Antibiotic Therapy for Positive Blood Cultures
Authors: Emilio V. Perez-Jorge, M.D., FACP, Steve D. Burdette, M.D., FACP
INTRODUCTION
The appropriate selection of empiric antibiotic therapy for positive blood cultures is a complex and difficult decision. Bacteria are only partially identified on Gram stain and speciation and susceptibility results will take an additional 24-48 hours after a culture is reported as positive. While patients with positive blood cultures may be bacteremic signifying a true infection, there is a subset of patients with positive blood cultures that are due to contamination and therefore does not indicate a true infection. Because of the high morbidity and mortality associated with bacteremia, prompt evaluation and appropriate empiric antibiotic treatment are of paramount importance (3). Once an organism has been fully identified, the antibiotic selection can be modified. The field of PCR-based detection of organism is rapidly becoming a helpful tool in the identification and diagnosis of blood-stream infections, especially those caused by Gram-positive cocci (25, 28, 33). Until molecular techniques for detection of bacteria in blood cultures are more readily available and easier to implement (25, 31), clinicians will have to continue to rely on the Gram stain as the preliminary source of data for making empiric therapeutic decisions.
The Infectious Diseases Society of America (IDSA) and other organizations have issued recommendations for the treatment of bacteremia in the context of catheter-related infections that include both empirical and specific organism-based treatment protocols. However, no guidelines exist regarding the initial selection of antibiotics based on the preliminary results of the Gram stain. This chapter attempts to provide general recommendations for such a clinical situation.
OBTAINING BLOOD CULTURES
The proper technique for obtaining blood cultures requires thorough cleaning of the skin surface with 70% alcohol followed by iodine-based preparation or 0.5 % chlorhexidine for 1-2 minutes (19, 23). Care should be taken not to contaminate the venipuncture site after preparation and prior to phlebotomy. The culture bottle should be cleansed with 70% alcohol and blood injected without needle change. Except for pediatric blood cultures, at least 2 bottles with 8-10cc of blood per bottle are required for proper specimen processing. In spite of the fact that the sensitivity of the blood cultures increases proportionally to the amount of blood drawn, the current automated systems provide an adequate accuracy with 8-10 cc of blood volume (4). At least 10-15 minutes should elapse between collection of the first and second blood specimen. Given the increasing use of empiric antibiotics, the sensitivity of the 2 blood cultures specimen is not always optimal. Some have suggested that at least as many as four sets of blood cultures may be needed to achieve better detection rates (16).
While the prevalence of central lines has decreased, the use of peripherally-inserted central catheters (PICC) has increased significantly. Catheter-related infections account for an estimated 11-37% (1) of nosocomial bacteremias. The most recent review of nosocomial bloodstream infections, released by the National Healthcare Safety Network (NHSN) in 2007 (10) estimates that the rate of infection of central lines ranges from 1.5 to 6.8 per 1,000 central line days. The data published by the Nosocomial Infection Surveillance System (NISS) in 2004, revealed that catheter-related bloodstream infections were responsible for aproximately 24% of all bloodstream infections (32).
Drawing Blood Culture Specimens off Central Catheters
Most microbiologists recommend against the drawing of blood cultures from central catheters due to possible colonization of the catheter by skin flora. While this is true, the use of catheter-drawn blood cultures continues to increase due to practical reasons (less patient discomfort, no alternative way to obtain blood specimens, etc). In order to identify a catheter-related source of the bacteremia, research studies have validated the role of quantitative cultures (higher colony counts are typically found in infected catheters than in peripheral blood) and “differential time to positivity” (specimens drawn from infected catheters tend to become positive earlier than peripheral blood samples). The appropriate interpretation of both quantitative cultures and differential time to positivity will require that at least one of the specimens is collected by venipuncture from a peripheral vein. The laboratory must ensure that both samples were drawn and incubated at the same time and that both volumes were the same. While these have demonstrated utility in research studies they are not routinely utilized in clinical practice and the diagnosis of catheter related bacteremia relies on a clinical diagnosis and catheter tip culture, (with a catheter tip colony count greater than 15 being generally recognized as significant). Table 1 includes a list of the most common bacteria associated with catheter-related blood stream infections (CR-BSI) (11, 15, 18, 24, 32).
RISK FACTORS FOR BACTEREMIA
Table 2 lists the significant risk factors for bacteremia. In general, these apply to all bacteria; though some risk factors are more specific to certain bacteria (e.g. liver disease is mainly associated with Gram-negative bacteremia).
BACTEREMIA VERSUS CONTAMINATION
Contamination of blood culture specimens submitted to the microbiology laboratory is not uncommon and may be due to improper aseptic technique or colonization of an IV catheter from which the blood is obtained. Differentiating contamination from true bacteremia is sometimes difficult. Factors indicative of true bacteremia include: patient’s clinical history and physical findings, presence of risk factors for bacteremia (Table 2), body temperature and leukocyte count. Staphylococcus aureus and Candida sp. isolated from the blood should never be considered a contaminant. While the differentiation between bacteremia and contamination is mostly a clinical judgment based on clinical scenario, the number of positive cultures and the final identification of the bacteria are used to help make a differentiation between the two situations. The following microbiologic factors would be suggestive of contamination rather than true bacteremia:
1. When typical commensal organisms of the skin flora are isolated: coagulase-negative Staphylococcal species, certain Streptococci and Gram-positive bacilli. If the patient has an intravenous catheter, the probability of true infection is increased, even with common skin contaminants. Table 3 lists the percentage by which some common bacteria can cause false positive blood cultures due to contamination.
2. When only one of the blood cultures is positive out of a set of 2 or more. The validity of this statement is directly proportional to the number of negative blood cultures and depends on the organism identified. This criteria is used specially when evaluating coagulase-negative Staphylococcal species bacteremia, however there are conflicting reports as of its accuracy and predictive value (21, 29).
3. When the antibiotic susceptibility pattern of one organism isolated from the blood cultures is different from the pattern of the other organisms in the same or subsequent set of cultures (as long as the organisms are of the same species). For example, if 2 sets of blood cultures are both positive for Staphylococcus epidermidis but one set shows sensitivity to oxacillin and ciprofloxacin while the other set shows resistance to these 2 antibiotics, it is likely that both sets of blood cultures are contaminated.
GRAM STAIN BASED APPROACH
Identification of bacteremia is only one component of the patient evaluation. It is essential that the source of bacteremia be identified to assist with the necessary diagnostic testing as well as to help determine the duration of therapy. Occasionally, the potential source can be identified based on the gram stain results (Gram-positive diplococci are consistent with Streptococcus pneumoniae secondary to pneumonia or severe sinusitis; Gram-negative bacteremia with associated pyuria suggests a genitourinary source, etc). However, in most circumstances, the potential source may not be clear until the identification of the bacterial species is made.
Based on gram stain characteristics, this monograph divides the organisms into five categories: 1) Gram-positive cocci, 2) Gram-positive bacilli, 3) Gram-negative bacilli, 4) anaerobes and 5) Candida species. We will not address gram negative cocci as they are rarely a cause of bacteremia (except for Neisseria meningitides which can be a devastating infection). Table 4 includes a list of the most common pathogens isolated from monomicrobial nosocomial bloodstream infections (BSI) in decreasing order of frequency (32).
Gram-positive Cocci
The classification of Gram-positive cocci is based on bacterial staining as seen on the Gram stain. They form clusters (staphylococcus) or chains (streptococcus). Clustered Gram-positive cocci can be further differentiated based on the coagulase and catalase reactions (see Figure 1 below) which can be performed approximately 24 hours after being incubated on blood agar. For the purpose of limiting our review to the Gram-positive cocci that most frequently cause bacteremia, we will focus on the species that are clinically significant: these include S. aureus and coagulase-negative staphylococcus in the staphylococci group, viridans group Streptococcus, and Streptococcus pneumoniae in the streptococci group, and Enterococci.
Gram-positive Cocci in Clusters:
Staphylococcus aureus
Source of Infection: Staphylococcus aureus bacteremia may be primary (no evident source of infection) or secondary, in which the origin of the infection is a distant organ or system. A blood culture positive for Staphylococcus aureus is always considered a true bacteremia and should never be considered a contaminant due to the potential adverse consequences of not treating it. Skin, musculoskeletal system (bones/disks/joints) and endocardium are the main sources of secondary bacteremia. Additionally, an important source of secondary S. aureus bacteremia is seeding the bloodstream from an infected central catheter. It may be difficult to differentiate primary versus secondary bacteremia, especially when the source of secondary bacteremia is the musculoskeletal system. Complicating this distinction is the fact that S. aureus has a predilection to adhere to and cause infection in any kind of prosthetic material (catheters, orthopedic hardware, endovascular grafts, ventriculoperitoneal shunts, etc.) with the development of a biofilm; therefore a primary bacteremia can cause a distant infection of prosthetic material and then a subsequent secondary bacteremia.
Evaluation: The minimal evaluation in any patient with S. aureus bacteremia should include a thorough physical examination including skin, joints, and any peripheral and central vascular catheters. A chest X-ray and an imaging of any areas of musculoskeletal complaints should be considered. Some endorse a transesophageal echocardiogram (TEE) for all patients. We suggest consideration for a TEE in those with risk factors for endocarditis (Table 5), persistent bacteremia or primary bacteremia without an obvious source. The overall estimated risk of endocarditis in S. aureusbacteremia is 10-15%, but has been reported as high as 44-51% in patients with the risk factors listed in Table 5. Considering this, most clinicians will order at least a transthoracic echocardiogram (TTE) realizing that the sensitivity of the TTE is less than 50%. Patients diagnosed with S. aureus bacteremia should generally have follow-up blood cultures 3-5 days after initiation of appropriate antibiotic therapy to ensure that the bacteremia has resolved.
Treatment: While β-lactams have historically been effective for the treatment of S. aureus infections, the emergence of methicillin-resistant Staphylococcus aureus (MRSA) makes beta-lactams practically obsolete while susceptibility results are pending. The IDSA recently published guidelines for the treatment of MRSA infections, including bacteremia. Numerous studies have demonstrated that proper empiric therapy does have significant impact on adverse consequences from Staphylococcus aureusbacteremia. We therefore suggest initial empirical therapy with IV vancomycin or daptomycin that can be de-escalated to anti-staphylococcal β-lactams such as nafcillin, oxacillin or cefazolin once the susceptibility results are available. Daptomycin is not indicated for pulmonary infections therefore a respiratory source of bacteremia should be ruled out prior to initiating therapy with this antibiotic. Vancomycin is inferior to beta-lactams or daptomycin for the treatment of methicillin-sensitive S. aureus (5). Linezolid or Tigecycline are not considered first line antibiotics for S. aureus bacteremia due to its bacteriostatic properties and lack of clinical data suggesting its efficacy for bacteremia.
The duration of antibiotic therapy for S. aureus bacteremia must be at least 2 weeks of an intravenous agent. If the bacteremia is secondary to a distant source of infection or in the context of septic embolization, recommended therapy would be at least 4-6weeks. Selected patients with S. aureus bacteremia can be treated for 2 weeks if they meet the following criteria: (1) removal of intravascular catheters that were present during the bacteremia, (2) endocarditis is excluded with TEE, (3) absence of implanted prosthesis (prosthetic valves, cardiac devices or arthroplasties, (4) follow up cultures obtained 2-4 days after initiation of therapy are negative and (5) resolution of fever and absence of localizing symptoms or signs suggestive of metastatic staphylococcal infection (9, 20). Though lacking clinical evidence to suggest benefit, if the patient presents with a severe infection or sepsis, consideration for combination therapy which could include 2 antistaphylococcal agents (vancomycin, daptomycin or linezolid in combination) or the addition of gentamicin for synergy during the initial 3-5 days.
Coagulase-Negative Staphylococci
Source of Infection: The most common source of coagulase-negative Staphylococcal bacteremia is an infected central line. Coagulase-negative Staphylococcal species is the most commonly found organism in bacteremia of any etiology (primary or secondary) and is particularly prevalent as an etiologic agent for catheter-related bloodstream infections. Unfortunately, coagulase-negative Staphylococcal species, especially S. epidermidis, are also the most common skin contaminants found in blood cultures. Applying the rules mentioned in the section of bacteremia vs. contamination can help differentiate between true bacteremia and blood culture contamination.
Evaluation: Primary coagulase-negative Staphylococcal species bacteremia is rare. The most common species causing bacteremia is S. epidermidis. Frequently the bacteremia is secondary to an infected IV catheter; therefore, close attention should be paid to infected peripheral or central vascular catheters. The removal of the catheter is recommended, but not necessary unless it is a pacemaker or a tunneled catheter with clinical signs of infection. Some species of coagulase-negative Staphylococcal species deserve special attention as they are associated with other diseases that can secondarily cause bacteremia. S. saprophyticus is a common cause of UTI in women, S. lugdunensis is associated with native valve endocarditis, meningitis and skin and soft tissue infections, with endocarditis being the most serious. S. haemolyticus is also associated with native valve endocarditis and meningitis. Given these associated clinical conditions, a urine analysis, repeated blood cultures and an echocardiogram may be necessary.
Treatment: If an isolate of coagulase-negative Staphylococcal species is found to be β-lactamase negative, then penicillin is the drug of choice. The semi-synthetic antistaphylococcal penicillins (oxacillin and nafcillin) are derivatives of penicillin that are poorly hydrolyzed by staphylococcal ß-lactamase and are the drugs of choice among the penicillin group. However, a majority of coagulase-negative Staphylococcal species isolated during a hospitalization will be methicillin-resistant; therefore vancomycin or daptomycin would be appropriate choices. Duration of treatment varies with the underlying clinical scenario. Based on the 2009 IDSA recommendations (20), we suggest at least 5-7 days of antibiotic therapy, possibly longer depending on the clinical scenario (unable to remove catheter, prosthetic material present). If there is no concern for endocarditis (or it has been ruled out) or endocarditis equivalent, antibiotics can be administered orally after 3-4 days of IV therapy.
Gram-positive Cocci in Chains:
Enterococcus
Source of Infection: Their normal habitat is the gastrointestinal tract, although they can be isolated from the oropharynx, genitor-urinary tract, and skin. Enterococcus faecalis is the most common human pathogen, but Enterococcus faecium has become increasingly prevalent in hospital-acquired infections. As it may colonize the skin, especially after a prolonged hospitalization, it may also be a cause of contaminated blood cultures in the proper clinical setting. Clinically significant sources of enterococcal bacteremia include urinary tract infections, intra-abdominal infections, infected vascular catheters and endocarditis. Enterococcal species are the fourth most common cause of catheter related bacteremias.
Evaluation: In addition to the blood cultures, urine analysis and a focused abdominal exam should be performed in all patients with enterococcal bacteremia. Imaging studies (CT scan, HIDA, biliary and renal ultrasound) should be considered depending on the clinical scenario. If the suspicion for endocarditis is present (Table 5) then echocardiography (TTE and possibly TEE) should be performed. Patients should likely have repeat blood cultures to ensure clearance of bacteremia, especially if endocarditis is of concern.
Treatment: Enterococci are inherently resistant to many classes of antibiotics that are active against other gram positive cocci, including cephalosporins, macrolides, and clindamycin. Antibiotics with varying degrees of in vitro activity against enterococci include the penicillins (especially penicillin and ampicillin but NOT nafcillin), glycopeptides (vancomycin and teicoplanin), carbapenems (imipenem and meropenem but NOT ertapenem), daptomycin, quinupristin/dalfopristin and linezolid. Other antibiotics have some activity but should not be routinely utilized for the treatment of serious enterococcal infections such as bacteremia. These include the tetracyclines (tetracycline, minocycline and doxycycline), quinolones (including ciprofloxacin, moxifloxacin and gemifloxacin), chloramphenicol, and rifampin. The penicillins and the glycopeptides have the best activity, and ampicillin typically has greater in vitro killing ability than vancomycin. Ampicillin is the drug of choice if the isolate is susceptible. For penicillin allergic patients or in patients with ampicillin-resistant strains, vancomycin would be the first line therapy. For bacteremia caused by vancomycin-resistant enterococci (VRE), the choices include daptomycin and quinupristin/dalfopristin. While use of linezolid for penicillin-sensitive enterococcal bacteremia, should not be routine, VRE bacteremia is one exception in which this antibiotic would be a therapeutic option (34).
Two questions remain controversial in the treatment of enterococcal bacteremia in the absence of endocarditis: duration of treatment and need for synergistic antibiotics. If the patient does not have endocarditis, then 7-10 days of IV antibiotic treatment may be sufficient but otherwise, a longer course is recommended. The need for concurrent synergistic aminoglycoside therapy is only recommended for the treatment of endocarditis, but not for uncomplicated enterococcal bacteremia. Enterococci have intrinsic low-level resistance to the aminoglycosides due to the decreased ability of these agents to penetrate the cell wall, but this can be overcome by the addition of cell wall-active agents (such as the penicillins and glycopeptides) that result in synergistic killing of the organisms. However, it is important to remember is that high level aminoglycoside resistance (HLAR) has been demonstrated in some strains of enterococci. In cases of endocarditis by enterococci with HLAR, ampicillin and ceftriaxone in combination has been used with some success (14).
Viridans-group Streptococci (VGS)
Source of Infection: The viridans-group streptococci are in general low virulence pathogens, but like any commensal organisms, careful evaluation of skin and oral structures is necessary before VGS bacteremia is considered non-clinically significant. Some of the important species of viridans-group Streptococci include S. milleri, S. sanguis, S. mitis and S. bovis. Transient bacteremias may occur following dental manipulation and frequently are of no consequence in patients without predisposing conditions. It has been estimated that only 21-50% of the positive blood cultures for VGS are clinically significant. Viridans-group Streptococci used to be the most common cause of native valve endocarditis and late onset prosthetic valve endocarditis but nowadays S. aureus has taken over as the most common organism (12). VGS have also been associated with serious pyogenic infections, bacteremia in neutropenic patients, neonatal sepsis, and septicemia/shock syndrome (also known as "α strep shock syndrome"). The risk factors for VGS bacteremia include: neutropenia, oral mucositis, irradiation to the oral cavity, antibiotic prophylaxis with trimethoprim-sulfamethoxazole and fluoroquinolones, intravenous hyperalimentation, high dose chemotherapy and female sex (30).
Evaluation: Any viridans-group streptococci bacteremia requires consideration of repeated blood cultures to rule outendocarditis (only if multiple sets of blood cultures are positive) and a detailed dental and skin examination. An echocardiogram may be indicated if the possibility of endocarditis exists. Consideration of abdominal imaging studies (CT scan or ultrasound) to rule out intra-abdominal abscesses (liver, spleen, peritoneal) should also be entertained.
Treatment: Any viridans-group Streptococci bacteremia considered clinically significant will require empirical coverage for endocarditis until that condition is ruled out. Beta-lactams are active against viridans-group Streptococci and are the drugs of choice. For the PCN allergic patient, vancomycin and clindamycin can be utilized. If the allergic reaction to penicillin was not anaphylactic or IgE mediated, ceftriaxone may be an appropriate and well tolerated alternative. Two weeks of antibiotic therapy will be sufficient unless treating endocarditis or other complicated infections (liver abscesses, brain abscess, etc) in which a minimum of 4-6 weeks are required (2 weeks of PCN plus gentamicin may be appropriate for treatment of uncomplicated endocarditis). Therapy with oral antibiotics (beta-lactams preferred) is reasonable for non-intravascular infections after an initial course of intravenous therapy.
Other Streptococci
Other streptococcal species besides enterococci and viridans-group stretococci are occasionally isolated in blood cultures. They include Streptococcus pneumoniae, β-hemolytic Streptococcus and others. S. pneumoniae is a diplococcus associated with pneumonia, meningitis, peritonitis and other severe infections. Isolation of this organism is always significant and should be treated. S. pneumoniae is still relatively susceptible to beta-lactams, which remain a first line therapy. Alternative agents in areas of high penicillin resistance include macrolides or quinolones. For ß-hemolytic streptococcus (Group A Streptococcus, Group B Streptococcus, etc) beta-lactams are the first line antibiotic therapy.
Gram-positive Bacilli
Many Gram-positive bacilli are part of the normal skin flora thereby able to contaminate blood cultures or colonize intravenous catheters. Identifying these organisms in a blood culture may be suggestive of false-positive results. They include Propionibacterium acnes, Corynebacterium species and Bacillus species. It needs to be remembered that these bacteria may be pathogenic in the context of selected clinical conditions such as endocarditis, prosthetic heart valves, joint infection, neutropenia, etc.
The most common species of Gram-positive bacilli isolated in blood culture specimens will be briefly described since a more detailed description is beyond the scope of this chapter.
Corynebacterium Species:
While diphtheria is a well-described upper respiratory infection, isolation of Corynebacterium spp. (often called diphtheroids) from patients without the typical respiratory symptoms, usually indicates skin contamination. There are exceptions to this, for example, patients with central catheters or risk factors for endocarditis when associated with multiple positive blood cultures for the same species or neutropenic patients in which C. jeikeium is isolated. In this population, the possibility for clinically significant bacteremia should be considered. Corynebacterium are susceptible to penicillins, vancomycin, erythromycin and clindamycin.
Propionibacterium species (Anaerobe):
Propionibacterium acnes is a common skin inhabitant, therefore a common blood culture contaminant. If a decision to treat is made, which would be unusual, the antibiotics that would be most effective include penicillins, tetracyclines and macrolides. It has been associated with upper extremity prosthetic joint infections, therefore a musculoskeletal evaluation is in order in patient with Propionibacterium bacteremia.
Bacillus species (Excluding Anthrax):
Despite the widespread distribution of Bacillus organisms, they are rarely implicated with actual infections and are more frequently isolated as a culture contaminant. An exception would be patients with symptoms of endocarditis or ocular infections in which B. cereus has been implicated. It also a well known cause of intravascular catheter related infections. Treatment with penicillin and clindamycin is usually effective for most Bacillus species when therapy is indicated.
Gram-Negative Bacilli
The presence of Gram-negative bacilli in blood is always significant. The list of Gram-negative bacilli that can cause bacteremia is rather extensive, but the microbiology lab can be very helpful in providing initial information that will allow narrowing the possibilities and selecting appropriate empiric antibiotic therapy. Once the blood cultures have been inoculated onto agar plates and growth has been demonstrated (typically over 24 hours), there are several tests that can provide useful information prior to the final species identification. Based on the growth in McConkey plates, the Gram-negative bacilli can be divided between lactose fermenters or non-fermenters. Further evaluation with indole and oxidase tests can also help in predicting which bacteria are expected to be identified. In Figure 2, a simplified algorithm for identification of Gram-negative bacilli is shown. It is purposefully limited to the most common Gram-negative bacilli isolated in both community-acquired and nosocomial bacteremia (15, 18).
Source of Infection: In the general population (non-ICU patients), the urinary tract is the most common origin for Gram-negative bacilli bacteremia, followed by the gastrointestinal and respiratory tracts. In ICU patients, the urinary tract still predominates but central lines and wound infections follow in frequency, preceding the GI and respiratory tract. The risk factors for Gram-negative bacilli bacteremia are listed in Table 6.
Evaluation: Any bacteremia will need an evaluation of the GU and GI tracts. Urine analysis, CBC and possibly a KUB radiograph are required in any patient with Gram-negative bacilli bacteremia. If the patient is in the ICU, central lines and postoperative wounds should be evaluated. In immunosuppressed patients, it may be necessary to perform CT scans or an ultrasound to evaluate occult intestinal or urinary problems that may not be clinically apparent.
Treatment: If the Gram-negative bacilli bacteremia is community-acquired, initial therapy with a third generation cephalosporin or a fluoroquinolone is appropriate. The key to deciding initial antibiotic therapy is a review of recent antibiotics (3 months). If the patient has received antibiotics within the previous 3 months the decision to choose therapy from an alternative class is encouraged, as the potential for bacterial resistance is high. If the bacteremia is health-care associated, the patient has recently been administered antibiotics (especially quinolones), has known malignancy or an immunocompromised state, it is recommended that Pseudomonas sp. be covered with the initial choice of antibiotics. Most clinicians will empirically treat potential Pseudomonas bacteremia with two drugs from different antibiotic classes. This is due to the fact that Pseudomonas sp. can quickly develop beta-lactam resistance. A combination of an anti-pseudomonal β-lactam (piperacillin-tazobactam, imipenem/meropenem or cefepime) plus an aminoglycoside (or anti-pseudomonal quinolone such as ciprofloxacin) would be the initial standard therapy. De-escalation of the initial regimen to a single appropriate antibiotic is recommended once the culture and sensitivity results are available (2, 26). Combination therapy has also been advocated for bacteremia due to Enterobacter and Klebsiella pneumonia, especially when there is concern for an extended spectrum beta-lactamase producing species (6). The emergence of resistant enterobacteriae able to produce Klebsiella pneumonia carbapenemases (KPC) and New Delhi Metallo-beta lactamase (NDM-1) is a growing global public health threat. Clinically unstable patients should also receive combination therapy upon the identification of gram negative bacteremia. Penicillin allergic patients may be treated with aztreonam (with or without either an aminoglycoside or ciprofloxacin).
The optimal duration of antibiotic therapy for Gram-negative bacilli bacteremia is not clear but should be dictated by the clinical response and the underlying etiology of bacteremia. Most clinicians will treat for at least 2 weeks, especially if Pseudomonas sp. is isolated. Oral therapy may be appropriate and is determined by susceptibility patterns of the isolated organism.
Anaerobes
Anaerobic bacteria can be divided for the purpose of initial microbiological identification in Gram-positive and Gram-negative. The gram positive species include cocci (Peptostreptococcus spp.) and rods (Actinomyces, Bifidobacterium, Eubacterium, Lactobacillus, Clostridium and Propionibacterium species). The gram negatives (only rods, no cocci) include Prevotella, Porphyromonas, Fusobacterium and Bacteroides species). Many aerobic bacteria will grow in the anaerobic culture media, therefore a positive anaerobic culture does not initially rule out a potential aerobic infection.
Source of Infection: Anaerobic bacteria normally inhabit the oral mucosa and the GI tract. In females, some anaerobic bacteria are normally found in the GU area. In the presence of anaerobic bacteremia the evaluation of the patient should include clinical and imaging studies of those areas. From a clinical standpoint, the presence of gas in any tissue is highly suggestive of an anaerobic infection.
Evaluation: Oral (attempting to identify poor dentition), GI and GU examination is mandatory in anaerobic bacteremia. Some anaerobes are associated with specific infections (e.g. Actinomyces in females with IUD/uterine infections,Fusobacterium in oropharyngeal/brain abscesses and Lemierre’s syndrome, Bacteroides in intra-abdominal infections).
Treatment: Most oral anaerobic bacteria are susceptible to penicillins while a majority of intra-abdominal anaerobes (specifically B. fragilis) are beta-lactamase positive and therefore resistant to beta-lactams unless an inhibitor is also administered (such as piperacillin/tazobactam). Some specific species are penicillin resistant (due to the development of beta-lactamases), therefore until a final identification is made it may be advisable to use alternative agents such as metronidazole, a carbapenem or a combination drug that includes a beta-lactamase inhibitor. There has been increasing resistance reported with clindamycin and gram-negative anaerobes so caution must be used with this drug, especially when treating anaerobes that may be from an abdominal source.
Candida Species
Source of Infection: The genus Candida contains > 100 different species, but only a limited number of these species routinely cause disease in humans (Table 7). Whatever the source of infection, Candidemia is always clinically significant and should be addressed immediately. Delaying treatment of candidemia results in significantly increased mortality and therefore early treatment is essential (13). Candida infection can develop in several areas: skin, urine, lungs, etc, but candidemia is commonly associated with infection of central catheters. The risk factors for candidemia include: IV catheters, neutropenia, hematological malignancies, ICU admission, TPN administration, abdominal surgeries, bowel perforation, immunosupression and HIV.
Evaluation: When clinically feasible, central lines shouldbe routinely removed as part of the management of Candidemia. Examination of the retina by an experienced physician/ophthalmologist is mandatory to rule out candida endophthalmitis, a common complication of candidemia. Blood cultures should be repeated until resolution of the candidemia is documented. Endocarditis is not as common with candidemia as it is with S. aureus bacteremia, therefore, evaluation with a TEE or TTE is not mandatory except in cases of documented persistent candidemia and septic embolization.
Treatment: The IDSA will be publishing guidelines addressing the treatment of candidemia in 2009. Until then, the current recommendations suggest that the choice of antifungal agent depends on 2 factors: a) presence of neutropenia or hemodynamic instability and b) local susceptibility data (22, 27).
For neutropenic or hemodynamically unstable patients, the recommended empiric therapy has previously been amphotericin B (especially the liposomal compounds). However, there is now adequate data that echinocandins (caspofungin, anidulafungin, micafungin) or triazoles (voriconazole) will provide adequate therapy with decreased risk of toxicity. In non-neutropenic and clinically stable patients, fluconazole is an appropriate initial therapy unless prevalence of C. glabrata or C. krusei in the hospital is high, or if the patient has previously received an azole, in which case an echinocandin or amphotericin B would be the most appropriate empiric agent. In both neutropenic and non-neutropenic patients, removal of central lines in the presence of candidemia is of paramount importance. The main risk factors for non-albicans Candidemia are previous fluconazole and central line exposure. Total parenteral nutrition seems to be associated mostly with C. albicans blood stream infection but not with non-albicans Candidemia (7). Figure 3 summarizes a treatment algorithm for candidemia according to the 2009 IDSA guidelines.
Candida species isolated from blood should be routinely sent for susceptibility testing to assist with appropriate therapy (though controversy does exist on this issue). Candidemia needs to be treated for a minimum of 2 weeks from the time that blood cultures become negative unless an intravascular infection such as endocarditis is identified, then therapy is often extended to at least 6 weeks.
CONCLUSION
Until the development of new PCR techniques that facilitate early identification of microorganisms is widely implemented, the clinician will continue to rely on the gram stain data for appropriate selection of antibiotic therapy. In life-threatening infections, early treatment can significantly affect the prognosis and mortality. Table 8 includes a simplified approach to choice of primary and alternative antimicrobial agents depending on the results of the gram stain. As we learn the advantages that the new PCR and molecular technology can provide, we will need to continue to use our clinical and microbiological skills to select the appropriate empiric antibiotic and to provide the best care to our patients.
REFERENCES
1. Antimicrobial Therapy and Vaccines. Antimicrobe.org.
2. Basseti M, Righi E, Viscoli C. Pseudomonas aeruginosa Serious Infections: Mono or Combination Antimicrobial Therapy. Curr Med Chem, 2008. 15: 517-522 [PubMed]
3. Bearman GM, Wenzel RP. Bacteremias: A Leading Cause of Death. Arch Med Res 2005 Nov-Dec; 36(6):646-59. [PubMed]
4. Bouza E, Sousa D, Rodriguez-Creixems M, et al. Is the Volume of Blood Cultured Still a Significant Factor in the Diagnosis of Bloodstream Infections?. J Clin microbiol. 2007;45:2765-2769. [PubMed]
5. Chang FY, Peacock JE Jr, Musher DM et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine 2003;82: 333-9 [PubMed]
6. Chow JW, Yu VL. Combination antibiotic therapy vs. monotherapy for gram-negative bacteremia: a commentary. Int J Antimicrob Ag 1999;II:7-12. [PubMed]
7. Chow JK, Golan Y, Ruthazer R, et al. Factors Associated with Candidemia Caused by Non-albicans Candida Species Versus Candida albicans in the Intensive Care Unit. Clin Infec Dis. 2008;46:1206-13. [PubMed]
8 Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America.http://www.journals.uchicago.edu/doi/pdf/10.1086/596757
9. Cosgrove SE, Fowler VG. Management of Methicillin-Resistant Staphylococcus aureus Bacteremia. Clin Infec Dis. 2008;46(Suppl 5): S386-93. [PubMed]
10. Edwards J, Peterson K, Andrus M, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control 2007;35:290-301. [PubMed]
11. Fatkenheuer G, Cornely O, Seifert H. Clinical management of catheter-related infections. Clinical Microbiology and Infection. 2002;8:545-9. [PubMed]
12. Fowler VG, Miro JM, Hoen B, et al. Staphylococcus aureus Endocarditis; a Consequence of Medical Progress. JAMA. 2005;293:3012-21. [PubMed]
13. Garey KW, Regi M, Pai MP, et al. Time to initiation of Fluconazole Therapy Impacts Mortality in Patients with Candidemia; A Multi-institutional Study. Clin Infect Dis. 2006;43:25-31. [PubMed]
14. Gavalda J, Len O, Miro JM, et al. Brief communication: treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann Intern Med 2008 Apr 17;146(8): 574-9. [PubMed]
15. Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41: 848-54.[PubMed]
16. Lee A, Mirrett S, Reller LB, Weinstein MP. Detection of Bloodstream Infections in Adults: How Many Blood Cultures are Needed? J Clin Microbiol. 2007;45:3546-3548. [PubMed]
17. Liu C, Bayer A, Cosgrove S, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus Aureus Infections in Adults and Children.Clin Infect Dis 2011;52:1-28 [PubMed]
18. Luzzaro F, Vigano EF, Fossati D, et. Al. Prevalence and drug susceptibility of pathogens causing bloodstream infections in northern Italy: a two-year study in 16 hospitals. Eur J Clin Microbiol Infect Dis. 2002;21:849-55. [PubMed]
19. Mandel GL, Bennet JE, Dolin R. Mandell, Bennet & Dollin: Principles and Practice of Infectious Diseases. Sixth Edition. 2005. Elsevier Publishers.
20. Mermel LA, Allon M, Bouza E, et al. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45.[PubMed]
21. Mirrett, S, Weinstein M, Reimer L, et al. Relevance of the Number of Positive Bottles in Determining Clinical Significance of Coagulase-Negative Staphylococci in Blood Cultures. J of Clin Microbiol 2001:3279-3281. [PubMed]
22. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for Treatment of Candidiasis (IDSA Guidelines). Clin Infect Dis. 2004;38:161-189. [PubMed]
23. Reller LB, Sexton DJ. Technique of obtaining blood cultures for the detection of bacteremia. In: UpToDate, Rose BD (Ed), UpToDate, Waltham, MA, 2007.
24. Rodriguez-Baňo J. Selection of Empiric Therapy in Patients with Catheter-related Infections. Clin Microbiol Infect 2002;8:275-81. [PubMed]
25. Ruimy R, Dos-Santos M, Raskine L, et al. Accuracy and Potential Usefulness of Triplex Real-Time PCR for Improving Antibiotic Treatments of Patients with Blood Cultures Showing Clustered Gram-Positive Cocci on Direct Smear. J of Clin Microbiol. 2008:46:2045-2051. [PubMed]
26. Safdar N, Handelsman J, Maki, D. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Inf Dis. 2004;4:520-7. [PubMed]
27. Spellberg B, Filler S, Edwards J. Current Treatment Strategies for Disseminated Candidiasis. Clin Infect Dis 2006;42:244-51.[PubMed]
28. Tissari P, Zumla A, Tarkka E, et al. Accurate and rapid identification of bacterial species from positive blood cultures with a DNA-based microarray platform: an observational study. The Lancet, 2010;375:224-30 [PubMed]
29. Tokars J. Predictive value of Blood Cultures Positive for Coagulase-Negative Staphylococci: Implications for Patient Care and Health Care Quality Assurance. Clin Infect Dis 2004:39:333-41. [PubMed]
30. Tunkel A, Sepkowitz K. Infections Caused by Viridans Streptococci in Patients with Neutropenia. Clin Infect Dis 2002;34:1524-9. [PubMed]
31. Wiesinger-Mayr H, Vierlinger K, Pichler R, Kriegner A, Hirschl A, Presterl E, Bodrossy L, Noehammer C. Identification of human pathogens isolated from blood using microarray hybridization and signal pattern recognition. BMC Microbiology, 2007;7:78. [PubMed]
32. Wisplinghoff H; Bischoff T; Tallent SM; Seifert H; Wenzel RP; Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309-317. Epub 2004 Jul 15. [PubMed]
33. Wllinghausen N, Kochem AJ, Disque C, et al. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. J Clin Microbiol. 2009 Sep;47(9):2759-65. Epub 2009 Jul 1 [PubMed]
34. Zyvox package insert. https://www.pfizerpro.com/sites/ppro/pages/products/zyvox_home.
Tables
Table 1. Organisms Associated with Nosocomial Catheter-Related Blood Stream Infections (CR-BSI).
Organism |
Comments |
|---|---|
Most common pathogen in CR-BSI |
|
Increasing prevalence of MRSA strains |
|
Gram-negative bacilli |
Among the resistant gram negative bacilli,Acinetobacter baumannii predominates in Europe. In USA Pseudomonas aeruginosa seems to be more prevalent |
E. faecalis is more common than e. faecium |
|
C. albicans is the most common species |
|
Other bacteria including diphteroids, viridans streptococci, Micrococcus, and more rarely, fungi other than Candida spp. |
|
The organisms are listed in order of decreasing frequency. From multiple sources (6, 9,11, 20, 21)
Table 2: Risk Factors for Bacteremia
· Advanced age · Corticosteroids · Immunosuppressing medications (transplant patients, rheumatologic diseases, etc) · Chronic liver disease · Chronic renal failure (especially if on hemodialysis) · Hematological malignancies · HIV infection · Intravenous catheters · Intravenous drug use · Loss of skin integrity · Malnutrition and hypoalbuminemia · Neutropenia · Parenteral nutrition |

Table 3. Contamination versus True Infection Rates for Specific Organisms.
Organism |
False positives |
|---|---|
Bacillus spp. |
>90% |
Coag-negative Staphylococcus spp. |
>90% |
Propionibacterium spp. |
>90% |
Corynebacterium spp. |
>80% |
Viridans streptococci |
50% |
Clostridium spp. |
40% |
Staphylococcus aureus spp. |
25% |
Enterococcus spp. |
15% |

Table 4. Organisms Causing Nosocomial Blood Stream Infections by Frequency.
Organism |
Percent of BSI |
|---|---|
Coagulase-negative Staphylococcal spp. |
31.3% |
Staphylococcus aureus |
20.2% |
Enterococcus spp. |
9.4% |
Candida spp. |
9.0% |
Escherichia coli |
5.6% |
Klebsiella spp. |
4.8% |
Pseudomonas aeruginosa |
4.3% |
Enterobacter spp. |
3.9% |
Serratia spp. |
1.7% |
Acinetobacter baumannii |
1.3% |
Table 5. Risk Factors for Infective Endocarditis
Non-cardiac |
Cardiac |
|---|---|
· IV drug abuse · Male · Advancing age · Recent dental surgery or other invasive procedures · Nosocomial bacteremia · Permanent venous access lines · Surgically constructed pulmonary shunts |
· Degenerative valvular lesions · Congenital heart disease · Prosthetic valves · Mitral valve prolapse with insufficiency · Rheumatic heart disease · Previous infective endocarditis · Hypertrophic cardiomyopathy |

Table 6: Risk Factors for Gram Negative Bacilli Bacteremia
|

Table 7. Characteristics of the Major Candida spp.
Species |
Frequency |
Virulence |
Clinical Associations |
|---|---|---|---|
42% - 65% |
High |
Most common in all settings |
|
11% - 25% |
High |
Cancer |
|
7% - 15% |
Low |
Cancer |
|
7% - 18% |
Variable |
Plastic devices, hyperalimentation |
|
1% - 4% |
Low |
Cancer |
|
C. lusitaniae |
1% - 2% |
Low |
Cancer |
Shown are frequency estimates for the species causing invasive disease.
Table 8. Primary and Alternative Empiric Antibiotic Choices for Bacteremia
|
Primary |
Alternative |
|---|---|---|
Gram-positive cocci in clusters* |
Vancomycin OR daptomycin^ |
Nafcillin OR cefazolin (once MRSA ruled out)
|
Gram-positive cocci in chains |
Ampicillin OR vancomycin |
Ceftriaxone** OR daptomycin
|
Gram-negative bacilli in a clinically unstable patient, immunosupressed, history of malignancy or a patient with health care associated infection
|
Cefepime + ciprofloxacin (ortobramycin) OR piperacillin/tazobactam+ ciprofloxacin (or tobramycin) |
Aztreonam + tobramycin (or ciprofloxacin OR imipenem + ciprofloxacin (or tobramycin) |
Gram-negative bacilli in a clinically stable patient
|
Ceftriaxone OR piperacillin/tazobactam |
Aztreonam OR ciprofloxacin |
Anaerobes |
Piperacillin/tazobactam ORmetronidazole
|
Clindamycin OR Imipenem |
Candida sp. |
Echinocandin OR voriconazole |
^daptomycin not indicated if patient clinically has pneumonia.
*consider the addition of a second agent such as gentamicin if patient is clinically unstable or endocarditis is likely
**Do not use if enterococcus is a potential cause of infection
“May be used as a first line agent when patient is hemodynamically stable and no previous azoles administered
Figure 1: Staphylococcal spp. are Always Catalase Positive while Streptococcal spp. are Catalase Negative
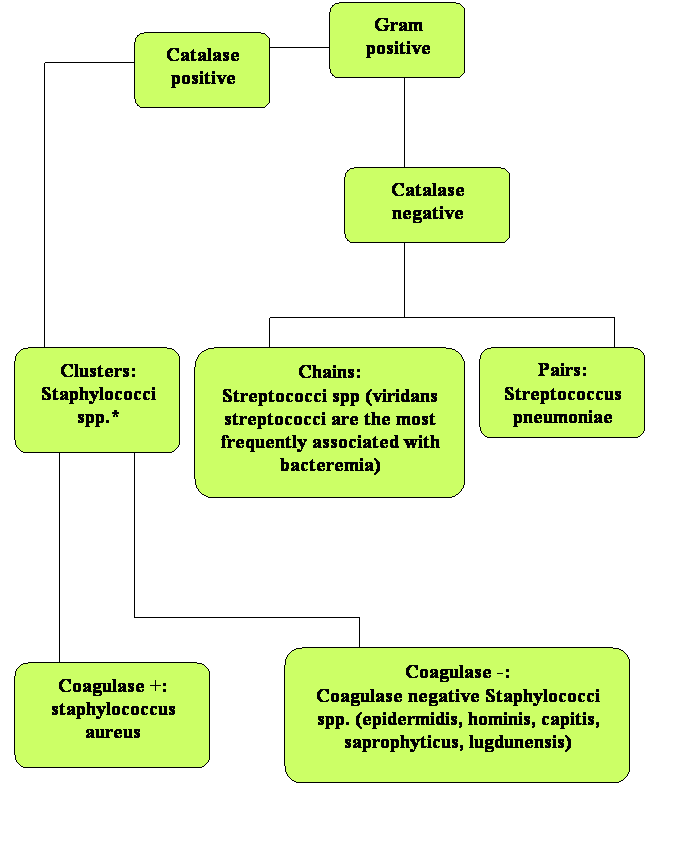
Figure 2: Classification of Gram Negative Bacilli
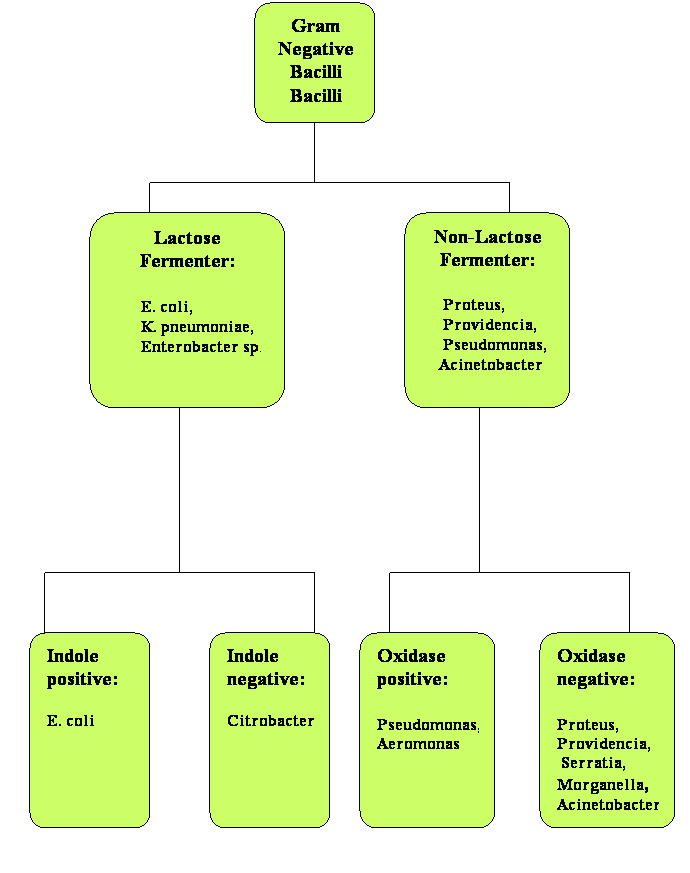
Figure 3. Algorithm for the Treatment of Candidemia

Baskin MN, et al. Bacteremia Risk and Outpatient Management of Febrile Patients with Sickle Cell Disease. Pediatrics 2013;131:1035-1041.
