Candidiasis in Solid Organ Transplantation
Authors: Luis Espinosa-Aguilar, Graeme N. Forrest
Introduction
Despite the major advances in prolonging allograft and recipient survival, Candida sp. remain one of the most common causes of fungal infections in solid organ transplant recipients, and is associated with significant early morbidity and mortality after transplantation (18).
Epidemiology and Risk Factors
The incidence of invasive mycoses following SOT ranges from 5% to 42 % with the most common fungi being Candida and Aspergillus (55). Candida is the most common cause of invasive fungal infection in liver, pancreas, renal and small bowel transplant recipients while aspergillosis is higher in heart and lung transplant recipients (26). Renal transplant recipients have the lowest incidence of invasive fungal infections and small bowel transplant have the highest risk. Candida represents the most common cause of fungal infection in the early period after transplant (24) ( Figure 1). Mortality from invasive Candida infections ranges from 11-81% in the solid organ transplant population (56) (Table 1) and this is even higher if the patient is located in an intensive care unit (44, 49).
Liver Transplant
Liver transplant recipients as described are high risk for invasive candidiasis which accounts for more than 90% of invasive fungal infections in liver transplant recipients. The major risk factors in these patients are related to technical complexity of the surgery, prolonged operation time, multiple blood transfusions, cold ischemic time of the donor organ and the use of Roux-en-Y biliary anastomosis (7, 39).
Other major risk factors that have been identified include re-transplantation, post-transplantation dialysis, antibiotic prophylaxis for spontaneous bacterial peritonitis, encephalopathy and postoperative bacterial infection (22, 53).
Pancreas Transplant and Small Bowel Transplant
Pancreas transplant recipients also have high rates on invasive Candida infections, ranging from 6-38%. Intra-abdominal abscesses and deep wound or surgical site infections have been documented at around 7-14% of pancreas transplant recipients. Risk factors in these patients include donor age, enteric versus bladder drainage, pancreas after kidney transplantation, preoperative peritoneal dialysis, and pancreatic re-transplantation (4,30). The incidence of candidiasis in small bowel transplant recipients ranges form 28-55%. It is related to disruption in the integrity of the gastrointestinal tract (anastomotic leak), requirement of increased immunosuppression and high incidence of CMV infection.
Renal and Lung Transplants
Candidiasis in renal transplant recipients represents 76-95% of fungal infections in this group and is mostly confined to the genitourinary tract with disseminated infection occurring in fewer than 5%. Risk factors for fungal infections in these patients are diabetes, prolonged pre-transplant dialysis and rejection (24). Lung transplant recipients are frequently colonized with Candida and the use of extracorporeal membrane oxygenation in the post transplant period is associated with a higher rate of candidiasis. A recently published study including 1305 thoracic organ transplant recipients, between 1980 and 2004, identified 76 episodes of invasive candidiasis with an overall incidence of 5.2%. Incidence was higher in lung and lung-heart transplant compared to heart transplant recipients. The incidence and attributable mortality from invasive candidiasis decreased over time in all thoracic organ transplant recipients (50).
Candida Species
Candida albicans remains the most frequent species associated with infection despite widespread usage of fluconazole for over 15 years (61). However, Candida glabrata and Candida tropicalis have emerged as major causative pathogens in the transplant recipient (54). There are multiple other species that can be obtained from transplant recipients such as: C. parapsilosis, C. krusei, C. lusitaniae, C. rugosa, C. kefyr and C. guillermondi. In a large database analysis which included 2019 patients with candidemia, C. glabrata was the organism most associated with infection of solid organ transplant recipients and the incidence of candidemia caused by non-Candida albicans species (54.4%) was greater than infections caused by C. albicans (45.6%) (20). The increased frequency of non-C. albicans isolates could be explained because of: widespread use of fluconazole in the pre and post-transplant period, prolonged pre-transplant hospitalization and use of broad spectrum antibiotics (20).
Pathogensis
Candida infections are derived from endogenous flora. Colonization with Candida sp. is more frequent among transplant patients compared to non-transplant patients (9). Conditions that predispose colonization or overgrowth of the hepatobiliary, gastrointestinal and genitourinary tract can facilitate fungemia and dissemination, especially with mucosal injury or bleeding during surgical manipulation. Translocation across the gut mucosa, manipulation of the gastrointestinal tract and impaired hepatic reticuloendothelial function are predisposing factors (18). In pancreas transplant recipients who have bladder drainage, urinary colonization pre-transplantation because of diabetes and liberation of digestive enzymes into the surgical site predispose to invasive candidiasis (30). Candida can enter the bloodstream by direct penetration from the epithelium after tissue damage, or by dissemination from biofilms formed on medical devices introduced into the patient’s organism (catheters, endoprotheses, artificial joints or central nervous system shunts) (18, 55). Candida albicans main virulence factors are hydrolytic enzymes, adhesions and ability to form biofilms (25). Immunomodulatory and immunosuppressive viruses, like CMV and HHV-6, facilitate superinfections with opportunistic fungi. Production of suppressive cytokines that alter lymphocyte and macrophage function is the mechanism responsible. HHV-6 also decreases immunologic response from CD4+ and CD8+ lymphocytes. Immunosuppressive therapy, especially with corticosteroids is a major risk factor for invasive candidiasis (22, 39, 55). Use of Alemtuzumab for treatment of acute graft rejection, conferred and increased risk of invasive fungal infections, mostly esophageal candidiasis within 3 months after the start of therapy (40). ![]()
Clinical Presentation
Mucosal Disease
Mucosal candida infection is the most common clinical presentation in patients with solid organ transplant, especially oral candidiasis, but the predominant site of involvement varies with the type of organ transplantation. Candida esophagitis is also common and is characterized by dysphagia, odynophagia and chest pain (24). Oral candidiasis is very common in organ transplant recipients and is greater in the first month after transplantation, however can be present for many months later (9, 24). There may be also be a close correlation with the presence of underlying cytomegalovirus (CMV) infection, or this may reflect the depth of immunosuppression with the two organisms closely tied together (35). Mostly, Candida superficially coats the orophaynx, however on rare occasions oral Candida may cause severe erosive lesions without underlying herpes virus infections (47). Oral thrush may be present in only 43% of cases of esophageal candidiasis and 21.4% of patients may be asymptomatic while 54% will have dysphagia (16). Vaginal candidiasis may also be increased in female organ transplant recipients and is frequently missed without regular screening (2).
Urinary Disease
Candiduria occurs frequently in renal transplant recipients and could represent colonization but may also be a sign of disseminated infection and has been associated with reduced survival rates, but may reflect underlying severity of disease. Pyelonephritis and fungal ball formation within the genitourinary system are other potential complications. In a review of candiduria in renal transplant recipients most were asymptomatic and C. glabrata occurred in 51% of cases (48). Predictors identified for candiduria were female gender, intensive care unit admission, and antibiotic use in prior month, urinary catheter, diabetes and neurogenic bladder. They noted that treatment did not improve clearance or outcomes in this population (48). There is very little supporting data with other organ transplant recipients on whether candiduria is more than a colonizer, but should be evaluated as to whether it is part of systemic disease in all of these patients (17, 24, 64).
Intra-abdominal Disease
In liver transplant patients common presentations of invasive candidiasis are: intra-abdominal abscesses, wound infections, peritonitis and candidemia (23, 64). Peritonitis is also common in pancreas transplant recipients, secondary to manipulation of the intestines and biliary tree (55). Most of these infections occur due to post-operative anastomotic leaks and ensuring mucosal integrity can reduce these complications.
Candidemia and Other Invasive Disease
In the U.S, Candida is considered the fourth most common cause of nosocomial bloodstream infection, with an attributable mortality close to 40% (66). Candidemia is frequently related to the presence of central venous catheters, followed by translocation from a gastro-intestinal source (24, 55). A review of 2935 SOT transplant patients with bloodstream infections between 2003 and 2005, found that Candida was responsible for 4% of all bloodstream infections in this population, being more frequent on lung transplants (18%) and less frequent on heart transplants (3%) (34). Excluding coagulase negative staphylococci, Candida was the 7th most common organism isolated from blood cultures in their study (34). Candidemia is a common problem after small bowel transplantation (15). A sepsis syndrome that fails to respond to treatment may accompany disseminated disease (24). Secondary effects of candidemia that should be evaluated in organ transplant recipients, especially when there are persistently positive cultures include endophthalmitis, endocarditis, meningitis and septic arthritis (6, 32, 36, 43, 45, 62).
Pneumonia
Lung and heart-lung transplant recipients could have tracheobronchitis to pneumonitis, less frequently anastomotic dehiscence, mediastinitis and mycotic aneurysm. In lung transplant recipients, Candida isolated from the donor lung bronchial segment should be monitored as can lead to fungal tracheobronchitis or anastomotic infection or dehiscence if left untreated (21). Candida pneumonia is a rare entity and diagnosis is difficult as obtaining a sputum culture without evidence of invasion frequently reflects oropharyngeal colonization (14, 21, 41). ![]()
Diagnosis
Distinguishing Candida colonization from infection in solid organ transplant recipients is difficult. With colonization frequent in this population the progression to infection while on immunosuppression, or from their prior debility from underlying illness can increase the risk of invasive infection (22, 26, 50, 55, 64). The same risk factors associated with invasive disease are associated with Candida colonization in both the donor and the recipient, especially with small bowel, liver, lung and lastly kidney (24). The presence of Candida in sterile sites (i.e. blood, cerebrospinal and vitreal fluid) is clearly infection, while its presence in post-surgical specimens is less clear, especially with the presence of drains (38). For non sterile specimens, useful criteria for defining whether there is infection or colonization with Candida include: 1) a compatible process that explains the hosts present clinical condition, 2) histopathologic evidence of tissue damage and/or inflammation in non-sterile areas, 3) radiological findings consistent with the process (1, 19, 38). With the increased incidence of non-C. albicans infections causing candidemia in this population, the use of rapid identification with polymerase chain reaction or peptide nucleic acid fluorescence in situ hybridization (PNA FISH) probes could assist in appropriate targeted therapy (33, 46, 52). Both have rapid turnaround in a couple of hours, with PNA FISH able to identify the five most common Candida species (46).
Antifungal Prophylaxis, Pre-emption or Prevention
Antifungal prophylaxis in organ transplant recipients is controversial, with most studies having numerous limitations and a standardized approach to antifungal prophylaxis for these patients does not exist (56). A meta analysis of 1497 SOT patients showed that antifungal prophylaxis did not reduce mortality (42). Some centers use antifungal prophylaxis for mucocutaneous candidiasis but this approach has not been based on documented evidence. Oral prophylaxis with non-absorbable antifungal agents has shown inconsistent results. A recently published meta analysis regarding use of nystatin as prophylaxis for invasive fungal infections in immunosuppressed patients (including patients with liver transplant) didn’t demonstrate any benefit compared to placebo and was inferior compared to fluconazole prophylaxis (13). The risk of invasive candidiasis in renal or heart transplant recipients is too low to require prophylaxis.
Liver transplant recipients with at least 2 risk factors, including re-transplantation, creatinine greater then 2mg/dl, choledochojejunostomy, prolonged intraoperative time, intraoperative use of more 40 U of blood products and fungal colonization detected at least 2 days before and 3 days after transplantation have been identified as being at high risk for invasive candidiasis and antifungal prophylaxis is recommended (37). A meta-analysis of 10 trials with 1106 liver transplant patients demonstrated that fluconazole reduced invasive fungal infections in approximately 75%, but didn’t show any reduction in mortality. Fluconazole prophylaxis also did not significantly increase colonization or infection with azole resistant fungi (42). A randomized trial on 212 liver transplant recipients that compared fluconazole (400 mg/day for 10 weeks) against placebo, revealed reduced fungal colonization and fungal infection (superficial and invasive infection) by most Candida species, except C.glabrata. Overall mortality rates were again similar but fewer deaths related to invasive fungal infection were seen in the fluconazole group than in the placebo group (65). Another study comparing fluconazole (100 mg/day for 4 weeks) to oral nystatin showed that fluconazole reduced Candida colonization and superficial infections, and trended towards a reduction of invasive fungal infections (29). A randomized trial using itraconazole decreased the rate of invasive fungal infection from 24% to 4% in liver transplant recipients (51). A prospective, non-comparative, open-label trial on antifungal prophylaxis with caspofungin (21 days of prophylaxis) in 71 high risk liver transplant patients, showed that only two patients experienced an invasive fungal infections (Mucor and Candida surgical wounds infection). The overall incidence of documented invasive fungal infection was 2.8. Overall 8 patients (11%) died during the study period and no death was attributable to an invasive fungal infection or caspofungin prophylaxis (12). A survey of 106 liver transplant centers in North America using an electronic questionnaire reported of the sites that responded (63%) 91% used routine antifungal prophylaxis, 28% universal prophylaxis and 72 % targeted high risk patients with fluconazole being the most commonly used agent (57). A retrospective study in pancreas transplantation recipients revealed a 6% frequency of intra-abdominal fungal infection in patients who received fluconazole (400mg/day for 7 days) compared to a 10% frequency for those who did not receive fluconazole prophylaxis (p=not significant). There are no randomized trials of antifungal prophylaxis among small bowel transplant recipients, but the risk for invasive Candida infection is high and most experts recommend the use of antifungal prophylaxis. The current Infectious Diseases Society of America (IDSA) guidelines on Candida treatment recommends use of fluconazole at a dosage of 200-400 mg daily or a lipid formulation of Amphotericin B at a dosage of 1-2 mg/kg daily , each at least for 7-14 days, is recommended as postoperative prophylaxis for pancreas, small bowel and high risk liver transplant recipients (38) (Table 2). ![]()
Treatment
Mucosal candidiasis, Oropharyngeal candidiasis, can be treated with clotrimazole troche (5 x days). Also nystatin suspension, fluconazole or itraconazole are alternatives. Vulvovaginal candidiasis can be treated with several topical antifungal agents that are effective for most not complicated cases. Also a single dose of 150 mg of fluconazole is effective. For esophageal candidiasis, oral fluconazole is the preferred agent for 14-21 days, however in patients with prior fluconazole exposure or known fluconazole resistance, an echinocandin should be used. Posaconazole orally at 400 mg twice a day for 28 days appears to be promising in resistant patients (38).
Urinary Candidiasis
Treatment of choice for urinary candidiasis, proven cystitis and pyelonephritis, is with oral fluconazole. It is primarily excreted in urine and achieves excellent urine levels with a recommended duration of treatment of 2 weeks. In the case of fluconazole resistance or clinical failure, uses of systemic amphotericin B or oral flucytosine are alternatives, but they are limited because of toxicity and development of resistance in the case of flucytosine (38, 63). Reports of successful use of echinocandins have been documented in small series of patients (28, 58), but these agents are not currently recommended because of poor urinary concentrations and limited clinical data. Removing an indwelling catheter is also part of the treatment and imaging of the kidneys to exclude abscess or a fungus ball is prudent.
Disseminated Candidiasis
For treatment of candidemia or invasive candidiasis the IDSA guidelines recommend initial treatment with fluconazole or and echinocandin. In patients with moderately severe to severe illness or recent azole exposure the recommendation is to use an echinocandin empirically until Candida sp. identification (3, 38). Transition from an echinocandin to fluconazole is recommended for patients who have isolates that are likely to be susceptible to fluconazole and are clinically stable. Amphotericin B deoxycholate or a lipid formulation of amphotericin B (AmB) are alternatives if there is intolerance to or limited availability of other antifungal (27, 38). Voriconazole is effective but it offers little advantage over fluconazole and is recommended as stepdown to oral therapy for selected cases of candidiasis due to C. krusei or voriconazole susceptible C. glabrata. For infection due to C. glabrata an echinocandin is preferred and for infection due to C. parapsilosis treatment with fluconazole is recommended. The duration varies related to the site of infection. For candidemia the recommended duration is for 2 weeks after documented clearance and resolution of symptoms. Also the removal of central intravenous catheters is strongly recommended. All patients with candidemia should have a retinal examination, preferably performed by an ophthalmologist within a week of first culture. Candida endophthalmitis is treated with an amphotericin B formulation combined with flucytosine for advancing lesions or fluconazole could be used for less severe cases. The recommended duration of therapy is at least 4 to 6 weeks (10). For Candida endocarditis, valve replacement and treatment with a lipid amphotericin B product with flucytosine or an echinocandin is recommended and treatment should continue for at least 6 weeks after valve surgery (59). For patients who cannot undergo valve replacement long term suppression with fluconazole is recommended (38).
Drug Toxicity and Drug Interactions
In general antifungals are associated with diverse types of toxicities, most commonly hepatic, renal, hematologic, electrolyte abnormalities and infusion related. Each class is associated with a set of unique adverse events. Amphotericin B preparations are related to nephrotoxicity and infusion related reactions and the azoles are more closely related to liver toxicities (8).
After initiation of azoles in solid organ transplant recipients, close monitoring of their immunosuppressive agents will be required. Azole antifungal agents are substrate and inhibitors of cytochrome P450 enzymes. Fluconazole and voriconazole increase levels of cyclosporine and tacrolimus. As voriconazole increases the levels of sirolimus ≥ 100% it is recommended to avoid simultaneous use (5). Also, after discontinuation of azole therapy, dose adjustment of immunosuppressive therapy is required to prevent rejection. The echinocandins are very safe with solid organ transplantation, however caspofungin has some minor interactions that require dosing adjustments, however micafungin appears to be safe and effective, but sirolimus monitoring is recommended (11, 60). Hepatic enzymes monitoring with the echinocandins, especially in liver transplantation (31).
References
1. Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ; Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer; Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002; 34:7-14. [PubMed]
2. Badiee P, Kordbacheh P, Alborzi A, Zeini F, Mirhendy H, Mahmoody M. Fungal infections in solid organ recipients. Exp Clin Transplant 2005;3:385-389. [PubMed]
3. Bennett JE. Echinocandins for candidemia in adults without neutropenia. N Engl J Med. 2006; 355:1154-1159. [PubMed]
4. Berger N, Wirmsberger R, Kafka R, Margreiter C, Ebenbichler C, Stelzmueller I, Margreiter R, Steurer W, Mark W, Bonatti H. Infectious complications following 72 consecutive enteric-drained pancreas transplants. Transpl Int. 2006; 19:549-557. [PubMed]
5. Bruggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, Burger DM. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009; 48:1441-1458. [PubMed]
6. Choi IS, Kim SJ, Kim BY, Joh JW, Kim YI, Lee SK, Huh WS, Oh SY, Kim DJ, Kim YG, Kim MK, Ko YH, and Lee BB. Candida polyarthritis in a renal transplant patient: case report of a patient successfully treated with amphotericin B. Transplant Proc. 2000; 32:1963-1964. [PubMed]
7. Collins LA, Samore MH, Roberts MS, Luzzati R, Jenkins RL, Lewis WD, Karchmer AW. Risk factors for invasive fungal infections complicating orthotopic liver transplantation. J Infect Dis. 1994; 170:644-652. [PubMed]
8. Dodds Ashley E, Lewis R, Lewis J, Martin C, Andes D. Pharmacology of systemic fungal agents. Clin Infect Dis. 2006; 43:S28-S39. [PubMed]
9. Dongari-Bagtzoglou A, Dwivedi P, Ioannidou E, Shaqman M, Hull D, Burleson J. Oral Candida infection and colonization in solid organ transplant recipients. Oral Microbiol Immunol. 2009; 24:249-254. [PubMed]
10. Essman TF, Flynn HW, Jr., Smiddy WE, Brod RD, Murray TG, Davis JL, Rubsamen PE. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg.Lasers 1997; 28:185-194. [PubMed]
11. Forrest GN, Rasetto F, Akpek G, Philosophe B. Safety and efficacy of micafungin in transplantation recipients. Transplantation 2006; 82:1549. [PubMed]
12. Fortun J, Martin-Davila P, Montejo M, Munoz P, Cisneros JM, Ramos A, Aragon C, Blanes M, San JR, Gavalda J, Llinares P. Prophylaxis with caspofungin for invasive fungal infections in high-risk liver transplant recipients. Transplantation 2009; 87:424-435. [PubMed]
13. Gotzsche PC, Johansen HK. Nystatin prophylaxis and treatment in severely immunodepressed patients. Cochrane.Database.Syst.Rev. 2002; CD002033. [PubMed]
14. Grossi P, Farina C, Fiocchi R, Dalla GD. Prevalence and outcome of invasive fungal infections in 1,963 thoracic organ transplant recipients: a multicenter retrospective study. Italian Study Group of Fungal Infections in Thoracic Organ Transplant Recipients. Transplantation 2000;70:112-116. [PubMed]
15. Guaraldi G, Cocchi S, Codeluppi M, Di BF, De RN, Masetti M, Venturelli C, Pecorari M, Pinna AD, Esposito R. Outcome, incidence, and timing of infectious complications in small bowel and multivisceral organ transplantation patients. Transplantation 2005; 80:1742-1748. [PubMed]
16. Gupta KL, Ghosh AK, Kochhar R, Jha V, Chakrabarti A, Sakhuja V. Esophageal candidiasis after renal transplantation: comparative study in patients on different immunosuppressive protocols. Am J Gastroenterol. 1994; 89:1062-1065. [PubMed]
17. Hadley S, Huckabee C, Pappas PG, Daly J, Rabkin J, KauffmanCA, Merion RM, Karchmer AW. Outcomes of antifungal prophylaxis in high-risk liver transplant recipients. Transpl Infect Dis. 2009; 11:40-48. [PubMed]
18. Hadley S, Karchmer AW. Fungal infections in solid organ transplant recipients. Infect Dis Clin North Am 1995; 9:1045-1074. [PubMed]
19. Hibberd PL, Rubin RH. Clinical aspects of fungal infection in organ transplant recipients. Clin Infect Dis. 1994; 19 Suppl 1:S33-S40. [PubMed]
20. Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009; 48:1695-1703. [PubMed]
21. Horvath J, Dummer S, Loyd J, Walker B, Merrill WH, Frist WH. Infection in the transplanted and native lung after single lung transplantation. Chest 1993; 104:681-685. [PubMed]
22. Husain S, Tollemar J, Dominguez EA, Baumgarten K, Humar A, Paterson DL, Wagener MM, Kusne S, Singh N. Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled study. Transplantation 2003; 75:2023-2029. [PubMed]
23. Iinuma Y, Senda K, Fujihara N, Saito T, Takakura S, Kudo T, Kiuchi T, Tanaka K, Ichiyama S. Surgical site infection in living-donor liver transplant recipients: a prospective study. Transplantation 2004; 78:704-709.[PubMed]
24. Infectious Diseases Community of Practice. Fungal Infections. Am J Transplant 2004; 4 (suppl 10), 110-134. [PubMed]
25. Karkowska-Kuleta J, Rapala-Kozik M, Kozik A. Fungi pathogenic to humans: molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim Pol. 2009; 56:211-224. [PubMed]
26. Kubak BM. Fungal infection in lung transplantation. Transpl Infect Dis. 2002; 4 Suppl 3:24-31. [PubMed]
27. Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, Sekhon JS, Freire A, Ramasubramanian V, Demeyer I, Nucci M, Leelarasamee A, Jacobs F, Decruyenaere J, Pittet D, Ullmann AJ, Ostrosky-Zeichner L, Lortholary O, Koblinger S, Diekmann-Berndt H, Cornely OA. Micafungin Invasive Candidiasis Working Group.. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519-1527. [PubMed]
28. Lagrotteria D, Rotstein C, Lee CH. Treatment of candiduria with micafungin: A case series. Can J Infect Dis Med Microbiol. 2007; 18:149-150. [PubMed]
29. Lumbreras C, Cuervas-Mons V, Jara P, del Palacio A, Turrión VS, Barrios C, Moreno E, Noriega AR, Paya CV. Randomized trial of fluconazole versus nystatin for the prophylaxis of Candida infection following liver transplantation. J Infect Dis. 1996; 174:583-588. [PubMed]
30. Lumbreras C, Fernandez I, Velosa J, Munn S, Sterioff S, Paya CV. Infectious complications following pancreatic transplantation: incidence, microbiological and clinical characteristics, and outcome. Clin Infect Dis. 1995; 20:514-520. [PubMed] ![]()
31. Marr KA, Hachem R, Papanicolaou G, Somani J, Arduino JM, Lipka CJ, Ngai AL, Kartsonis N, Chodakewitz J, Sable C. Retrospective study of the hepatic safety profile of patients concomitantly treated with caspofungin and cyclosporin A. Transpl Infect Dis. 2004; 6:110-116. [PubMed]
32. Masoud M, Nasser NJ, Karban A, Edelstein S. Candida parapsilosis septic arthritis in a renal transplant patient. J Clin Rheumatol. 2008; 14:56. [PubMed]
33. McMullan R, Metwally L, Coyle PV, Hedderwick S, McCloskey B, O'Neill HJ, Patterson CC, Thompson G, Webb CH, Hay RJ. A prospective clinical trial of a real-time polymerase chain reaction assay for the diagnosis of candidemia in nonneutropenic, critically ill adults. Clin Infect Dis. 2008; 46:890-896. [PubMed]
34. Moreno A, Cervera C, Gavaldá J, Rovira M, de la Cámara R, Jarque I, Montejo M, de la Torre-Cisneros J, Miguel Cisneros J, Fortún J, López-Medrano F, Gurguí M, Muñoz P, Ramos A, Carratalá J. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant 2007; 7:2579-2586. [PubMed]
35. Olczak-Kowalczyk D, Pawłowska J, Cukrowska B, Kluge P, Witkowska-Vogtt E, Dzierzanowska-Fangrat K, Wrześniewska D, Smirska E, Grenda R. Local presence of cytomegalovirus and Candida species vs oral lesions in liver and kidney transplant recipients. Ann Transplant 2008; 13:28-33. [PubMed]
36. Papanicolaou GA, Meyers BR, Fuchs WS, Guillory SL, Mendelson MH, Sheiner P, Emre S, Miller C. Infectious ocular complications in orthotopic liver transplant patients. Clin Infect Dis. 1997; 24:1172-1177. [PubMed]
37. Pappas PG, Andes D, Schuster M, Hadley S, Rabkin J, Merion RM, Kauffman CA, Huckabee C, Cloud GA, Dismukes WE, Karchmer AW. Invasive fungal infections in low-risk liver transplant recipients: a multi-center prospective observational study. Am J Transplant 2006; 6:386-391. [PubMed]
38. Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD; Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48:503-535. [PubMed]
39. Patel R, Portela D, Badley AD, Harmsen WS, Larson-Keller JJ, Ilstrup DM, Keating MR, Wiesner RH, Krom RA, Paya CV. Risk factors of invasive Candida and non-Candida fungal infections after liver transplantation. Transplantation 1996; 62:926-934. [PubMed]
40. Peleg AY, Husain S, Kwak EJ, Silveira FP, Ndirangu M, Tran J, Shutt KA, Shapiro R, Thai N, Abu-Elmagd K, McCurry KR, Marcos A, Paterson DL. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis. 2007; 44:204-212. [PubMed]
41. Petrocheilou-Paschou V, Georgilis K, Kontoyannis D, Nanas J, Prifti H, Costopoulos H, Stamatelopoulos S. Pneumonia due to Candida krusei. Clin Microbiol Infect. 2002; 8:806-809. [PubMed]
42. Playford EG, Webster AC, Sorrell TC, Craig JC. Systematic review and meta-analysis of antifungal agents for preventing fungal infections in liver transplant recipients. Eur J Clin Microbiol Infect Dis. 2006; 25:549-561.[PubMed]
43. Pruett TL, Rotstein OD, Anderson RW, Simmons RL. Tricuspid valve Candida endocarditis. Successful treatment with valve-sparing debridement and antifungal chemotherapy in a multiorgan transplant recipient. Am J Med. 1986; 80:116-118. [PubMed]
44. Pugliese F, Ruberto F, Cappannoli A, Perrella SM, Bruno K, Martelli S, Marcellino V, D'Alio A, Diso D, Rossi M, Corradini SG, Morabito V, Rolla M, Ferretti G, Venuta F, Berloco PB, Coloni GF, Pietropaoli P. Incidence of fungal infections in a solid organ recipients dedicated intensive care unit. Transplant Proc. 2007; 39:2005-2007. [PubMed]
45. Ralph ED, Hussain Z. Chronic meningitis caused by Candida albicans in a liver transplant recipient: usefulness of the polymerase chain reaction for diagnosis and for monitoring treatment. Clin Infect Dis. 1996; 23:191-192. [PubMed]
46. Reller ME, Mallonee AB, Kwiatkowski NP, Merz WG. Use of peptide nucleic acid-fluorescence in situ hybridization for definitive, rapid identification of five common Candida species. J Clin Microbiol. 2007; 45:3802-3803. [PubMed]
47. Romano C, Ghilardi A, Carcagni MR, De Aloe GB. A case of oral erosive candidosis in a kidney transplant patient. Mycoses 2004; 47:524-526. [PubMed]
48. Safdar N, Slattery WR, Knasinski V, Gangnon RE, Li Z, Pirsch JD, Andes D. Predictors and outcomes of candiduria in renal transplant recipients. Clin Infect Dis. 2005; 40:1413-1421. [PubMed]
49. Salavert M. Prophylaxis, pre-emptive or empirical antifungal therapy: which is best in non-lung transplant recipients? Int J Antimicrob.Agents 2008; 32 Suppl 2:S149-S153. [PubMed]
50. Schaenman JM, Rosso F, Austin JM, Baron EJ, Gamberg P, Miller J, Oyer PE, Robbins RC, Montoya JG. Trends in invasive disease due to Candida species following heart and lung transplantation. Transpl Infect Dis. 2009; 11:112-121. [PubMed]
51. Sharpe MD, Ghent C, Grant D, Horbay GL, McDougal J, David CW. Efficacy and safety of itraconazole prophylaxis for fungal infections after orthotopic liver transplantation: a prospective, randomized, double-blind study. Transplantation 2003; 76:977-983. [PubMed]
52. Shepard JR, Addison RM, Alexander BD, Della-Latta P, Gherna M, Haase G, Hall G, Johnson JK, Merz WG, Peltroche-Llacsahuanga H, Stender H, Venezia RA, Wilson D, Procop GW, Wu F, Fiandaca MJ. Multicenter evaluation of the Candida albicans/Candida glabrata peptide nucleic acid fluorescent in situ hybridization method for simultaneous dual-color identification of C. albicans and C. glabrata directly from blood culture bottles. J Clin Microbiol. 2008; 46:50-55. [PubMed]
53. Shi SH, Lu AW, Shen Y, Jia CK, Wang WL, Xie HY, Zhang M, Liang TB, Zheng SS. Spectrum and risk factors for invasive candidiasis and non-Candida fungal infections after liver transplantation. Chin Med.J 2008; 121:625-630. [PubMed]
54. Silveira FP, Husain S. Fungal infections in solid organ transplantation. Med Mycol. 2007; 45:305-320. [PubMed]
55. Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am. 2003; 17:113-34. [PubMed]
56. Singh N. Antifungal prophylaxis in solid-organ transplant recipients: considerations for clinical trial design. Clin Infect Dis. 2004; 39 Suppl 4:S200-S206. [PubMed]
57. Singh N, Wagener MM, Cacciarelli TV, Levitsky J. Antifungal management practices in liver transplant recipients. Am J Transplant 2008; 8:426-431. [PubMed]
58. Sobel JD, Bradshaw SK, Lipka CJ, Kartsonis NA. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis. 2007; 44:e46-e49. [PubMed]
59. Steinbach WJ, Perfect JR, Cabell CH, Fowler VG, Corey GR, Li JS, Zaas AK, Benjamin DK, Jr. A meta-analysis of medical versus surgical therapy for Candida endocarditis. J Infect. 2005; 51:230-247. [PubMed]
60. Theuretzbacher U. Pharmacokinetics/pharmacodynamics of echinocandins. Eur J Clin Microbiol Infect Dis. 2004; 23:805-812. [PubMed]
61. van Hal SJ, Marriott DJ, Chen SC, Nguyen Q, Sorrell TC, Ellis DH, Slavin MA. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transpl Infect Dis. 2009; 11:122-127. [PubMed]
62. van Hal SJ, Stark D, Harkness J, Marriott D. Candida dubliniensis meningitis as delayed sequela of treated C. dubliniensis fungemia. Emerg Infect Dis. 2008; 14:327-329. [PubMed]
63. Alvarez-Lerma F, Nolla-Salas J, Leon C, Palomar M, Jorda R, Carrasco N, Bobillo F. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003; 29:1069-1076. [PubMed]
64. Viviani MA, Tortorano AM, Malaspina C, Colledan M, Paone G, Rossi G, Bordone G, Pagano A. Surveillance and treatment of liver transplant recipients for candidiasis and aspergillosis. Eur J Epidemiol. 1992; 8:433-436. [PubMed]
65. Winston DJ, Pakrasi A, Busuttil RW. Prophylactic fluconazole in liver transplant recipients. A randomized, double-blind, placebo-controlled trial. Ann.Intern.Med. 1999; 131:729-737. [PubMed]
66. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin.Infect.Dis. 2004; 39:309-317. [PubMed]![]()
Figure 1: Timeline for Onset of Fungal Infections
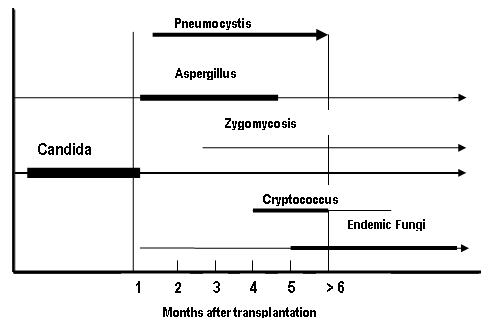
Figure adapted from Infectious Diseases Guidelines, Am J Transplant (24)
Table 1. Incidence of Candidiasis per Organ Transplant
| Type of transplant | Incidence of invasive fungal infection | Proportion of IFI caused by Candida | Most common fungal infection |
|---|---|---|---|
| Renal | 0-20% | 76-95% | Candida |
| Liver | 5-42% | 62-91% | Candida |
| Pancreas | 10-40% | > 90% | Candida |
| Heart | 5-20% | 8-25% | Aspergillus |
| Lung | 15-35% | 43-72% | Candida/Aspergillus |
| Small bowel | 40-59% | 80-100% | Candida |
Derived from Cited References: (24,49,54,55)
Table 2. Prophylaxis Recommendations Against Candida Infections in Solid Organ Transplantation
| Type of transplant | First line | Alternatives | Duration |
|---|---|---|---|
| Liver * | Fluconazole 200-400 mg/day | Echinocandin ± or LAmb | 2-4 weeks |
| Pancreas | Fluconazole 200-400 mg/day | LAmb# | 2-4 weeks |
| Small bowel | Fluconazole 200-400 mg/day | LAmb | 2-4 weeks |
* high risk: retransplantation, creatinine greater then 2mg/dl, choledochojejunostomy, prolonged intraoperative time, intraoperative use of more 40 U of blood products and fungal colonization (37).
± Echinocandin depends on hospital formulary and all are intravenously given: Caspofungin at 70 mg load then 50 mg daily or micafungin 100 mg daily have most clinical information (11, 12, 57)
# Lamb = lipid formulation of amphotericin B. Doses recommended are 3-5 mg/kg.
Zaoutis T. et al. A Prospective, Multicenter Study of Caspofungin for the Treatment of Documented Candida or Aspergillus Infections in Pediatric Patients. PEDIATRICS. 2009 Mar;123(3):877-84.
Sceinfeld, NS. Skin Disorders in Elderly Persons: Identifying Fungal Infections. Infect Med. 2007:509-515
Oliveri S, et al. Experience with the Platelia Candida ELISA for the diagnosis of invasive candidosis in neonatal patients.Clin Microbiol Infect. 2008 Apr;14(4):391-3. Epub 2008 Jan 11.
Harrington A. Differentiation of Candida albicans from non-albicans yeast directly from blood cultures by Gram stain morphology. Eur J Clin Microbiol Infect Dis 2007;26:325-329.
Colombo AL, et al. Caspofungin Use in Patients with Invasive Candidiasis caused by Common Non-albicans Candida Species: Review of the Caspofungin Database. Antimicrob Agents Chemother 2010;Mar 15 [Epub ahead of print]
Betts RF, Nucci M, et al. A Multicenter, Double-Blind Trial of a High-Dose Caspofungin Treatment Regimen versus a standard Caspofungin Treatment Regimen for Adult Patients with Invasive Candidiasis. Clin Inf Dis. 2009;48:1676-84.
Pappas PG, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 2007;45:883-893.
Zaoutis T. et al. A Prospective, Multicenter Study of Caspofungin for the Treatment of Documented Candida or Aspergillus Infections in Pediatric Patients. PEDIATRICS. 2009 Mar;123(3):877-84.
Hamza et al. Single-Dose Fluconazole versus Standard Therapy for Oropharyngeal Candidiasis in HIV-Infected Patients: A Randomized, Double-Blind, Double-Dummy Trial. Clin Infect Dis. 2008 Nov 15;47(10):1270-6.
Betts RF, Nucci M, et al. A Multicenter, Double-Blind Trial of a High-Dose Caspofungin Treatment Regimen versus a standard Caspofungin Treatment Regimen for Adult Patients with Invasive Candidiasis. Clin Inf Dis. 2009;48:1676-84.
Legrand F, et al. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis. 2008;46:696-702.
Zaoutis T. et al. A Prospective, Multicenter Study of Caspofungin for the Treatment of Documented Candida or Aspergillus Infections in Pediatric Patients. PEDIATRICS. 2009 Mar;123(3):877-84.
Ruan SY, Lee LN, et al. Candida glabrata fungaemia in intensive care units. Clin Microbiol Infect 2007 Nov 28 [Epub ahead of print].
Sun RL, et al. Clinical characteristics and outcome of Candida keratitis. Am J Ophthalmol 2007;143:1043-1045.
Van Hal et al. Candida dubliniensis Meningitis as Delayed Sequela of Treated C. dubliniensis Fungemia. Emerg Infect Dis. 2008 February; 14(2): 327–329.
Hamza et al. Single-Dose Fluconazole versus Standard Therapy for Oropharyngeal Candidiasis in HIV-Infected Patients: A Randomized, Double-Blind, Double-Dummy Trial. Clin Infect Dis. 2008 Nov 15;47(10):1270-6.
Guided Medline Search for
Rex JH., Sobel JD., Powderly W. Candida species
Baron EJ. Yeast
Pappas PG, et al. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009;48:503-535.
Schaudin C, Stoodley P, Kainovic' A, O'Keeffe T, Costerton B, Robinson D, Baum M, Ehrlich G, Webster P. Bacterial Biofilms, Other Structures Seen as Mainstream Concepts. Microbe 2007;2:231-237.
Raad, I., Hanna, H. and Maki, D. Intravascular Catheter-related Infections: Advances in Diagnosis, Prevention and Management. The LANCET Infectious Diseases 2007; Vol.7, Issue 10, 645-657.
Singh N, Treatment of opportunistic mycoses: how long is long enough? Lancet Infect Dis 2003;3:703-08.
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
Logan McCool: The Discovery and Naming of Candida
Simi Vincent: Origin of the Names of Species of Candida
