Pneumocystis jirovecii in Transplant Recipients
Authors: Juan José Castón, Julian Torre-Cisneros
PATHOGEN
Pneumocystis jirovecii was formerly classified as a protozoon given the histological features of its cystic and trophozoitic form, its susceptibility to anti-protozoa drugs such as pentamidine, the absence of ergosterol in its membrane and the inefficacy of traditional antifungal drugs against it. In 1988, DNA analyses showed that Pneumocystis jirovecii’s rRNA is much more similar to Saccharomyces cerevisiae than to the rRNA sequences of protozoan species (9). Subsequently, the sequences of thousands of Pneumocystis genes became known and it was determined that 33% of these genes show clear analogy with the genes of Saccharomyces pombe. Thus, Pneumocystis was finally classified as an ascomycetous fungus.
Since its initial discovery, it has been shown that the different Pneumocystis populations have specific hosting species (humans, rats, bats, lambs and certain cetaceans). The proliferation of Pneumocystis is host species specific and is somewhat atypical for fungi; the precise reason for this are not fully understood. The form currently infecting human beings is Pneumocystis jiroveci[i] (in honor of Czech pathologist Otto Jirovec).
EPIDEMIOLOGY
Pneumocystis jirovecii may develop in both healthy and ill (HIV, chronic lung diseases, healthcare professionals and pregnant women) individuals. The prevailing hypothesis that pneumocystic jirovecii pneumonia results from reactivation of latent infection is widely questioned nowadays. It is believed that airborne and person-to-person transmission is the most likely mode of transmission of pneumocystic jirovecii pneumonia (18).
The risk of developing Pneumocystis jirovecii pneumonia (pneumocystic jirovecii pneumonia) is directly related to the intensity and duration of immunosuppression. The incidence of pneumocystic jirovecii pneumonia varies among different transplant types and centers and ranges between 3-15 % depending upon whether prophylaxis is employed or not. However, in the recent years, the incidence of pneumocystic jirovecii pneumonia has declined even at centers that do not routinely employ trimethoprim-sulfamethoxazole (TMP-SMZ) prophylaxis (11, 27). A key factor contributing most to pneumocystic jirovecii pneumonia development is the use of corticosteroids. Additional contributory variables include T-cell depletion, neutropenia and the type of immunosuppression used. In this context, it has been shown that mycophenolate mofetil mycophenolate mofetil may have intrinsic activity against Pneumocystis jirovecii, while tacrolimus enhances its growth in vitro (33).
The risk of Pneumocystis jirovecii infection is higher between the first and sixth months after transplantation and during periods of increased immunosuppression by means of corticosteroids or antilymphocyte antibodies aimed at treating graft rejection or graft-versus-host disease.
CLINICAL MANIFESTATIONS
Pneumocystic jirovecii pneumonia is characterized by fever, non-productive cough and progressive dyspnea. Although the appearance of insidious dyspnea is characteristic, clinical symptoms may arise within a week. Pneumonia usually appears during reduction of corticosteroid doses (39).
Radiologically, pneumocystic jirovecii pneumonia usually manifests as a bilateral interstitial infiltrates with perihilar or lower lobar predominance (Figure 1). Pneumatoceles may appear, especially in during episodes of relapse, which may communicate with the pleural space thus producing spontaneous pneumothorax. Pleural haemorrhage is rather infrequent and should lead to consideration of alternative diagnoses. Chest radiographs may appear normal in pneumocystic jirovecii pneumonia. In patients receiving inhaled pentamidine prophylaxis, preferential deposition of the drug in the lower lobes may lead to infiltration in the upper lobes that may mimic tuberculosis. In some cases pulmonary infection may manifest as obliterating bronchiolitis with organized pneumonia (19).
Although choroiditis has been reported in haematopoietic stem cell transplant recipients receiving inhalational pentamidine prophylaxis, extrapulmonary pneumocystosis is distinctly unusual in organ transplant recipients. Disease relapse while infrequent, is associated with poor outcome.
LABORATORY DIAGNOSIS
Pneumocystis jirovecii cannot be cultured. Diagnosis is based on fungus identification in clinically relevant samples. Bronchoalveolar lavage (BAL) is the procedure of choice due to its high sensitivity (89-98 %). Non-invasive samples (induced sputum, orophraryngeal smear) should be reserved for patients in whom BAL cannot be performed (21, 45). Fungus microscopic identification for Pneumocystis jirovecii is as the following.
MICROSCOPIC IDENTIFICATION
Staining methods
Grocott-Gomori methenamine silver stain (GMS) is a sensitive method for demonstrating the cystic forms but does not stain the trophic forms. Differentiation between Pneumocystis jirovecii and yeasts may be challenging at times.
Toluidine blue O stains cyst wall (not their content or trophic forms) and is more rapid than GMS. However, it requires high-quality samples. It may also be difficult to differentiate between Pneumocystis jirovecii and yeasts using this stain.
Calcofluor white (CW) is a quick staining method that requires only a fluorescence microscope and an expert observer to distinguish Pneumocystis jirovecii from yeasts (3, 31).
Calcofluor white and Grocott-Gomori methenamine silver stain can have positive and negative predictive values > 90 % when performed by a skilled observer on a high quality sample.
Immunofluorescence with monoclonal antibodies (IMA)
Immunofluorescence with monoclonal antibodies detects both cystic and trophic forms. Antibodies aimed at trophic forms show higher sensitivity, since these forms are more numerous than cysts. On the other hand, they are more expensive and slow. Merifluor IMA is more sensitive but less specific than Calcofluor white and Grocott-Gomori methenamine silver stain. Confirmation with a second assay is recommended in all positive immunofluorescence with monoclonal antibodies cases. The performance of these techniques depends on sample quality, microorganism burden, and the experience of the laboratory staff.
Molecular diagnosis
These techniques include polymerase chain reaction (PCR) methods aimed at detecting different DNA regions in P. jirovecii and are usually performed on respiratory samples. Real-time PCR is quicker with a lower sample contamination probability and higher specificity than nested PCR (2, 4, 10). Besides, it enables DNA quantification, and has greater sensitivity and specificity than staining techniques.
Other methods for messenger-RNA identification in fungi have also been developed: messenger-RNA, in spite of being more unstable than DNA has the feasibility to detect the microorganism. Its sensitivity and specificity in BAL samples are 100 % and 86 %, respectively.
Serum markers
Serum markers represent relatively non-invasive tests capable of detecting infection associated with low microorganism burden in sputum samples.
Lactate dehydrogenase (LDH) elevation is likely to be an inflammatory as opposed to an infection-specific marker.
Elevation of KL-6 antigen, expressed in type-II pneumocytes and bronchial epithelium cells. It has low specificity, since it is elevated in patients with interstitial pneumonitis. (37).
Elevation of (1,3)-β-D-glucan (BDG), which is a component of the fungal wall is the most reliable marker, as its sensitivity and specificity has been shown to be 92% and 86%, respectively. Furthermore, its positive and negative predictive value approaches 61% and 98%, respectively. Given that the (1,3)-β-D-glucan levels decline with successful treatment, the test may also be useful for monitoring the response to therapy (8, 20, 38).
THERAPY
The treatment of choice is trimethoprim-sulfamethoxazole (cotrimoxizole, TMP-SMX) (39).The doses usually administered for therapy are 20 mg/kg/day of TMP plus 100 mg/kg/day of SMX divided into 3-4 doses. The usual duration of the treatment is 21 days, with subsequent secondary prophylaxis. The most frequent TMP-SMX-related secondary effects are hematologic toxicity including neutropenia and thrombocytopenia, skin rashes and nephrotoxicity (16). These effects are frequently reversible by means of dose reduction or drug discontinuation.
Alternative Therapies
Pentamidine isethionate is the main alternative to TMP-SMX (Table 1) (34). It has a prolonged half-life, thus reaching high concentrations in the lung tissue. The usual dose ranges between 2-4mg/kg/day administered intravenously. Therapeutic concentrations of pentamidine are reached more slowly than with other drugs, so clinical improvement may not ensue for up to 7 days. The major adverse event associated with pentamidine use is renal dysfunction. Other frequently observed side-effects include hypo- and hyperglycemia, neutropenia, thrombocytopenia, gastrointestinal intolerance such as nausea and dysgeusia, and pancreatitis. The latter mainly occurs when the cummulative dose of pentamidine exceeds 3 g and can appear up to 2 months after drug discontinuation. Due to the aforementioned adverse sequelae and the possibility of pancreatic islet cell necrosis, the use of pentamidine is discouraged in pancreas transplant recipients. Inhaled pentamidine has lower clinical efficacy and is not recommended as therapy for established infection.
Atovaquone (750 mg in oral suspension tid-qid) may be useful to treat mild to moderately severe pneumocystic jirovecii pneumonia (Table 1). Its most frequent side-effect is skin rashes the incidence of which correlates with high atovaquone levels. The drug may also cause gastrointestinal intolerance and elevation of transaminase levels (7).
Apart from pentamidine or atovaquone monotherapy, there are some drug combinations which may be useful as alternative treatments against pneumocystic jirovecii pneumonia. These include dapsone (100 mg/day orally) plus trimethoprim (15 mg/kg/day orally and divided into 3 doses) (15). However, the toxicity profile of this combination resembles that of TMP-SMX and includes neutropenia, anemia, skin rashes, and hepatitis. Thus, a change from TMP-SMX to dapsone-trimethoprim combination is not deemed advisable. Other side-effects associated with dapsone-trimethoprim combination are fever, and hemolysis in presence of G6DP deficiency.
The combination of clindamycin (600-900 mg intravenously or orally every 6-8 h) and primaquine (15-30 mg of base/day orally) may be effective in mild cases. The main adverse effects of clindamycin include skin rashes, anemia, neutropenia and the development of C. difficile colitis. Primaquine must not be used in G6DP-deficient patients.
The combination of pyrimethamine (50-100 mg/day orally after a load dose of 100-120 mg) and sulfadiazine or trisulfapyrimidines (4-8 g/day) may be effective. This combination requires concomitant administration of folinic acid (10 mg/day).
The use of trimetrexate (45 mg/m2/day) and folinic acid (80 mg/m2/day) is an option for the treatment of mild pneumonia, although it has not been well studied in transplant setting. Its adverse effects include fever, skin rashes and elevated transaminase levels, and neutropenia in the absence of folinic acid supplementation. Finally, although clinical evidence is still scarce, available data suggest that the addition of caspofungin (an antifungal agent that acts on the cell wall by inhibiting beta-1,3-glucan synthesis) to TMP-SMX may yield synergistic activity in solid organ transplant recipients with severe PCP. This activity may be due the fact that beta-1,3-glucan is the chief component of the cyst forms of Pneumocystis (1, 14, 24, 36,43). Indeed echinocandins were effective in clearing cystic forms from the lungs of infected animals (30). However, given that beta-1,3-glucan is present only in the cyst forms, caspofungin shows lesser activity against the trophic forms.
Resistance to Drugs
In the recent years there has been an increasing concern about resistance to drugs widely used in pneumocystic jirovecii pneumonia prophylaxis and treatment. This resistance is the result of mutations in the antimicrobials' targets and has correlated with both prophylaxis and treatment failure, as well with higher death rates (13, 25, 26). Notable amongst these is the mutation in the enzyme dihydrofolate-reductase which may result in resistance to trimethoprim and pyrimethamine. Likewise, 30% of the patients receiving atovaquone as compared to 6% of those not receiving this agent have been shown to develop mutation in the cytochrome-b gene which may lead to atovaquone resistance. Dihydropteroate synthase (DHPS) gene mutations impair the activity of dapsone and sulfamethoxazole. These mutations have been observed in 61-100 % of the patients receiving prophylaxis compared to 11-47% of patients not receiving prophylaxis.
ADJUNCTIVE THERAPY
A high proportion of pneumocystic jirovecii pneumonia cases may develop disease progression despite appropriate antimicrobial treatment. The use of steroids during the first 72 hours reduces morbidity and mortality rates in patients in whom arterial PO2 is below 70 mHg or in whom the Pa2/FiO2 ratio is below 350 (5, 28). Although the optimum steroid dose has not been determined yet, doses of 40-60 mg of prednisone or prednisolone are usually administered every 12 hours. After 5 days, the dose should be reduced to 40 mg/day for 5 additional days and subsequently to 20 mg/day until completion of 21 days of treatment.
PROPHYLAXIS
Prophylaxis against pneumocystic jirovecii pneumonia has proven highly effective in transplant recipients (12). The highest risk of developing pneumocystic jirovecii pneumonia is in the first 6 months after solid organ transplantation, during periods of neutropenia, during receipt of corticosteroid doses over 20 mg/day for >4 weeks, and in transplant patients requiring augmented immunosuppression for allograft rejection.
Primary prophylaxis against pneumocystic jirovecii pneumonia is typically employed for the first 6 to 12 months after transplantation in kidney transplant recipients and indefinitely in heart, intestinal and lung transplant recipients. In liver transplant recipients, prophylaxis is usually continued until at least 1 year after transplantation. In patients with pneumocystic jirovecii pneumonia and in whom a reduction in immunosuppression is not feasible, secondary prophylaxis must be maintained indefinitely.
The drug of choice for pneumocystic jirovecii pneumonia prophylaxis is TMP-SMX. Several dosage regimens have been used and have proven equally efficacious. These include a daily single tablet (80 mg of TMP/400 mg of SMX), a daily double-strength tablet (160 mg of TMP/800 mg of SMX) and a double-strength tablet three days a week (42). It should be noted that TMP-SMX prophylaxis not only prevents P. jirovecii infections but may potentially prevent infections due to a number of opportunistic pathogens such as Toxoplasma gondii, Listeria spp., Isospora belli, Nocardia asteroides and Legionella (29).
The main limitations of TMP-SMX prophylaxis are the development of hematologic toxicity and its possible interference with ganciclovir (increased potential for bone marrow suppression in patients with renal insufficiency), azathioprine (neutropenia and thrombocytopenia) and cyclosporine (mild elevation of serum creatinine levels and immunosuppressive effect decreased) (6, 17, 32, 40, 44). Toxicity usually develop in the first month of employment of prophylaxis and may be masked by the use of high corticosteroid doses for graft rejection. In case of cutaneous allergy or drug related rashes, desensitization may be attempted. In most cases however, toxicity warrants discontinuation of the drug.
In patients with TMP-SMX intolerance, alternative prophylactic options include pentamidine (300 mg of inhaled or intravenously-administered pentamidine every 3-4 weeks) which is usually well-tolerated (22, 35). However, breakthrough pneumocystis has been reported during prophylaxis with this drug, especially if an insufficient number of doses have been administered, in case of increased immunosuppression, or in the setting of CMV infection. The main secondary effects of pentamidine are hypoglycemia when used intravenously, cough and bronchospasm when used as an aerosolized. Additional prophyaxis effective against toxoplasmosis must be used when pentamidine prophylaxis is employed post-transplant.
Dapsone, with and without trimethoprim or pyrimethamine is another alternative against P. jirovecii in transplant recipients (15). Due to its relatively short half-life, dapsone is generally administered in 50-100 mg dosages daily. Like TMP-SMX, dapsone is useful as prophylaxis against toxoplasmosis. G6PD levels should be checked prior to employing dapsone as pneumocystic jirovecii pneumonia prophylaxis and the use of this agent should be deferred in patients with G6PD deficiency.
Atovaquone is another alternative option (23). Besides being well-tolerated in most cases an interference with cyclosporine metabolism has not been reported with this agent. Since prophylaxis failures have been reported in transplant patients when atovaquone is administered in low doses, 1500-2250 mg/day doses are advisable.
Clindamycin plus pyrimethamine or other agents such as primaquine may also be used in transplant recipients, although the failure rates are higher than withwith TMP-SMX and a higher incidence of C. difficile pseudo membranous colitis and anemia (especially in G6DP-deficiency patients) has been reported.
Finally, in liver transplant recipients, weekly pyrimethamine-sulphadoxine combination has proven effective as prophylaxis (41). The role of agents such as quinolones or azithromycin for pneumocystic jirovecii pneumonia prophylaxis remains undefined. Finally, up to date attempts to develop a vaccine have proven unsuccessful.
INFECTION CONTROL MEASURES
Although P. jirovecii is potentially transmissible from person to person via airborne transmission, no evidence exists to support respiratory isolation of transplant recipients with pneumocystic jirovecii pneumonia.
REFERENCES
1. Annaloro C, Della Volpe A, Usardi P, Lambertenghi Deliliers G. Caspofungin treatment of Pneumocystis pneumonia during conditioning for bone marrow transplantation. Eur J Clin Microbiol Infect Dis 2006; 25: 52-54. [PubMed]
2. Alvarez-Martínez MJ, Miró JM, Valls ME, Moreno A, Rivas PV, Solé M, Benito N, Domingo P, Muñoz C, Rivera E, Zar HJ, Wissmann G, Diehl AR, Prolla JC, de Anta MT, Gatell JM, Wilson PE, Meshnick SR; Spanish PCP Working Group. Sensitivity and specificity of nested and real-time PCR for the detection of Pneumocystis jirovecii in clinical specimens. Diagn Microbiol Infect Dis 2006; 56: 153-60. [PubMed]
3. Alvarez F, Bandi V, Stager C, Guntupalli KK. Detection of Pneumocystis carinii in tracheal aspirates of intubated patients using calcofluor-white (Fungi-Fluor) and immunofluorescence antibody (Genetic Systems) stains. Crit Care Med 1997; 25: 948-52. [PubMed]
4. Bandt D, Monecke S. Development and evaluation of a real-time PCR assay for detection of Pneumocystis jirovecii. Transpl Infect Dis 2007; 9: 196-202. [PubMed]
5. Bozzete SA, Sattler FR, Chiu J, Wu AW, Gluckstein D, Kemper C, Bartok A, Niosi J, Abramson I, Coffman J. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 1990; 323: 1451-57. [PubMed]
6. Bradley PP, Warden GD, Maxwell JG, Rothstein G. Neutropenia and thrombocytopenia in renal allograft recipients treated with trimethoprim-sulfamethoxazole. Ann Intern Med 1980;93:560-2. [PubMed]
7. Colby C, McAfee S, Sackstein R, Finkelstein D, Fishman J, Spitzer T.A. Prospective randomized trial comparing the toxicity and safety of atovaquone with trimethropim/sulfamethoxazole as Pnemocystis carinii pneumonia prophylaxis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 1999; 24: 897-902. [PubMed]
8. Cuetara MS, Alhambra A, Chaves F, Moragues MD, Pontón J, del Palacio A. Use of a serum (1->3)-beta-D-glucan assay for diagnosis and follow-up of Pneumocystis jirovecii pneumonia. Clin Infect Dis 2008; 47: 1364-66. [PubMed]
9. Edman JC, Kovacs JA, Masur H, Santi DV, Elwood HG, Sogin ML. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature 1988; 334: 519-22. [PubMed]
10. Fillaux J, Malvy S, Alvarez M, .Accuracy of a routine real-time PCR assay for the diagnosis of Pneumocystis jirovecii pneumonia. J Microbiol Methods 2008; 75: 258-61. [PubMed]
11. Garcia-Gil D, Moreno A, Miro JM, Valls ME, Vilardell J, Rimola A, Grande L, Rvira M, Claramonte J, Soriano E. Pneumocystis carinii pneumonia in the transplant recipient. Enfer Infecc Microbiol Clin 1996; 14: 296-99. [PubMed]
12. Gren H, Paul M, Vidal M, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV inmmunocompromised patients. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD005590. [PubMed]
13. Hauser PM, Sudre P, Nahimana A, Francioli P; Study Group. Prophylaxis failure is associated with a specific Pneumocystis carinii genotype. Clin Infect Dis 2001;33:1080-82. [PubMed]
14. Hof H, Schnülle P. Pneumocystis jirovecii pneumonia in a patient with Wegener´s granulomatosis treated efficiently with caspofungin. Mycoses 2008; 51: 65-67. [PubMed]
15. Hughes WT. Use of dapsone in the prevention and treatment of Pneumocystis jirovecii pneumonia: a review. Clin Infect Dis 1998; 27: 191-204. [PubMed]
16. Hughes WT, Lafon SW, Scott JD, Masur H. Adverse events associated with trimethoprim-sulfamethoxazole and atovaquone during the treatment of AIDS-related Pneumocystis jirovecii pneumonia. J Infect Dis 1995; 171: 1295-1301. [PubMed]
17. Jung D, AbdelHameed MH, Hunter J, Teitelbaum P, Dorr A, Griffy K. The pharmacokinetics and safety profile of oral ganciclovir in combination with trimethoprim in HIV- and CMV- seronegative patients. Br J Clin Pharmacol 1999; 47: 255-59. [PubMed]
18. Keely SP, Stringer JR. Sequences of Pneumocystis carinii hominis strains associated with recurrent pneumonia vary at multiple loci. J Clin Microbiol 1997; 35: 2745-47. [PubMed]
19. Krajicek BJ, Thomas CF Jr, Limper AH. Pneumocystis pneumonia: current concepts in pathogenesis, diagnosis and treatment. Clin Chest Med 2009; 30: 265-78. [PubMed]
20. Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment JAMA 2009; 301: 2578-85. [PubMed]
21. Kroe DM, Kirsch CM, Jensen WA. Diagnostic strategies for Pneumocystis carinii pneumonia. Semin Respir Infect 1997; 12: 70-78. [PubMed]
22. Marras TK, Sanders K, Lipton JH, Messner HA, Conly J, Chan CK. Aerosolized pentamidine prophylaxis for Pneumocystis carinii pneumonia after allogeneic marrow transplantation. Transpl Infect Dis 2002; 4: 66-74. [PubMed]
23. Meyers B, Borrego F, Papanicolau G. Pneumocystis carinii pneumonia prophylaxis with atovaquone in trimethoprim-sulfamethoxazole-intolerant orthotopic liver transplant patients: a preliminary study. Liver Transpl 2001; 7: 750-51. [PubMed]
24. Mu XD, Que CL, He B, Wang GF, Li HC. Caspofungin in salvage treatment of severe pneumocystis pneumonia: case report and literature review. Chin Med J 2009; 122: 996-99. [PubMed]
25. Nahimana A, Rabodonirina M, Bille J, Francioli P, Hauser PM. Mutations of Pneumocystis jirovecii dihydrofolate reductase associated with failure prophylaxis. Antimicrob Agents Chemother. 2004;48:4301-5. [PubMed]
26. Navin TR, Beard DB, Huang L, del Rio C, Lee S, Pieniazek NJ, Carter JL, Le T, Hightower A, Rimland D. Effects of mutations in Pneumocystis carinii dihydropteroate synthase gene on outcome of P. carinii pneumonia in patients with HIV-1: a prospective study. Lancet. 2001;358:545-9. [PubMed]
27. Neff RT, Jindal RM, Yoo DY Hurst FP, Agodoa LY, Abbott KC. Analysis of USRDS: Incidence and risk factors for Pneumocystis jirovecii pneumonia. Transplantation 2009; 88: 135.41. [PubMed]
28. Pareja JG, Garland R, Koziel H. Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest 1998; 113: 1215-24. [PubMed]
29. Podzarnezer D, Salazar A, Jimenez J, Consiglio E, Sandin M, Casanova A, Rufí G, Gudiol F. Intermittent trimethoprim-sulfametoxazole compared with dapsone-pyrimethamine for the simultaneous primary prophylaxis of Pneumocystis jirovecii and toxoplasmosis in patients infected with HIV. Ann Intern Med 1995; 122: 755-61 [PubMed]
30. Powles MA, Liberator P, Anderson J, Karkhanis Y, Dropinski JF, Bouffard FA, Balkovec JM, Fujioka H, Aikawa M, McFadden D, Schmatz D. Efficacy of MK-991 (L-743,872), a semisynthetic Pneumocandin, in murine models of Pneumocystis carinii. Antimicrob Agents Chemother 1998; 42: 1985-89. [PubMed]
31. Procop GW, Haddad S , Quinn J, Wilson ML, Henshaw NG, Reller LB, Artymyshyn RL, Katanik MT, Weinstein MP. Detection of Pneumocystis jirovecii in respiratory specimens by four staining methods. J Clin Microbiol 2004; 42: 3333-35. [PubMed]
32. Ringden O, Myrenfors P, Klintmalm G, Tyden G, Ost L. Nephrotoxicity by co-trimoxazole and cyclosporin in transplanted patients. Lancet 1984;1:1016-7. [PubMed]
33. Ritter ML, Pirofski L. Mycophenolate mofetil: effects on cellular immune subsets infectious complications and antimicrobial activity. Transpl Infect Dis 2009; 11: 290-97. [PubMed]
34. Satter PR, Cowan R, Nielsen DM, Ruskin J. Trimethoprim-sulfametoxazole compared with pentamidine for treatment of Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a prospective non crossover study. Ann Intern Med 1988; 109: 280-87. [PubMed]
35. Saukkonen K, Garland R, Koziel H. Aerosolized pentamidine as alternative primary prophylaxis against Pneumocystis carinii pneumonia in adult hepatic and renal transplant recipients. Chest 1996; 109: 1250-55. [PubMed]
36. Schmatz DM, Romancheck MA, Pittarelli LA, Schwartz RE, Fromtling RA, Nollstadt KH, Vanmiddlesworth FL, Wilson KE, Turner MJ. Treatment of Pneumocystis carinii pneumonia with 1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci USA 1990; 87: 5950-54. [PubMed]
37. Tanaka M, Tanaka K, Fukahori S, Fujimatsu Y, Jojima H, Shiraishi K, Honda J, Oizumi K. Elevation of serum KL-6 levels in patients with hematological malignancies associated with cytomegalovirus or Pneumocystis carinii pneumonia. Hematology 2002; 7: 105-108. [PubMed]
38. Tasaka S, Hasegawa N, Kobayashi S, Yamada W, Nishimura T, Takeuchi T, Ishizaka A. Serum indicators for the diagnosis of Pneumocystis pneumonia. Chest 2007; 131: 1173-80. [PubMed]
39. Thomas CF Jr, Limper AH. Pnemocystis pneumonia. N Engl J Med 2004; 350: 2487-98. [PubMed]
40. Thompson JF, Chalmers DH, Hunnisett AG, Wood RF, Morris PJ. Nephrotoxicity of trimethoprim and cotrimoxazole in renal allograft recipients treated with cyclosporine. Transplantation 1983;36:204-6. [PubMed]
41. Torre-Cisneros J, De la Mata M, Lopez-Cillero P, Sanchez-Guijo P, Miño G, Pera C. Randomized trial of weekly sulfadoxine/pyrimethamine vs. Daily low-dose trimethoprim-sulfamethoxazole for the prophylaxis or Pneumocystis jirovecii pneumonia after liver transplantation. Clin Infect Dis 1999; 29: 771-774. [PubMed]
42. Torre-Cisneros, De la Mata M, Lopez-Cillero P, Sanchez-Guijo, Miño G. Effectiveness of daily low-dose cotrimoxazole prophylaxis for Pneumocystis carinii pneumonia in liver transplantation-an open clinical trial. Transplantation 1996; 62: 1519-21. [PubMed]
43. Utili R, Durante-Mangoni E, Basilico C, Mattei A, Ragone E, Grossi P. Efficacy of caspofungin addition to trimethropim-sulfamethoxazole treatment for severe pneumocystis pneumonia in solid organ transplant recipients. Transplantation 2007; 84: 685-8. [PubMed]
44. Wallwork J, McGregor CG, Wells FC, Cory-Pearce R, English TA. Cyclosporin and intravenous sulphadimidine and trimethoprim therapy. Lancet 1983;1:366-7. [PubMed]
45. Wang Y, Doucette S, Qian Q, Kirby JE. Yield of primary and repeat induced sputum testing for Pneumocystis jirovecii in human immunodeficiency virus-positive and –negative patients. Arch Pathol Lab Med 2007; 131: 1582-84. [PubMed]
Figure 1. CT Scan Showing Ground Glass Infiltrate in a Patient with Pneumocystis jirovecii Pneumonia
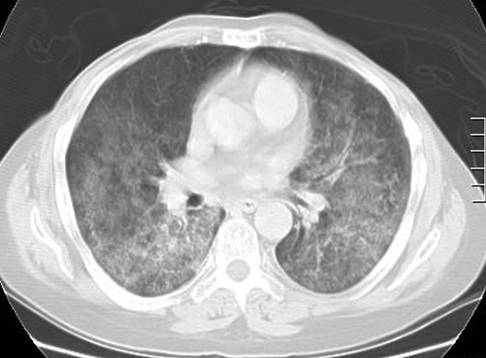
Table 1: Treatment of Pneumocystis jirovecii Disease
| Drug of choice | Dose |
|---|---|
| Trimethropim-sulfamethoxazole (iv/po) | TMP 15-20 mg/Kg/d + SMZ 75-100 mg/Kg/d (divided into 3-4 doses) |
| Alternative drugs | |
| Atovaquone | 750 mg (5 ml solution) tid p.o. |
| Dapsone& (po)+ TMP (po) | 100 mg/d + TMP 5 mg/Kg tid |
| Primaquine& (po) + Clindamycin (iv/po) | 30 mg base qd + 600 iv tid or 300-450 po qid |
| Pyrimethamine (po) + Sulfadiazine (po) | Load 50 mg bid 2 days then 25-50 mg/d Load 75 mg/Kg then 100 mg/Kg/d |
| Trimetrexate (iv) + Folinic acid (po/iv) | 45 mg/m2/d + 80 mg/m2/d |
| For acute illness with pAO2 <70 mmHg add prednisolone 40 mg bid for 5 days, 40 mg for 5 additional days and then 20 mg up to completing 21 treatment days | |
Choukri F, Menotti J, Sarfati C, Lucet JC, Nevez G, Garin YJ, Derouin F, Totet A. Spread of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis pneumonia. Clin Infect Dis. 2010 Aug 1;51(3):259-65.
Walzer PD et al. Early Predictors of Mortality from Pneumocystis jirovecii Pneumonia in HIV-infected Patients: 1985-2006. Clin Infect Dis. 2008 Feb 15;46(4):625-33.
Skelly MJ, et al. S-Adenosylmethionine levels in the Diagnosis of Pneumocystis carinii Pneumonia in Patients with HIV Infection. Clin Infect Dis 2008;46(3):467-471.
Huang L, Morris A, Limper AH, Beck JM; ATS Pneumocystis Workshop Participants. An Official ATS Workshop Summary: Recent advances and future directions in pneumocystis pneumonia (PCP). Proc Am Thorac Soc. 2006;3(8):655-64.
Guided Medline Search FOR
George P, Gingo MR, Morris A. Pneumocystis (carinii) jirovecii
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
Morris A et al. Is There Anything New in Pneumocystis jirovecii Pneumonia? Changes in P. jirovecii Pneumonia Over the Course of the AIDS Epidemic. Clin Infect Dis 2008;46:634-636.
Hawksworth DL. Responsibility in naming pathogens: the case of Pneumocystis jirovecii, the causal agent of pneumocystis pneumonia. Lancet Infect Dis. 2007 Jan;7:3-5; discussion 5.
