-8. [PubMed]
75. Craven DE, Steger KA. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect 1996;11:32-53. [PubMed]
76. D'Amico R, Pifferi S, Leonetti C, Torri V, Tinazzi A, Liberati A. Effectiveness of antibiotic prophylaxis in critically ill adult patients. BMJ 1998;316:1275-85. [PubMed]
77. de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomized controlled trial. Lancet 2003;362:1011-6. [PubMed]
78. DeRiso AJ, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest 1996;109:1556-61.[PubMed]
79. Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 1999;354:1851-8.[PubMed]
80. Dreyfuss D, Djedaini K, Weber P, Brun P, Lanore JJ, Rahmani J et al. Prospective study of nosocomial pneumonia and of patient and circuit colonization during mechanical ventilation with circuit changes every 48 hours versus no change. Am Rev Respir Dis 1991;143:738-43. [PubMed]
81. Driks MR, Craven DE, Celli BR, Manning M, Burke RA, Garvin GM et al. Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization. N Engl J Med 1987;317:1376-82. [PubMed]
82. Evans RS, Pestotnik SL, Classen DC, Clemmer TP, Weaver LK, Orme JF, Jr. et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998;338:232-8. [PubMed]
83. Ferrer M. Esquinas A, Arancibia F, Bauer TT, Gonzalez G, Carrillo A, et al. Noninvasive ventilation during persistent weaning failure. A randomized controlled trial. Am J Respir Crit Care Med 2003;168:70-76. [PubMed]
84. Gastinne H, Wolff M, Delatour F, Faurisson F, Chevret S. A controlled trial in intensive care units of selective decontamination of the digestive tract with nonabsorbable antibiotics. The French Study Group on Selective Decontamination of the Digestive Tract. N Engl J Med 1992;326:594-9. [PubMed]
85. Hammond JM, Potgieter PD, Saunders GL, Forder AA. Double-blind study of selective decontamination of the digestive tract in intensive care. Lancet 1992;340:5-9. [PubMed]
86. Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409-17. [PubMed]
87. Hess D. Prolonged use of heat and moisture exchangers: why do we keep changing things? Crit Care Med 2000 May;28:1667-8.[PubMed]
88. Heyland DK, Drover JW, MacDonald S, Novak F, Lam M. Effect of postpyloric feeding on gastroesophageal regurgitation and pulmonary microaspiration: results of a randomized controlled trial. Crit Care Med 2001;29:1495-501. [PubMed]
89. Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001;344: 481-7. [PubMed]
90. Holzapfel L, Chastang C, Demingeon G, et al. Randomized study assessing the systematic search for maxillary sinusitis in nasotracheal mechanically ventilated patients. Am J Respir Crit Care Med 1999;159:695-701.[PubMed]
91. Ibrahim EH, Mehringer L, Prentice D, Sherman G, Schaiff R, Fraser V et al. Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. JPEN J Parenter Enteral Nutr 2002;26:174-81. [PubMed]
92. Jensen LS, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte-depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet 1996;348:841-5. [PubMed]
93. Keenan SP. Noninvasive positive pressure ventilation in acute respiratory failure. JAMA 2000;284:2376-8. [PubMed]
94. Kirton OC, DeHaven B, Morgan J, Morejon O, Civetta J. A prospective randomized comparison of an in-line heat moisture exchang filter and heated wire humidifiers: rates of ventilator-associated early-onset (community- acquired) or late-onset (hospital-acquired ) pneumonia and incidence of endotracheal tube occlusion. Chest 1997;112:1055-9. [PubMed]
95. Kollef MH, Shapiro SD, Fraser VJ, Silver P, Murphy M, Trovillon E et al. Mechanical ventilation with or without 7-day circuit changes: a randomized controlled trial. Ann Intern Med 1995;123:168-74. [PubMed]
96. Kollef MH. Ventilator-associated pneumonia: A multivariate analysis. JAMA 1993;270:1965-70. [PubMed]
97. Kollef MH. The prevention of ventilator-associated pneumonia. N Engl J Med 1999;340:627-34. [PubMed]
98. Kollef MH, Shapiro SD, Boyd V, Silver P, von Harz B, Trovillion E et al. A randomized clinical trial comparing an extended-use hygroscopic condenser humidifier with heated-water humidification in mechanically ventilated patients. Chest 1998;113:759-67. [PubMed]
99. Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999;115:462-74. [PubMed]
100. Kollef MH, Skubas NJ, Sundt TM. A randomized clinical trial of continuous aspiration of subglottic secretions in cardiac surgery patients. Chest 1999;116:1339-46. [PubMed]
101. Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471-7. [PubMed]
102. Krueger WA, Lenhart FP, Neeser G, Ruckdeschel G, Schreckhase H, Eissner HJ et al. Influence of Combined Intravenous and Topical Antibiotic Prophylaxis on the Incidence of Infections, Organ Dysfunctions, and Mortality in Critically Ill Surgical Patients: A Prospective, Stratified, Randomized, Double-Blind, Placebo-controlled Clinical Trial. Am J Respir Crit Care Med 2002;166:1029-37. [PubMed]
103. Leal-Noval SR, Marquez-Vacaro JA, Garcia-Curiel A, Camacho-Larana P, Rincon-Ferrari MD, Ordonez-Fernandez A et al. Nosocomial pneumonia in patients undergoing heart surgery. Crit Care Med 2000;28:935-40.[PubMed]
104. Leal-Noval SR, Rincon-Ferrari MD, Garcia-Curiel A, Herruzo-Aviles A, Camacho-Larana P, Garnacho-Montero J et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest 2001;119:1461-8. [PubMed]
105. Lingnau W, Berger J, Javorsky F, Fille M, Allerberger F, Benzer H. Changing bacterial ecology during a five-year period of selective intestinal decontamination. J Hosp Infect 1998;39:195-206. [PubMed]
106. Mahul PH, Auboyer C, Jospe R, Ros A, Guerin C, el Khouri Z et al. Prevention of nosocomial pneumonia in intubated patients: Respective role of mechanical sugglottic secretions drainage and stress ulcer prophylaxis. Intensive Care Med 1992;18:20-5. [PubMed]
107. Marelich GP, Murin S, Battistella F, Inciardi J, Vierra T, Roby M. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator-associated pneumonia. Chest 2000;118:459-67. [PubMed]
108. Markowicz P, Ricard JD, Dreyfuss D, Mier L, Brun P, Coste F et al. Safety, efficacy, and cost-effectiveness of mechanical ventilation with humidifying filters changed every 48 hours: a prospective, randomized study. Crit Care Med 2000;28:665-71. [PubMed]
109. Misset B, Kitzis MD, Conscience G, Goldstein F, Fourrier A, Carlet J. Mechanisms of failure to decontaminate the gut with polymyxin E, gentamicin and amphotericin B in patients in intensive care. Eur J Clin Microbiol Infect Dis 1994;13:165-170. [PubMed]
110. Misset B, Kitzis MD, Mahe P, Conscience G, Goldstein FW, Fourrier A et al. Bacteriological side effects of gut decontamination with polymyxin E, gentamicin, and amphotericin B. Infect Control Hosp Epidemiol 1993;14:62-4. [PubMed]
111. Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, et al. Noninvasive mechanical ventilation in the weaning of patients with respiration failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998;128:721-728.[PubMed]
112. Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. N Engl J Med 2002;346:1715-22. [PubMed]
113. Niederman MS, Craven DE. Devising strategies for preventing nosocomial pneumonia--should we ignore the stomach? Clin Infect Dis 1997;24:320-3. [PubMed]
114. Nourdine K, Combes P, Carton MJ, Beuret P, Cannamela A, Ducreux JC. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med 1999;25:567-73. [PubMed]
115. Orozco-Levi M, Torres A, Ferrer M, Piera C, El-Ebiary M, Puig de la Bellacasa J et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med 1995;152:1387-90. [PubMed]
116. Pingleton SK, Hinthorn DR, Liu C. Enteral nutrition in patients receiving mechanical ventilation. Multiple sources tracheal colonization include the stomach. Am J Med 1986;80:827-32. [PubMed]
117. Pittet D, Hugonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet 2000 October 14;356:1307-12. [PubMed]
118. Prod hom G, Leuenberger P, Koerfer J, Blum A, Chiolero R, Schaller MD et al. Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine, or sucralfate as prophylaxis for stress ulcer. Ann Intern Med 1994;120:653-62. [PubMed]
119. Pugin J, Auckenthaler R, Lew DP, Suter PM. Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia. A randomized, placebo-controlled, double-blind clinical trial. JAMA 1991 May 22;265:2704-10. [PubMed]
120. Rello J, Diaz E, Roque M, Valles J. Risk factors for developing pneumonia within 48 hours of intubation. Am J Respir Crit Care Med 1999;159:1742-6. [PubMed]
121. Rello J, Sonora R, Jubert P, Artigas A, Rue M, Valles J. Pneumonia in intubated patients: Role of respiratory airway care. Am J Respir Crit Care Med 1996;154:111-5. [PubMed]
122. Rodriguez-Roldan JM, Altuna-Cuesta A, Lopez A, Carrillo A, Garcia J, Leon J et al. Prevention of nosocomial lung infection in ventilated patients: use of an antimicrobial pharyngeal nonabsorbable paste. Crit Care Med 1990;18:1239-42. [PubMed]
123. Rouby JJ, Laurent P, Gosnach M, Cambau E, Lamas G, Zouaoui A et al. Risk factors and clinical relevance of nosocomial maxillary sinisitis in the critically ill. Am J Respir Crit Care Med 1994;150:776-83. [PubMed]
124. Sirvent JM, Torres A, El-Ebiary M, Castro P, de Batlle J, Bonet A. Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med 1997;155:1729-34. [PubMed]
125. Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53(RR-3):1-36. [PubMed]
126. Thorens JB, Kaelin RM, Jolliet P, Chevrolet JC. Influence of the quality of nursing on the duration of weaning from mechanical ventilation in patients with chronic obstructive pulmonary disease. Crit Care Med 1995 November;23:1807-15. [PubMed]
127. Torres A, Gatell JM, Aznar E, El-Ebiary M, Puig de la Bellacasa J, Gonzalez J, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med 1995;152:137-41.[PubMed]
128. Torres A, Serra-Batlles J, Ros E, Piera C, Puig de la Bellacasa J, Cobos A et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med 1992;116:540-3. [PubMed]
129. Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am Rev Respir Dis 1998;157(2):531-9. [PubMed]
130. Tryba M. Risk of acute stress bleeding and nosocomial pneumonia in ventilated intensive care patients: Sucralfate versus antacids. Am J Med 1987;83:117-24. [PubMed]
131. Valles J, Artigas A, Rello J, Bonsoms N, Fontanals D, Blanch L et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med 1995;122:179-86. [PubMed]
132. Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion 1999;39:701-10. [PubMed]
133. Vamvakas EC, Carven JH. Exposure to allogeneic plasma and risk of postoperative pneumonia and/or wound infection in coronary artery bypass graft surgery. Transfusion 2002;42:107-13. [PubMed]
134. van den Berghe BG, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001;345:1359-67. [PubMed]
135. van Nieuwenhoven CA, Buskens E, van Tiel FH, Bonten MJ. Relationship between methodological trial quality and the effects of selective digestive decontamination on pneumonia and mortality in critically ill patients. JAMA 2001 July 18;286:335-40. [PubMed]
136. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomised, controlled trial of selective digestive deconatmination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:861-7. [PubMed]
137. Weinstein RA. Epidemiology and control of nosocomial infections in adult intensive care units. Am J Med 1991 September 16;91:179S-84S. [PubMed]
138. Wiener J, Itozaku G, Nathan C, Kabins SA, Weinstein RA. A randomized, double-blind, placebo-controled trial of selective digestive decontamination in a medical-surgical intensive care unit. Clin Infect Dis 1995;20:861-7. [PubMed]
Table 1. Clinical Pulmonary Infection Score
| Score | Day 0 | Day 3 | Score |
|---|---|---|---|
|
Temperature, ºC ³38.5º - 38.9º = 1 point ³39.0º - 36.0º = 2 points |
Temperature, ºC 38.5º - 38.9º = 1 point 39.0º - 36.0º = 2 points |
|
|
Blood leucocytes, mm-3 <4.000 or >11.000 = 1 point 50% band forms = add 1 point |
Blood leucocytes, mm-3 <4.000 or >11.000=1 point 50% band forms = add 1 point |
|
|
Tracheal secretions Presence of non-purulent tracheal secretions = 1 point Presence of purulent tracheal secretions = 2 points |
Tracheal secretions Presence of non-purulent tracheal secretions = 1 point Presence of purulent tracheal secretions = 2 points |
|
|
Oxygenation: PaO2/FIO2 >240 or ARDS = 0 point < 240 and no ARDS = 2 points |
Oxygenation: PaO2/FIO2 >240 or ARDS = 0 point < 240 and no ARDS = 2 points |
|
|
Pulmonary radiography No infiltrate = 0 point Diffuse or patchy infiltrate = 1 point Localized infiltrate= 2 points |
Pulmonary radiography No infiltrate = 0 point Diffuse or patchy infiltrate = 1 point Localized infiltrate= 2 points |
|
|
Microbiological Data Pathogenic bacterial cultured in rare or hight quantity or no growth = 0 point Pathogenic bacterial cultured in moderate or heavy quantity = 1 point Same pathogenic bacterial seenon Gram stain = add 1 point |
Microbiological Data Pathogenic bacterial cultured in rare or hight quantity or no growth = 0 point Pathogenic bacterial cultured in moderate or heavy quantity = 1 point Same pathogenic bacterialseen on Gram stain = add 1point |
|
Total Day #0 = _________ Total Day #3 = _________
Table 2. Differential Diagnosis for Pulmonary Infiltrates in ICU Patients
|
Table 3. Diagnostic Tests in the Workup of Pulmonary Infiltrates in the ICU
1. Respiratory Cultures
2. Two blood cultures 3. Pleural fluid culture if parapneumonic effusion. 4. Legionella pneumophila urinary antigen and Streptococcus pneumoniae urinary antigen. 5. CBC, electrolytes, hepatic and renal function tests. 6. Arterial blood gases 7. C-reactive protein (CRP) and procalcitonin. |
___________________________________________________________
Send samples to microbiology laboratory immediately (or if not possible, refrigerate at 4ºC for a maximum of one hour) Gram staining, intracellular organism counting (only in BAL and mini-BAL) and quantitative cultures *** should be done.*Collection of cultures should not delay the initiation of empiric treatment in patients with severe sepsis.
**These techniques may be performed by bronchoscopy by blinded procedures.
***Quantitative cultures are performed with the respiratory secretions obtained by transbronchial aspirate, BAL or PBS. The cut-off points for determining infection colonization are: Transbronchial aspirate 105CFU/mL; BAL 104 CFU/mL and PSB 103 CFU/mL.
Table 4. Risk Factors for Infection by Multiresistant Microorganisms
| Risk factors for Multi-Resistant Microorganisms |
|---|
|
Table 5: Initial Empiric Antibiotic Treatment For Hospital-Acquired Pneumonia or VAP Of Late Onset Or In Patients With Risk Factors For Resistance
| Microbial Etiology | Combination antibiotic treatment |
|---|---|
Microorganisms from Table 3 plus: Pseudomonas aeruginosa Klebsiella pneumoniae (ESBL+) Serratia marcescens Acinetobacter spp. Other nonfermentative GNB MRSA Legionella pneumophila
|
Antipseudomonal cephalosporin
(ceftazidime or cefepime)* or Carbapenem(imipenem, meropenem)* or Beta-lactam / betalactamase inhibitor(piperacillin / tazobactam)* + Antipseudomonal quinolone(ciprofloxacin, levofloxacin)** or Aminoglycoside** (amikacin) ± Linezolid or vancomycin*** |
GNB = Gram negative bacilli
ESBL = Extended-spectrum beta-lactamase
MRSA = Methicillin-resistant Staphylococcus aureus
*The choice of beta-lactam antibiotic is made as follows: patients who have not received any antipseudomonal beta-lactam antibiotic within the last 30 days are administered piperacillin/tazobactam or antipseudomonal beta-lactam cephalosporin. Patients who have received these prior antipseudomonal drugs are given a carbapenem. Patients with infection by ESBL-producing microorganisms are treated with carbapenem regardless of the results of the antibiogram.**For combination empiric therapy for multiresistant gram-negative bacilli, an antipseudomonal quinolone is used in cases of renal failure or concomitant use of vancomycin. In other settings combined empiric therapy is initiated with amikacin and is maintained for a 5 day period.
***Empiric therapy aimed at MRSA is initiated in patients with established colonization, previous infection by MRSA, or implementation of mechanical ventilation for more than 6 days. Our antibiotic of choice is vancomycin. However, linezolid is used in patients allergic to vancomycin, creatinine values ≥ 1.6 mg/dL or in patients presenting signs of infection after 48 hours of vancomycin therapy and in those in whom MRSA has been isolated.
Table 6. Initial Empiric Antibiotic Treatment In Hospital-Acquired Pneumonia or VAP of Early Onset In Patients Without Risk Factors For Resistance
| Probable Microorganism | Empiric Antibiotic |
|---|---|
Streptococcus pneumonia Haemophilus influenzae Enteric Gram-negative bacilli Escherichia coli Klebsiella pneumoniae Enterobacter spp. Proteus spp. |
Ceftriaxone or Levofloxacin
|
Table 7. Antibiotic Dosages and Timing
| Antibiotic | Doses | Interval of administration | Infusion time |
|---|---|---|---|
| Ceftriaxone | 1 g | 12 hours | 1 / 2 - 1 hour† |
| Levofloxacin | 750 mg | 12 hours* | 1 / 2 hour |
| Ceftazidime | 2 g | 8 hours | 2 - 3 hours† |
| Cefepime | 2 g | 8 hours | 2 - 3 hours† |
| Imipenem | 0.5 g | 6 hours | 1 hour† |
| Meropenem | 0.5 – 1 g | 6 hours | 2 - 3 hours† |
| Piperacillin/Tazobactam | 4 / 0.5 g | 6 hours | 2 - 3 hours† |
| Ciprofloxacin | 400 mg | 8 hours | 1 / 2 hour |
| Amikacin | 15 mg / Kg | 24 hours ** | 1 / 2 - 1 hour |
| Vancomycin | 1 g | 8-12 hours*** | 1- 3 hours* |
| Linezolid | 600 mg | 12 hours | 1 hour |
**Adjust the dosage according to PK / PD parameters
***Initiate this dose with 24 hours, measure trough blood levels prior to the following dosage and adjust the levels according to values.†For beta-lactam agents and vancomycin, continuous infusion should be considered.
Table 8. Definitions
A. SIRS: 2 or more of the following variables:
B. Bacteremia: bacteria within the blood stream (does not always lead to SIRS or sepsis) C. Sepsis: SIRS plus a documented or presumed infection. D. Severe sepsis: aforementioned sepsis criteria with associated organ dysfunction, hypoperfusion or hypotension. E. Sepsis induced hypotension: presence of a systolic BP <90 mmHg or a reduction of > 40 mmHg from baseline in the absence of other causes of hypotension.” F. Septic shock: Persistent hypotension and perfusion abnormalities despite adequate fluid resuscitation. G. Multiorgan dysfunction syndrome: state of physiological derangements in which organ function is not capable of maintaining homeostasis. |
Figure 1: Clinical Suspicion of Hospital-Acquired Pneumonia
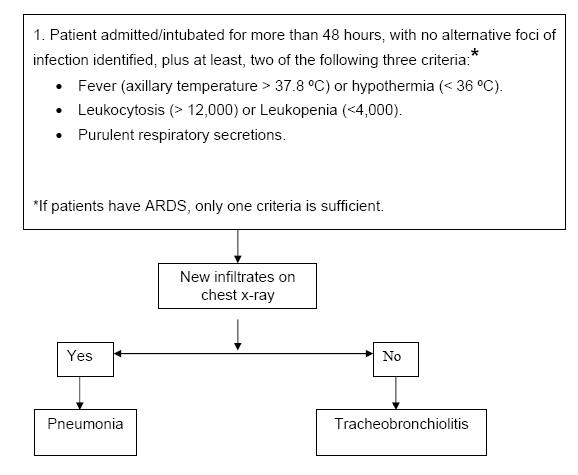
Figure 2: Algorithm for the Management of Patients with Pulmonary Infiltrates
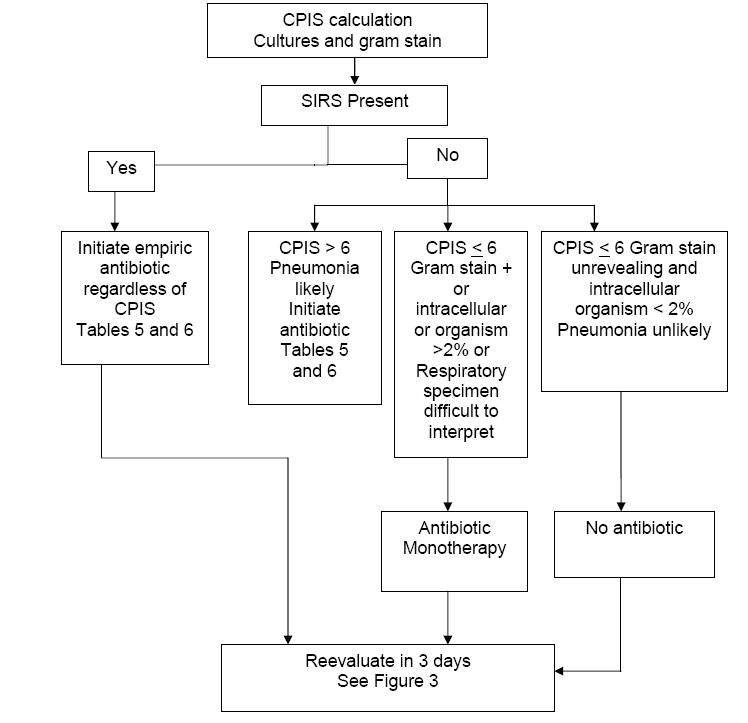
Figure 3: Follow up of Patients with Pulmonary Infiltrates
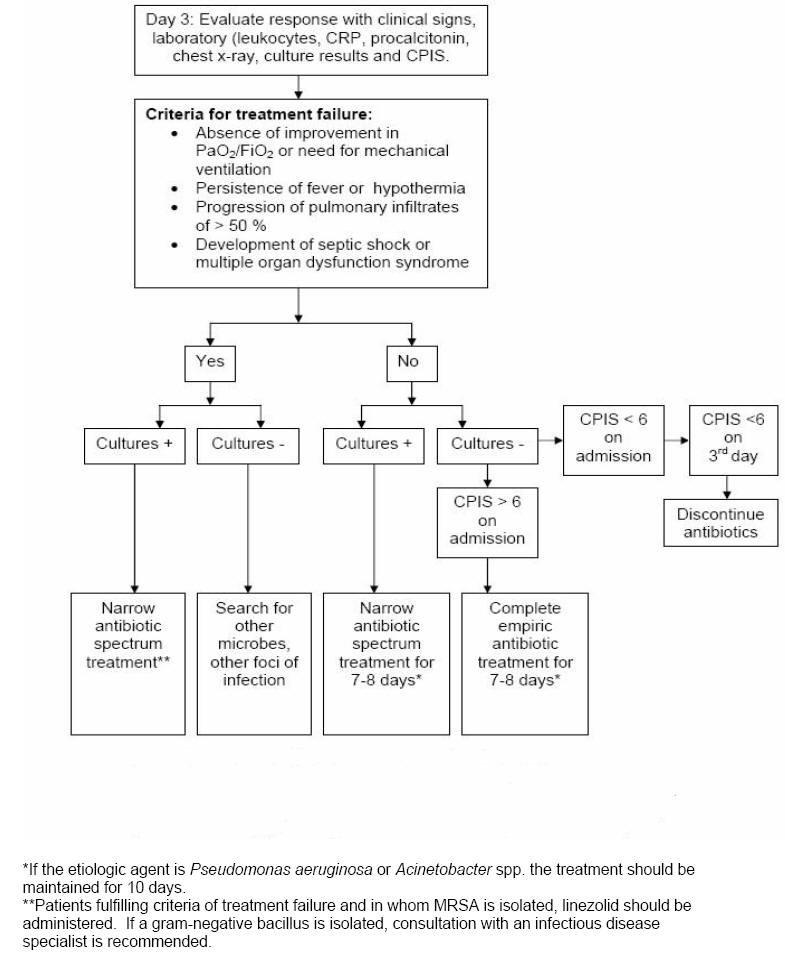
Figure 4: Evolution of the potentially pathogenic microorganisms present in the oropharyngeal flora related to the comorbidity, antibiotic treatment and colonization pressure.
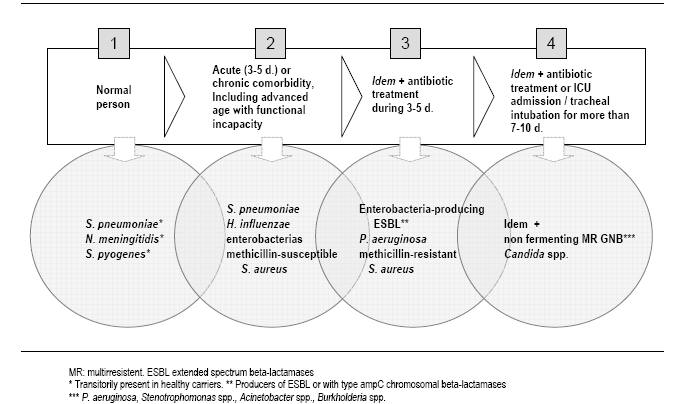
Figure 5: Empiric antibiotic treatment of VAP in patients without factors of infection by P. aeruginosa
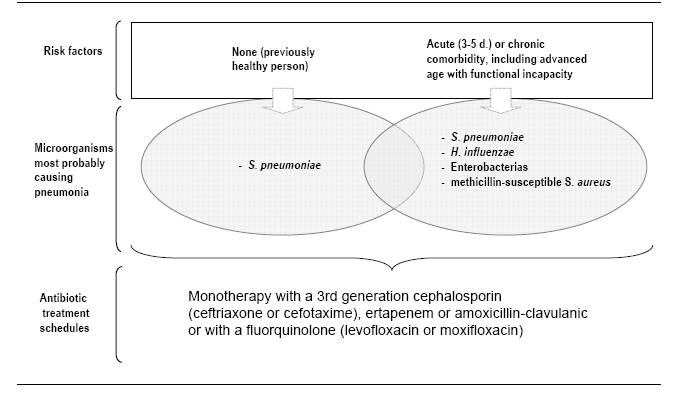
Figure 6: Empiric antibiotic treatment of VAP in setting of risk of infection by P. aeruginosa or by multiresistant microorganisms
What's New?
Giunta V, et al. ICU-acquired pneumonia with or without etiologic diagnosis: a comparison of outcomes. Crit Care Med 2013;41:2133-43.
Jung JY, et al. Effect of vancomycin plus rifampicin in the treatment of nosocomial methicillin-resistantStaphylococcus aureus pneumonia. Crit Care Med 2010;38:175-180.
Labelle AJ, et al. A Comparison of Culture-Positive and Culture-Negative Health-Care-Associated Pneumonia. Chest. 2010 May;137(5):1130-7. Epub 2009 Dec 4.
Tejerina E, Esteban A, et al. Accuracy of clinical definitions of ventilator-associated pneumonia: Comparison with autopsy findings. J Crit Care. 2009 Jul 8.
Kim A, Kuti JL, et al. Probability of Pharmacodynamic Target Attainment with Standard and Prolonged-InfusionAntibiotic Regimens for Empiric Therapy in Adults with Hospital-Acquired Pneumonia. Clin Ther. 2009 Nov;31:2765-78.
Nicasio AM, Eagye KJ, et al. Pharmacodynamic-Based Clinical Pathway for Empiric Antibiotic Choice in Patients with Ventilator-Associated Pneumonia. J Crit Care. 2010 Mar;25:69-77.
Eachempati SR, Hydo LJ, et al. Does De-Escalation of Antibiotic Therapy for Ventilator-Associated Pneumonia affect the Likelihood of Recurrent Pneumonia or Mortality in Critically Ill Surgical Patients? J Trauma. 2009 May;66:1343-8.
GUIDED MEDLINE SEARCH FOR
Reviews
Yu VL. Guidelines for hospital-acquired pneumonia and health-care-associated pneumonia: a vulnerability, a pitfall, and a fatal flaw. Lancet Infect Dis 2011;11:248-52.
Gilbert DN. Use of Plasma Procalcitonin Levels as an Adjunct to Clinical Microbiology. J Clin Microbiol 2010;48:2325-2329.
Guidelines on antimicrobial therapy of pneumonia in adults in Taiwan, revised 2006. J Microbiol Immunol Infect. 2007 Jun;40(3):279-83.
American Thoracic Society. Guidelines for the Management of Adults with Hospital-Acquired, Ventilator-Associated, and Healthcare-associated Pneumonia. Am J Respir Crit Care Med 2005;171:388-416.
