Empiric Antibiotic Selection
Authors: Christopher J Grace, MD, FACP, Brad Robinson, MPH, M.D.
Empiricism
Empiricism is the philosophical doctrine that states that all human knowledge comes from experience and theory is based upon observation. It is this philosophy that forms the basis of the modern scientific method. The empiric method of antibiotic selection makes use of this philosophy by using our observations of the patient (history, physical examination and laboratory test results) along with our past clinical experiences and the medical literature to scientifically select antibiotics. Empiric antibiotic selection starts with framing the question (creating the the problem list) that confronts us when caring for a patient, then generating a hypothesis (clinical and microbiological differential diagnosis) to answer the question. Antibiotics can then be rationally selected rather than using blind non-analytic intuition that often leads to the indiscriminant use of antibiotics. What follows is an approach to the empirical selection of antibiotics for the patient with fever or presumed infection. It is an attempt to break the investigative process down into sequential steps. The greater severity of illness and the more complex the clinical picture the greater the effort should be to proceed through this process step by step and as thoroughly as one can. Although it can be accepted that the sooner an antimicrobial is started the better, rarely if ever are infections true medical emergencies requiring instant antimicrobial administration. The time taken to carefully diagnose the patient and prudently select antibiotics will in the long run better serve the patient. It has been repeatedly shown that the indiscriminate and excessive use of antibiotics leads to increased patient toxicities, excessive costs and the emergence of antibiotic induced infections caused by Clostridium difficile, methicillin resistant Staphylococcus aureus (MRSA) and vancomycin resistant Enterococcus (VRE). The sequential steps involve (1) gathering the data, (2) organizing the data into clinical and microbiological differential diagnoses and (3) selecting antibiotics based on the most appropriate diagnosis. What follows is an overview of this diagnostic and therapeutic process. Sections detailing specific clinical syndromes will use this approach to develop differential diagnoses and to select appropriate empiric antibiotics.
Gather The Data
It is axiomatic that the patient should have a thorough history and physical examination. Do not accept what you are told by colleagues regarding the history, physical findings or laboratory information. They may be correct…but they may not be. Confirm or negate what you might have been told. Review the past medical record. Important clues may be there that will help clarify the data being collected. Review the radiographs yourself, preferably with a radiologist. For complex patients, especially those who have been ill for a number of days, have had multiple tests and antibiotic treatments, it is often helpful to organize the data on a flow sheet. See Figure 1. The flow sheet will allow you to see the clinical picture visually and note trends in temperature and laboratory test results that may give you a clue to the diagnosis or response to treatment. It is useful to write in the antibiotics used along the top of the temperature chart. This will help keep track of the antibiotics used and correlate their use with the temperature, white blood cell count and other laboratory abnormalities that you are using for your clinical markers (see below).
Organize The Data
This is a stepwise process to help organize your thoughts, bring order to complex problems and move away from the “pan culture and pan treat” approach. It is tempting to jump to the culture results and prescribe antibiotics based on those results. This simplistic approach however is fraught with difficulties and will lead to over prescribing and inappropriate use of antibiotics. Instead, the selection of antibiotics should proceed through a logical process that involves (a) creating a problem list, (b) developing a differential diagnosis for each problem in that list, (c) developing a microbiologic differential diagnosis specific for the infectious disease diagnoses identified and (d) selecting antibiotics based on the clinical and microbiologic diagnosis. Steps a-d should be worked through sequentially. See Figure 2. The selection of the antibiotic (d) should be the last step and never done prior to thinking out the problem. If steps a-c are thoroughly thought through, then antibiotic selection (d) is straightforward. Often, practitioners will jump from (a) to (d) without working through the problem. This “pan culture / pan treat” approach does a disservice to your patient by skipping the thought process needed for the correct selection of empiric antibiotics.
Develop a Problem List
The proper framing of the problem(s) will set the stage for developing a solid differential diagnosis. This will help bring order to the blizzard of data you have gathered and refine it to a succinct problem statement(s). The problem should be a single word or at most a very short phrase. The problem statement should not be a clinical diagnosis but a succinct statement describing the problem(s) from the history, physical or laboratory data. The problem can then be further assessed in the next step when a differential diagnosis is developed for the problem(s). This will help you avoid jumping to conclusions and missing other diagnoses that may be causing the problem. The less sure you are of the cause of the problems the more they should be separated and not lumped together. The problem list for a patient with fever and an abnormal chest x-ray should list both fever and abnormal chest x-ray. While it may be tempting to jump to the conclusion that the patient has pneumonia, the two problems may or may not be linked and should not be combined into a unifying diagnosis until both problems have been thoroughly thought through. The fever may be due to pneumonia, other non-pulmonary infections, drug reactions or deep venous thrombosis. The abnormal chest x-ray may be caused by pneumonia, atelectasis, congestive heart failure, non-infectious inflammatory changes from vasculitis, malignancy or chronic changes from years gone by. If one jumps to a clinical diagnosis without thinking through the problem list, the differential diagnosis of the problem will be lost and the correct diagnosis may be missed. Listing fever as the sole problem should be avoided since the differential diagnosis is so extensive. If fever is listed in the problem list it is best to link it to some other clue from the history and physical examination or laboratory assessment (rash, pyuria, localized pain etc.).Figure 3 shows the development of the diagnostic process for a patient presenting with two days of fever and cough. The patient was found to have leukocytosis, an abnormal chest x-ray and pyuria. Five problems are listed (fever, cough, abnormal chest x-ray, pyuria and leukocytosis) that may or may not be linked together. If the source of the fever is not clear at this step, it is best to list fever as a problem and link it to one of the other problems when creating the differential diagnosis in the next step. Though leukocytosis often parallels fever as a sign of infection, it is best to list it as a separate problem at this point in time since it may be also be attributable to other causes.
Develop a Clinical Differential Diagnosis
The differential diagnosis of each problem should them be outlined with the most likely diagnosis for each problem listed first. This is where one must “think at the end of ones pencil”. It is often worthwhile, even for the most experienced clinicians, to review medical texts and the literature to be sure that the differential diagnosis is complete. If you are not confident in your differential diagnosis or unsure of how to proceed, then appropriate consultation should be requested. It is worth trying to link all the problems into one unifying diagnosis, a philosophy known as Occam’s razor, but this is not always possible. To carry the current example further, Wegener’s granulomatosis can cause all the problems and may be worth considering. But, the acute onset, lack of nasal symptoms and the relative rarity of the syndrome itself make it an unlikely diagnosis. The old adage, “when you hear hoof beats, think of horses not zebras” , remains true. Figure 3 shows the differential diagnosis for the problems generated in step (a). After careful review of the history and physical examination, laboratory results and the abnormal chest x-ray, problem # 1, cough, was felt to be due to community acquired pneumonia, and not bronchitis or congestive heart failure. The second problem, fever, was most appropriately linked to the first problem, cough and the third problem, abnormal chest x-ray, as part of the pneumonic illness and less likely due to a drug fever, pyelonephritis, Wegener’s granulomatosis or leukemia. Fever could therefore be removed from the problem list. The third problem, abnormal chest x-ray, was most consistent with pneumonia and not atelectasis, congestive heart failure or Wegener’s granulomatosis and could be merged with the first and second problems. The fourth problem, pyuria, was related to asymptomatic bacteruria and not due to pyelonephritis or Wegener’s granulomatosis. Since the pyuria was asymptomatic, it was felt not to be clinically relevant and could also be removed from the problem list. The fifth and last problem, leukocytosis, could be caused by pneumonia, pyelonephritis, leukemia or corticosteroid use. The patient was not taking corticosteroids. The peripheral blood smear showed neutrophilia. The leukocytosis was felt most likely due to infection and linked to pneumonia. The problem list has now been pared down and confined to abnormal chest x-ray and the clinical diagnosis presumed to be community acquired pneumonia. The microbiologic diagnosis of this diagnosis can now be thought through.
Make a Microbiologic Diagnosis
Once a clinical diagnosis has been made, the most likely pathogens causing the infection can be linked to it. In the example of community acquired pneumonia, the most likely pathogens are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma pneumoniae, Chlamydia pneumoniae and viruses such as influenza. Although aerobic Gram negative bacilli and Staphylococcus aureus are possibilities, they are unlikely in this community acquired setting. It is here that you may now use your culture data, if available. It is best to interpret the culture based on the results of the accompanying Gram stain. A Gram stain without inflammatory cells suggests that the bacteria or fungi growing represent colonization or contamination. From Figure 3, it can be seen that the sputum Gram stain revealed white blood cells and gram positive cocci while the sputum culture grew Streptococcus pneumoniae.
It is also at this step that the notorious “backward step” can trip you up. See Figure 2. The “backwards step” is using a culture result to make a clinical diagnosis: For example, assuming that the patient with a positive urine culture has a urinary tract infection. The urine culture result led to the clinical diagnosis instead of the clinical diagnosis guiding appropriate interpretation of the culture. Thus, one went “backwards” from step (c) → (b) instead of “forwards” from (b) → (c). If you find yourself doing a “backwards step”, it is best to stop, add the culture result as a new problem and develop a differential diagnosis for the positive culture result. The differential diagnosis for a positive urine culture would include contamination and colonization in addition to infection. The most frequent “backwards step” is the over attribution of cultures from urine, skin, wound and sputum samples representing infections at these sites.
It is important to keep in mind that a positive culture may not only represent either contamination or colonization, but could also not identify the true pathogen. A patient with a polymicrobic diverticular abscess may only have Escherichia coli isolated from the blood culture while the abscess also harbors anaerobes, streptococci and enterococci. Reacting only to the E. coli isolated from the blood will result in under treatment of the abscess. Certain pathogens including viruses, Mycobacteria, Legionella, Mycoplasma, Francisella and Chlamydia do not grow on routine cultures and will be missed unless cultured on specific media or tissue cultures. Concrete interpretation of the culture may therefore lead to under treatment as well as over treatment. Thus, the culture must always be interpreted in light of the clinical diagnoses.
Select Antibiotics
The selection of antibiotics should be based on the presumed or documented microbiology that in turn had been based on the clinical diagnosis. If positive culture results are available and represent samples with demonstrated inflammation, they can be used to assist in antibiotic selection. Your antibiotic selection also needs to take into account the severity of the patients’ clinical illness (see below) coupled with any recent antibiotic usage and/or hospitalization that may place the patient at risk for resistant aerobic gram negative bacilli, MRSAand VRE. For the patient illustrated in Figure 3 with community acquired pneumonia, it is reasonable to choose a macrolide, a tetracycline, a second or third generation cephalosporin or a fluoroquinolone if the microbiological diagnosis had not been confirmed. If bacterial sensitivities are available to help guide your selection, then the selection of the antibiotic needs to proceed in a stepwise manner as outlined in Table 1. If the listed antibiotics have equivalent efficacy, then one should move onto the next criteria, toxicity. If several antibiotics have the same efficacy and toxicity, then adherence, antibiotic spectrum, risk of developing resistance and costs need to be factored into the decision. This selection process should continue moving from criteria (a) through criteria (f) until a drug is selected. The culture result in Figure 3 revealed a penicillin sensitive S. pneumoniae. It is most reasonable to use penicillin although any number of other antibiotics including cephalosporins and fluoroquinolones would have worked.
Antibiotic Efficacy
The designation “sensitive” (S) means that the organism isolated from the culture will be inhibited by that antibiotic. “Resistant” (R) suggests that the antibiotic will not inhibit the organism’s growth. The S/R system compares the minimal inhibitory concentration (MIC) of the isolated bacterium to blood and tissue levels that can be attained using standard dosing of that specific antibiotic. The S/R system establishes a “breakpoint” for the antibiotic. If the pathogen’s MIC is above that breakpoint, the antibiotic will not achieve blood levels adequate to inhibit the growth of the pathogen and it will be designated resistant. If the pathogen’s MIC is below the breakpoint, it will be designated sensitive. The sensitive category though will not tell you the exact MIC. It only tells you that achievable blood or tissue levels can be attained, using standard dosing of the antibiotic that will inhibit the bacterial growth. A bacterium sensitive to gentamicin will be inhibited by ≤ 4 µg/ml of gentamicin, the breakpoint for the antibiotic. Without seeing the exact MIC though, you would not know if the MIC is 4, 3, 2, 1 or 0.5 µg/ml. One should not compare the MIC of two antibiotics unless the blood and tissue levels of the two drugs are known and are similar. Otherwise you may be comparing “apples and oranges”. For example, ciprofloxacin with a MIC of 0.1 µg/ml for E. coli is not a better antibiotic choice than cephalexin that has an MIC of 1.0µg/ml despite the fact that there is a 10 fold difference in MICs. Ciprofloxacin 500mg orally has an attainable blood level (Cmax or peak plasma concentration) of 2.4 µg/ml. Cephalexin 500mg orally has an attainable peak plasma concentration of 15µg/ml. Both antibiotics have attainable blood and tissue levels well over the MIC of the antibiotic. If they are both sensitive, they should both be effective antibiotics choices. Ciprofloxacin, however, will not work better simply because it has a lower MIC. Generally, the acceptance of the sensitive vs resistant designation is adequate to use for antibiotic selection. The major exception to this generalization is infections involving the central nervous system. Infections in the central nervous system may not be adequately treated by some antibiotics designated sensitive due to antibiotic penetration blockade of some drugs by the blood brain barrier. A bacterium may be sensitive to an antibiotic but be ineffective if the drug does not cross the blood brain barrier.
Toxicity
If two or more antibiotics are both sensitive, then the next selection criteria should be based on the toxicity of the agents. The patient’s allergy history and past drug intolerance should be reviewed. Interaction with other drugs that the patient is taking should also be assessed.
Adherence
Taking multiple doses of an antibiotic daily can be challenging for some patients. If this appears to be an issue for the patient then selection of those agents that can be used once or twice a day may result in better adherence and overall better treatment outcome.
Spectrum
This is the “Why use a hammer when a fly swatter will do the job?” question. It is best to use the narrowest spectrum antibiotic as possible. This may help reduce toxicity, costs and subsequent emergence of bacterial resistance. Thus, from the previous example, it does not make sense from this perspective to use ciprofloxacin, which has activity against Pseudomonas sp., Enterobacter sp. and other resistant gram negative bacilli, to treat an E. coli that is sensitive to narrower spectrum ampicillinor cephradine.
Risk of Resistance
When an antibiotic is administered, it is distributed throughout the body. In addition to affecting the pathogen we are hoping to eradicate, the antibiotic affects the bacteria normally colonizing the mouth, gastrointestinal and genitourinary tract and skin.
Normal bacterial flora all those sites that are sensitive to the selected antibiotic will be eradicated thus leaving only resistant strains of bacteria and yeast. The broader the spectrum of the antibiotic, the greater the destruction of normal endogenous flora. The remaining resistant bacteria may cause problems for the patient both during the antibiotic treatment course (diarrhea, vaginal yeast infections) as well as in the future for your patient. It is best not to use antibiotics of broader spectrum than is needed.
Costs
In this day and age of spiraling health care costs it is prudent to use the least expensive antibiotic which you are able to. Those patients on fixed incomes and limited insurance coverage may not able to afford the newer more expensive antibiotics being marketed.
Severity of Illness
The initial empiric antibiotic selection needs to be made in the context of disease severity. For critically ill patients, there is less willingness to gamble that resistant pathogens may or may not be present. Symptoms and signs that would warrant more aggressive antibiotic empiricism include hypotension, leukopenia with increased band forms, thrombocytopenia, absolute neutrophil counts < 500 cells/mm3, severe immune compromise or a sepsis syndrome with altered mental status, acute renal insufficiency or disseminated intravascular coagulopathy. One needs to carefully assess the “risk benefit ratio” when selecting antibiotics. On one side of the scale is the need to appropriately treat the patient. On the other side of the scale is the concern for excessive toxicities, unnecessary breadth of spectrum and costs incurred by “overdoing” antibiotic coverage. Clinical judgment and experience are needed to properly balance this important decision.
Example (see Figure 4): A 72 year old women is admitted to hospital with fever, flank pain and vomiting. She has a temperature of 39.5º (C), left sided costovertebral angle tenderness and appeared acutely ill. Laboratories include a WBC = 21.5 cells/mm3 with 13% band forms, a platelet count of 125,000 cells/mm3 and a serum creatinine of 1.5 mg%. A urinalysis revealed 10-50 white blood cells/mm3 and positive nitrites. She was diagnosed with pyelonephritis. She has no allergies. She had recently been hospitalized, placing her at risk for harboring resistant pathogens such as Pseudomonas and Enterobacter species in addition to the more common E. coli, Klebsiella and Proteus species. Her degree of toxicity, bandemia, thrombocytopenia, age and previous hospitalizations warrant selection of antibiotics that would inhibit more resistant gram negative bacilli such as P. aeruginosa until cultures became available. The balance in this case leans in the direction of treating her for more resistant pathogens until the cultures are available in 24-48 hours. Intravenous ciprofloxacin was initiated. She clinically improved over the next 72 hours and was ready for transition to oral antibiotics. Table 2 shows the laboratory data concerning the E. coli isolated from her urine and blood. One transitions the patient from intravenous to oral antibiotics by working through the six steps of antibiotic selection as outlined in Table 1.
1. Efficacy: All five drugs will be equally efficacious.
2. Toxicity: Trimethoprim sulfamethoxazole may increase the serum creatinine. Ciprofloxacin can cause central nervous system toxicity.
3. Spectrum: Ciprofloxacin, trimethoprim sulfamethoxazole, cefpodoxime and amoxicillin-clavulanate have a broader spectrum than would be needed to eradicate the E. coli. Cephalexin would be the most appropriate choice as this fulfills the important goal of utilizing an effective antibiotic that has the narrowest spectrum.
4. Adherence: While twice daily ciprofloxacin and cefpodoxime may improve adherence, both drugs are expensive
5. Risk of Resistance. Generally the broader the spectrum antibiotic used, the more the endogenous normal flora of the patient will be disrupted. This may increase risk for colonization with MRSA, VRE and multi-resistant aerobic gram negative bacilli in addition to placing the patient at risk for Clostridium difficile colitis. Again, cephalexin would be most appropriate to fulfill this criterion.
6. Costs: All things being equal, the least expensive drug should be used. Since trimethoprim sulfamethoxazole is relatively contraindicated, cephalexin is a reasonable choice.
Thus in this example, it would be reasonable to change the patient over to oral cephalexin from the initial empiric antibiotic choice of intravenous ciprofloxacin. Using oral ciprofloxacin just because she responded to the initial intravenous ciprofloxacin is not sound reasoning. If adherence is a major issue the other twice daily drugs could be used though at greater potential toxicity and costs.
Choose Clinical Markers
Once a clinical diagnosis has been made and antibiotics selected, parameters should be chosen to judge the efficacy of the empiricism. Such clinical markers may include improvement in the illness defining signs and symptoms, a normalizing temperature curve, resolution of white blood count cell count abnormalities and enhancement of the patient’s general sense of well being and appetite. Monitoring the trends of the markers over time is more important than a single abnormality at any one point in time. As shown in Figure 4, a temperature of 38.7º C on day 3 of antibiotic therapy does not mean the patient is failing the antibiotic choice. The clinical examination, temperature and leukocytosis are all trending in the right direction. Despite the desire of both patients and providers for immediate improvement upon initiation of antibiotic therapy, it often takes 48-96 hours before initial signs of improvement are seen. Therefore, the physician must be patient and allow time for the antibiotic(s) to work. The physician must always “Be of Stout Heart”. That is, don’t panic and start switching antibiotics around if the patient does not respond as quickly as you, or the patient, had hoped. Instead, observe, reassess and re-balance the risks on a daily basis. If any of the chosen markers show improvement then most likely the antibiotic is working. If reliable cultures return, the empirically chosen antibiotics can be modified following the selection criteria outlined in Table 1.
Summary
When selecting empiric antibiotics
o Perform a thorough history and physical examination and review of the laboratory data
o Make a problem list
o Develop a clinical differential diagnosis for the problems in the list
o Develop a microbiological differential diagnosis for infections defined from the clinical diagnosis.
o Select antibiotics based on the microbiologic assessment. Prioritize efficacy, reduced toxicities, adherence, limited spectrum and lesser costs.
• If all things are equal, the antibiotic with the narrowest spectrum, least toxicities and cost should be selected.
• Be aware of making “backwards steps”. Do not use culture results to define a clinical diagnosis.
• Do not be concrete when interpreting culture results, but instead interpret the culture in the light of the clinical diagnosis.
• “Be of stout heart”. Give the antibiotics time to work. Carefully follow the clinical markers over 48-96 hours while judging antibiotic efficacy.
Tables
Figure 1: Example of flow diagram used for data collection and assessment.
The columns represent separate days. The example represents only seven days. The actual flow sheet used in our practice has fourteen days. The top section of the flow is a temperature grid. The rows along the left side are events or data that can be trended chronologically along with the temperature. Antibiotic(s) used are written across the top of the temperature grid.
WBC-white blood cells, POLY/Band=percent polymorphonuclear WBC and band forms of WBC, Lymph/Mono/EO=percent lymphocytes, mononuclear and eosinophil cells, Hct/Plt-hematocrit and platelet count, BUN/Creat=blood urea nitrogen and serum creatinine, ESR/CRP-erythrocyte sedimentation rate and C-reactive protein, AST/ALT-serum aspartate aminotransferase/serum glutamic-pyruvic transminase, LDH/ALK PHOS=lactate dehydrogenase and alkaline phosphatase, CONG/UN BILI=conjugated and unconjugated bilirubing. LEVEL=drug levels being followed. Blank rows are for other laboratory tests that may be followed.
Figure 2: Four sequential steps (a-d) for the diagnosis and treatment of infections.
The solid line represents the appropriate sequence to follow in the diagnosis and treatment of an infectious disease. The dashed lines are pathways that should be avoided. The “pan culture / pan treat” approach eliminates the thinking process and will lead to the indiscriminate use of unnecessarily broad spectrum antibiotics. Using the culture to establish the clinical diagnosis is a “backwards step” since it moves the diagnosis process upstream instead of downstream. Selection of antibiotics should be the final step and only given thought once both a clinical and microbiologic diagnosis has been given serious thought and properly outlined.
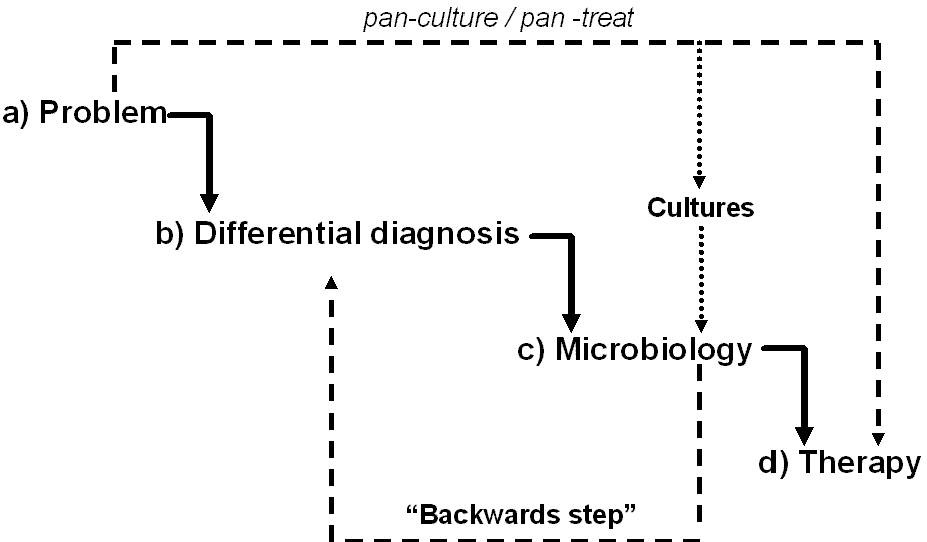
Figure 3: Clinical assessment of the patient with fever, abnormal chest x-ray, pyuria and leukocytosis.
From the history, physical examination and laboratory data, four problems (a) are identified. Each problem has a clinical differential diagnosis (b). Based on clinical judgment, several of the listed diagnoses can be eliminate (NO). Once a clinical diagnosis is established, the microbiology (c) can be considered based on the medical literature. Culture results can be used to better define the microbiologic diagnosis. Selection of antibiotics (d) is the last step and should be based on the clinical diagnosis and microbiology.
CXR = chest x-ray, WBC= white blood cells, CHF = congestive heart failure, GPC = Gram positive cocci, (S) = sensitive
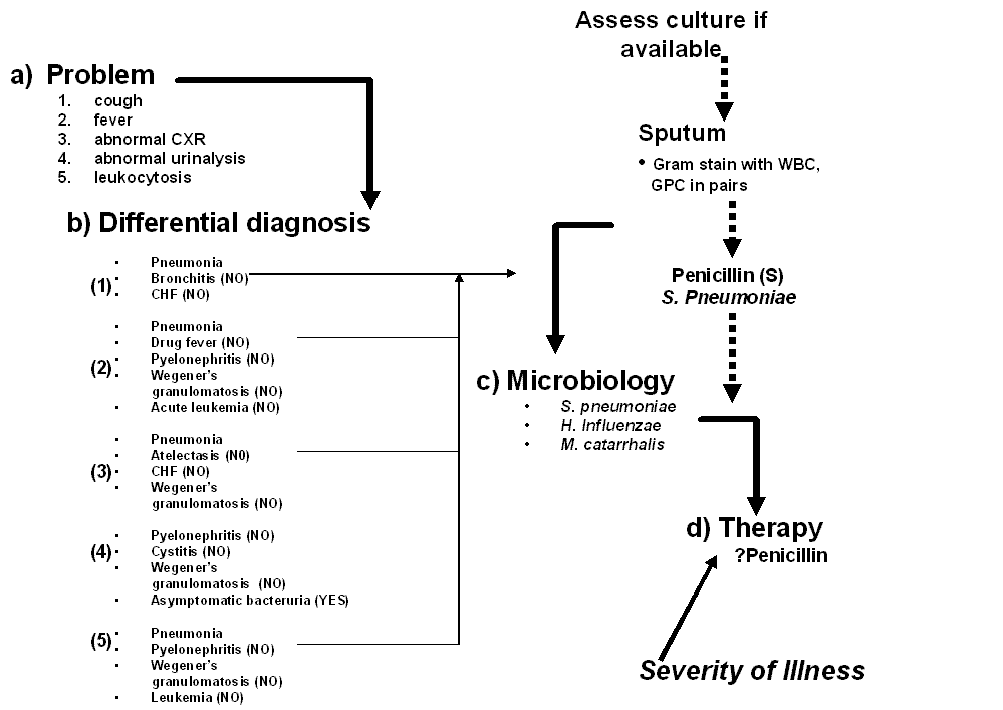
Figure 4: Flow sheet illustration of a patient with bacteremic pyelonephritis.
Ciprofloxacin is empirically selected as the initial antibiotic. See text. Over the next 72 hours, the patient’s temperature, WBC and sense of well being improve. The blood and urine cultures drawn upon admission grow E. coli after 48 hours. In changing from intravenous to oral antibiotics, the spectrum is appropriately narrowed to cephalexin to complete a fourteen day course.
CVAT= costovertebral angle tenderness, CXR = chest x-ray, BC = blood culture, UC = urine culture, WBC = white blood cell count, IV = intravenous, PO = per os or oral.
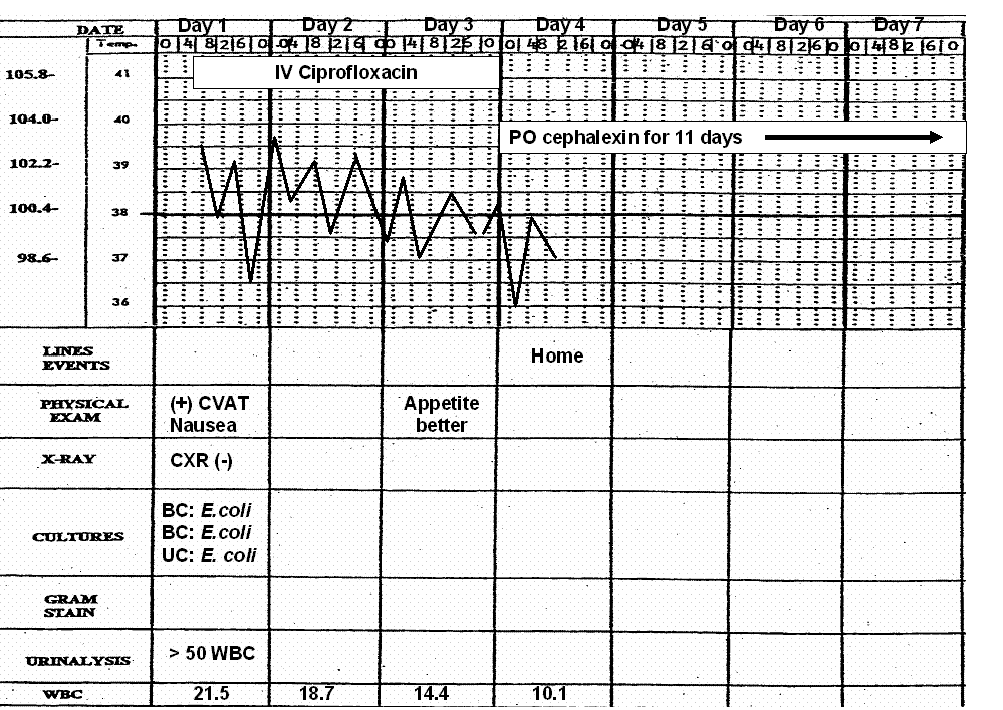
Table 1: Issues to be addressed during antibiotic selection
a. Antibiotic efficacy
b. Toxicity
c. Spectrum
d. Adherence
e. Risk of resistance
f. Costs
Table 2. Selection of oral antibiotics in a patient recovering from a bacteremic pyelonephritis caused by E. coli.
Antibiotic |
S/R |
Dose |
MIC (µg/ml) |
Peak Serum level (µg /mg) |
Cost for 14 days5 |
Comment |
|---|---|---|---|---|---|---|
Cephalexin1 |
S |
500 mg qid |
1.0 |
15 |
$22 |
Active Well tolerated Inexpensive |
Trimethoprim-sulfamethoxazole |
S |
1DS3 q12h |
1.04 |
______ |
$9 |
Active Inexpensive Broad spectrum not needed May increase serum creatinine |
Ciprofloxacin |
S |
500 mg 12h |
0.1 |
2.4 |
$122 |
Active Expensive Broad spectrum andPseudomonasactivity not needed |
Cefpodoxime2 |
S |
400 mg q12h |
0.5 |
4.5 |
$158 |
Active Expensive Broad spectrum not needed |
Amoxicillin-clavulanate |
S |
875mg/125mg q12h |
1.0 |
12 |
$165 |
Active Expensive Broad spectrum not needed May cause diarrhea |
DS = double strength, CNS=central nervous system
1first generation cephalosporin
2second generation cephalosporin
3trimethoprim 160 mg/sulfamethoxazole 800 mg
4MIC of trimethoprim with sulfamethoxazole at a 1:20 ratio
5www.drugstore.com

