Table 1: Hepatitis B Proteins
|
Protein |
Open Reading
Frame |
Region |
RNA Transcript |
|
Pre-core |
C (core) |
Pre-core |
3.5 kb |
|
HBcAg |
C (core) |
Core |
3.5 kb |
|
DNA polymerase |
P (polymerase) |
Pol |
3.5 kb |
|
Large (L) HBsAg |
S (surface
envelope) |
Pre-S1, Pre-S2,
S |
2.4 kb |
|
Middle (M) HBsAg |
S (surface
envelope) |
Pre-S2, S |
2.1 kb |
|
Small (S) HBsAg |
S (surface
envelope) |
S |
2.1 kb |
|
HBxAg |
X |
X |
0.7 kb |
Abbreviations:
HBcAg: hepatitis B core antigen, HBsAg: hepatitis B surface antigen, HBxAg:
hepatitis B x antigen.
Table 2: Guidelines for
Hepatocellular Carcinoma Surveillance in HBsAg Carriers
|
Guideline |
Recommended
surveillance population |
|
AASLD, 2010 |
Africans and
African Americans
Asian males >40
years old
Asian females
>50 years old
Family history
of HCC
Cirrhosis
|
|
U.S. Algorithm,
2008 |
Africans >20
years old
Asian males >40
years old
Asian females
>50 years old
Asians >30 years
old with presumed vertical or horizontal
acquisition of HBV early in life
Family history
of HCC
Cirrhosis
>40 years old
with elevated ALT and/or HBV DNA level >2,000
IU/mL
|
|
WGO, 2010 |
African males
>20 years old
Asian males >40
years old
Asian and
African females >50 years old
Family history
of HCC
Cirrhosis
|
|
APASL, 2010 |
Cirrhosis |
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase;
APASL, Asia Pacific Association for the Study of the Liver; HBsAg, hepatitis B surface antigen; HBV,
hepatitis B virus; HCC, hepatocellular carcinoma; WGO, World Gastroenterology Association.
Table 3: Phases of Chronic
Hepatitis B Infection
|
Phase of
infection |
Serum ALT |
HBeAg |
Anti-HBe |
Serum HBV DNA |
Liver Histology |
|
Immune tolerance
|
Normal |
Positive |
Negative |
Very high |
Minimal to no
activity |
|
Immune clearance
(HBeAg-positive)
|
Elevated |
Positive |
Negative |
Variable; high
or fluctuating |
Significant
activity |
|
Inactive HBsAg
carrier
|
Normal |
Negative |
Positive |
Very low or
undetectable |
Minimal to no
activity |
|
Reactivation (HBeAg-negative) |
Elevated,
fluctuating |
Negative |
Positive |
Variable; lower
than HBeAg-positive infection |
Significant
activity |
Abbreviations: ALT, alanine aminotransferase; HBeAg, hepatitis
B e antigen; Anti-HBe, antibody against hepatitis B e antigen; HBV, hepatitis B
virus.
Table 4:
Guidelines for Initiation of Antiviral Therapy in Chronic Hepatitis B
|
|
EASL Guidelines |
APASL Guidelines |
AASLD Guidelines |
U.S. Algorithm
(78)* |
|
HBeAg-Positive |
HBV DNA >2,000
IU/mL and /or ALT >ULN
•
Consider liver biopsy if ALT >ULN or HBV DNA
>2,000 IU/mL; initiate therapy if moderate to
severe histologic disease |
HBV DNA >20,000
IU/mL and ALT >2x ULN
• Obtain
liver biopsy if age >40 and HBV DNA >20,000 IU/mL
with ALT <2x ULN; initiate therapy if moderate
to severe histologic disease |
HBV DNA >20,000
IU/mL and ALT >2x ULN
• Observe
3 to 6 months; initiate therapy if no
spontaneous HBeAg loss
•
Consider liver biopsy if HBV DNA >20,000 IU/mL,
ALT ≤2x ULN, and age >40 or family history of
HCC
•
Consider therapy if histologic disease and ALT
≤2x ULN
|
HBV DNA ≥20,000
IU/mL and ALT >ULN
•
Consider liver biopsy if HBV DNA ≥20,000 IU/mL,
normal ALT, and age >35; initiate therapy if
histologic disease
•
Consider therapy if known histologic disease,
regardless of ALT
|
|
HBeAg-Negative |
HBV DNA >2,000
IU/mL and /or ALT >ULN
•
Consider liver biopsy if ALT >ULN or HBV DNA
>2,000 IU/mL; initiate therapy if moderate to
severe histologic disease
|
HBV DNA >2,000
IU/mL and ALT >2x ULN
• Obtain
liver biopsy if age
≥40 and
HBV DNA >2,000 IU/mL with ALT <2x ULN; initiate
therapy if moderate to severe histologic disease |
HBV DNA >20,000
IU/mL and ALT >2x ULN
•
Consider liver biopsy if HBV DNA >2,000 IU/mL
and ALT >ULN; initiate therapy if histologic
disease |
HBV DNA ≥2,000
IU/mL and ALT >ULN
•
Consider liver biopsy if HBV DNA ≥2,000 IU/mL,
normal ALT, and age >35
•
Consider therapy if known histologic disease,
regardless of ALT
|
|
Cirrhosis |
Decompensated or
compensated:
Any detectable HBV DNA |
Decompensated:
Any detectable HBV DNA
Compensated: HBV
DNA >2,000 IU/mL |
Decompensated:
Any detectable HBV DNA
Compensated:
HBV DNA >2,000 IU/mL
•
Consider therapy if HBV DNA <2,000 IU/mL and ALT
>ULN
|
Decompensated:
Any detectable HBV DNA
Compensated:
HBV DNA ≥2,000 IU/mL
•
Consider therapy if HBV DNA <2,000 IU/mL,
regardless of ALT
|
|
Immunosuppression or Chemotherapy |
HBsAg-positive
•
Initiate oral antiviral therapy prior to onset
of chemotherapy and continue for 12 months
following completion
HBsAg-negative,
anti-HBc-positive
• Monitor
ALT and HBV DNA; initiate oral antiviral therapy
if confirmed reactivation by HBV DNA positivity |
HBsAg positive
•
Initiate oral antiviral therapy prior to onset
of chemotherapy and continue for at least 12
weeks following completion |
HBsAg-positive
•
Initiate oral antiviral therapy at onset of
chemotherapy and continue for 6 months following
completion; longer duration of therapy if HBV
DNA >2,000 IU/mL |
HBsAg-positive
•
Initiate oral antiviral therapy several weeks
prior to onset of chemotherapy and continue for
6 months following completion; longer duration
of therapy if HBV DNA ≥2,000 IU/mL |
Adapted from European Association for the Study of the Liver (50), Liaw et al. (98), Lok and McMahon (113), and Keeffe et al. (78).
*ULN of ALT in the U.S. Algorithm defined by 30 IU/mL in men and 19 IU/mL in women.
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; anti-HBc, antibody to hepatitis B core antigen; APASL, Asian Pacific Association for the Study of the Liver; EASL, European Association for the Study of the Liver; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; ULN, upper limit of normal.
Table 5: Serologic Assessment
of Hepatitis B Infection
|
|
Serologic marker |
|||
|
|
HBsAg |
Anti-HBs |
Anti-HBc (IgM) |
Total Anti-HBc |
|
Acute infection
|
+ |
– |
+ |
+ |
|
Chronic
infection
|
+ |
– |
– |
+ |
|
Resolved
infection, immune
|
– |
+ |
– |
+ |
|
Previous
vaccination, immune
|
– |
+ |
– |
– |
|
Unexposed,
susceptible |
– |
– |
– |
– |
Abbreviations: HBsAg, hepatitis B surface antigen; Anti-HBs,
antibody against hepatitis B surface antigen; Anti-HBc, antibody against
hepatitis B core antigen.
Table 6: Antiviral Agents Approved for Treatment of Chronic Hepatitis B Infection
|
Antiviral agent |
Drug class |
Dose |
Duration |
|
Entecavir* |
Nucleoside
analogue |
0.5 mg daily |
Variable |
|
Tenofovir* |
Nucleotide
analogue |
300 mg daily |
Variable |
|
Lamivudine |
Nucleoside
analogue |
100 mg daily |
Variable |
|
Adefovir |
Nucleotide
analogue |
10 mg daily |
Variable |
|
Telbivudine |
Nucleoside
analogue |
600 mg daily |
Variable |
|
Interferon
alfa-2b |
Immune modulator |
5 million IU
daily or 10 million IU three times weekly |
48 weeks |
|
Pegylated
interferon alfa* |
Immune modulator |
1.5 mcg/kg
weekly** (or) 180 mcg weekly*** |
48 weeks |
* Preferred agent
** Pegylated interferon alfa-2b is given at a dose of 1.5
mcg/kg weekly
*** Pegylated interferon alfa-2a is given at a dose of 180 mcg
weekly
Table 7: Efficacy of
Antiviral Therapy for HBeAg-Positive Chronic Hepatitis B
|
Antiviral Agent |
HBV DNA negative |
HBeAg loss |
HBeAg
seroconversion |
HBsAg loss |
|
Entecavir* |
67 |
22 |
21 |
2 |
|
Tenofovir* |
76 |
22 |
21 |
3 |
|
Pegylated
Interferon Alfa* |
10-25 |
29-30 |
22-27 |
3-5 |
|
Lamivudine |
36-44 |
17-32 |
16-21 |
0 |
|
Adefovir |
13-21 |
24 |
12 |
0 |
|
Telbivudine |
60 |
26 |
22-23 |
0 |
|
Emtricitabine |
39 |
14 |
12 |
0 |
*Preferred antiviral
agents
Table 8: Efficacy of
Antiviral Therapy for HBeAg-Negative Chronic Hepatitis B
|
Antiviral Agent |
HBV DNA negative |
HBsAg loss |
|
Entecavir* |
90 |
0 |
|
Tenofovir* |
93 |
0 |
|
Pegylated
Interferon Alfa* |
52-63 |
4 |
|
Lamivudine |
60-73 |
0 |
|
Adefovir |
51-64 |
0 |
|
Telbivudine |
88 |
0 |
|
Emtricitabine |
79 |
0 |
*Preferred
antiviral agents
Table 9:
Management of Drug Resistance Associated with Hepatitis B Therapy
|
Antiviral drug
resistance |
Management |
|
Lamivudine
|
Continue
lamivudine and add adefovir or tenofovir
Switch to
tenofovir or tenofovir/emtricitabine
|
|
Adefovir
|
Continue
adefovir and add lamivudine or telbivudine
Switch to or add
entecavir (only if no lamivudine resistance)
Switch to
tenofivir or tenofovir/emtricitabine
|
|
Telbivudine |
Continue
telbivudine and add adefovir or tenofovir
Switch to
tenofovir/emtricitabine
|
|
Entecavir
|
Switch to or add
adefovir or tenofovir
Switch to
tenofovir/emtricitabine
|
Adapted from Lok and McMahon
(113),
and Keeffe et al.
(78).
Table 10: Hepatitis B Vaccines
|
Patient
Group |
Recombivax HB |
Engerix-B |
Twinrix*** |
Pediarix**** |
|
Children |
5mcg* |
10mcg |
– |
10mcg |
|
Adults
(≥20 yrs) |
10mcg |
20mcg |
20mcg |
– |
|
Immunocompromised or dialysis patients |
40mcg |
40mcg** |
– |
– |
Adapted from Mast et al.(121)
All hepatitis vaccines are administered as an intramuscular injection given in 3
doses at 0, 1, and 6 months, unless otherwise specified.
*Adolescents ages 10-15 yrs can receive an alternate regimen
of 10mcg given in 2 doses at months 0 and 4-6.
**Dosing regimen of 40mcg given in 4 doses at months 0, 1, 2,
and 6.
***Combined hepatitis A and hepatitis B vaccine. Approved for
adults (>18 yrs). Alternate accelerated regimen involves 20mcg given in 4 doses
at days 0, 7, 21 to 30, and at one year.
****Combined hepatitis B vaccine with diphtheria, tetanus,
pertussis, and poliomyelitis vaccines; approved for ages 0-6 yrs given in 3
doses at 0, 2, and 4 months.
Table 11: Candidates for
Hepatitis B Vaccination
|
Patient Group |
|
All infants
within 24 hours of birth
Health care
workers
Individuals at
risk of occupational exposure
Travelers to HBV
endemic areas with high prevalence
Injection drug
users
Men who have sex
with men
Persons with
multiple sex partners
Household and
sexual contacts of persons with known chronic
HBV infection
Incarcerated
individuals
Dialysis
patients
Solid organ
transplant recipients
Persons with
underlying chronic liver disease or HIV
infection
|
Abbreviations: HBV, hepatitis B virus; HIV, human
immunodeficiency virus
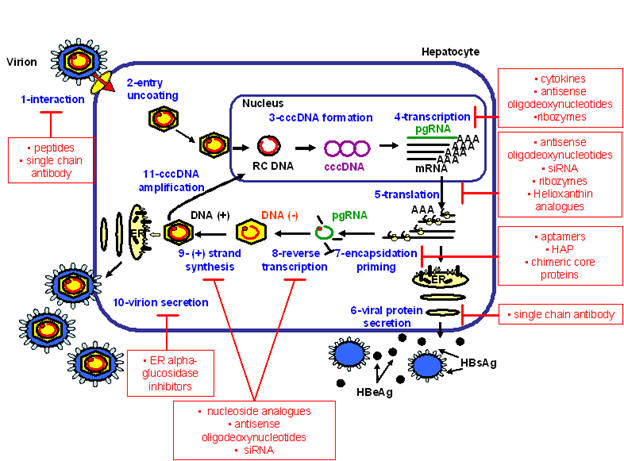
Figure 1: Hepatitis B Life Cycle

The HBV life cycle involves a sequence of events allowing for viral entry into hepatocytes and import of HBV DNA into the hepatocyte nucleus, followed by viral replication, assembly, and excretion of mature virions into the extracellular space (96). Molecular targets of antiviral therapy based on the HBV life cycle are noted, including classes of investigational antiviral agents and corresponding sites of activity. Abbreviations: cccDNA, covalently closed circular double-stranded DNA; HAP, heteroaryldihydropyrimidines; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; pgRNA, pre-genomic RNA; siRNA, short interfering RNA.
Figure 2. Hepatitis B Genome
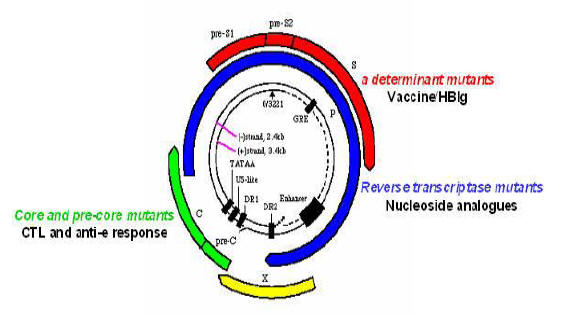
The organization of the HBV genome is shown, including the four overlapping open reading frames
(S, surface envelope; C, core; P, polymerase; X) involved in translation of HBV RNA into viral proteins.
Common HBV mutants are also shown. Abbreviations: CTL,
cytotoxic T lymphocyte; HBIg, hepatitis B immunoglobulin.
Figure 3: Risk of Hepatocellular Carcinoma Based on Hepatitis B Viral Load
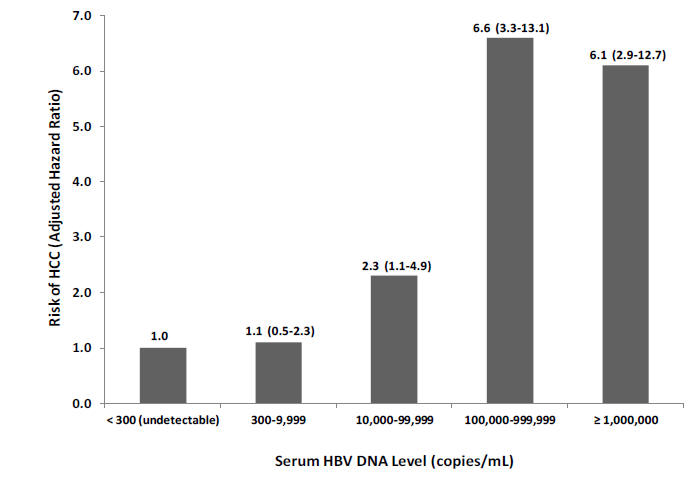
Multivariable adjusted hazard ratios (95% confidence interval) are given, indicating risk of hepatocellular carcinoma based on a Cox
proportional hazards model involving all participants from the REVEAL-HBV study (n=3653). Abbreviations: HBV, hepatitis B virus;
HCC, hepatocellular carcinoma.
Figure 4:
Efficacy
of Antiviral Therapy for Hepatitis B
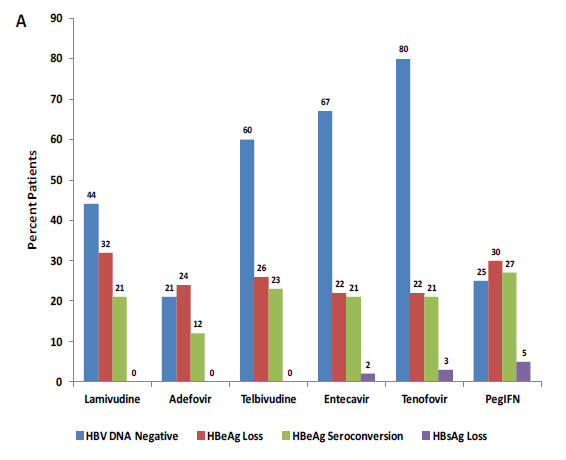
Optimal efficacy reported with antiviral agents in the treatment of chronic hepatitis B is shown based on prospective clinical trials.
One-year data are provided for treatment endpoints in A) HBeAg-positive (46, 73, 89, 118) and B) HBeAg-negative infection (46, 118, 120, 147).
Treatment endpoints include HBV DNA negativity, HBeAg loss, HBeAg seroconversion, and HBsAg loss. Abbreviations: HBeAg, hepatitis B
e antigen; HBV, hepatitis B virus; HBsAg, hepatitis B surface
antigen; PegIFN, pegylated interferon alfa.
Figure 5: Antiviral Drug Resistance Associated with Nucleoside and Nucleotide Analogues
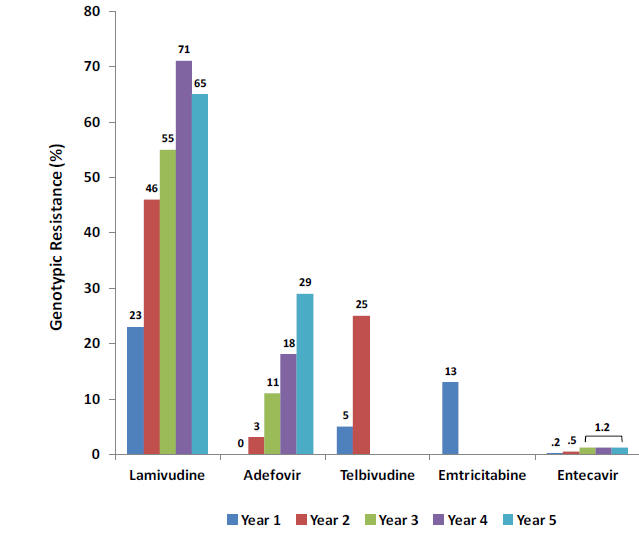
Genotypic resistance, defined by the presence of known HBV mutations resulting in treatment failure, is shown by
year and specific nucleotide or nucleoside analogue (63, 87, 97, 102, 112, 119, 159).