Lymphatic filariasis: Wucheria bancrofti and Brugia species
Authors: Pascal Magnussen, M.D., DTM&H
Parasitology
Lymphatic filariasis is caused by the filarial nematodes of the species Wuchereria bancrofti [Life Cycle], Brugia malayi [Life Cycle] and Brugia timori that are transmitted by the bite of a number of mosquito species such as Anopheles, Culex and Mansonia and Ochlerotatus (5). When biting infective third stage larvae break out of the mosquito proboscis and enter the skin through the puncture wound. The parasite then develops over a period of 6-12 months in the human host. Adult filarial worms reside in the afferent lymphatics close to draining lymph glands. The female adult worms of Wuchereria bancrofti measures 6.5 mm x 0.2-2.8 mm while adult male worms measure 4 mm x 0.1 mm) and estimates of the lifespan of adult worms vary from 8-10 years (23, 54). Adult worms of B. malayi are practically identical with W. bancroftiwhile adult worms of B. timori may be distinguished from the two other species based on differences in the cuticulumm and number of an arrangement of spicules. Adult filarial worms are the cause of pathology. Female worms produce thousands of microfilariae (6-8 μm x 200-300 μm) daily which circulate in the blood. Microfilariae of W. bancrofti and B. malayi show nocturnal periodicity (23) in most endemic areas except form the Western Pacific and New Caledonia where microfilariae are either non-periodic or semi-periodic. Circulating microfilariae cause pathology in special conditions such as tropical pulmonary eosinophilia (24).
Epidemiology
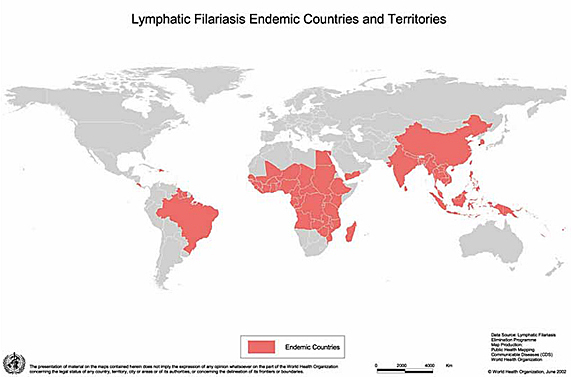
In 2007 it was estimated that 120 million people were infected and the disease is endemic in more than 80 countries and territories (Map). On a global scale, lymphatic filariasis is thought to be the second leading cause of long term/permanent disability. An estimated 40 million people have clinical disease (21). Characteristic patterns of disease, circulating antigenaemia and microfilaraemia are found in endemic populations (29, 30, 49, 51). Usually, microfilaraemia appears in children approx 5 years of age, rising with age and level off around the age of 30 years. The prevalence rarely exceeds 40-50% in any age group. The prevalence of specific circulating antigens is higher than for microfilariae in all age groups and has been found in children < two years of age in the absence of microfialraemia. Sub-clinical pathology with dilatation and dysfunction of lymphatics in young children infected with both W. bancrofti and B. malayi has been registered using lymphoscintigraphy and Doppler ultrasonography (15, 46, 47). Clinical disease develops around the onset of puberty with recurrent acute adenolymphangitis attacks. Hydrocele appears at the same age as well as early stages of lymphoedema. The prevalence of clinical disease rises steadily with age and in areas with high transmission the majority of older males may suffer from hydrocele.
Exposure to intense transmission over many years is necessary for the infection to establish itself in the host and visitors to endemic areas therefore rarely acquire an infection with microfilaraemia.
The epidemiology of W. bancrofti and B. malayi infections varies in different geographical settings (23). Differences in vectorial capacity and density are major factors influencing prevalence and intensity of infection and clinical manifestations. There are also inherent differences in the parasite. Some strains of W. bancrofti and B. malayi show nocturnal periodicity of the microfilariae (only circulating in the blood at night time), being adapted to transmission by night biting Anopheles and Culex mosquitoes. In the South Pacific Islands a diurnal subperiodic form of W. bancrofti adapted to day biting Aedesmosquitoes is found. The nocturnal sub-periodic form of B. malayi is transmitted by Mansonia mosquitoes while B. timori has nocturnal periodicity and is only transmitted by A. barbirostris.
Map of lymphatic filariasis-endemic countries and territories, 2004.
Clinical Manifestations
Lymphatic filariasis is characterized by a wide spectrum of clinical manifestations. At one end of the spectrum we find a group of people with microfilariae in the blood but without any symptoms. Some of these persons will remain asymptomatic for many years or even for the rest of their lives. However, when using diagnostic methods such as Doppler ultrasonography and lymphoscintigraphy, substantial sub-clinical pathology may be found (lymphangiectasia and lymph transport dysfunction) in these patients (Figure 1) (1, 16, 32). Recently the same type of subclinical pathology has been found in amicrofilaraemic but circulating antibody positive young children (15, 46, 47).
Acute manifestations of lymphatic filariasis are characterized by signs and symptoms of adenolymphangitis, presenting with fever swollen, tender regional lymph glands and inflammation of afferent lymph vessels. Patients suffer repeated episodes including systemic symptoms like fever and general malaise. Localized enlargement of lymph nodes draining the affected body parts appears. The lymph nodes are initially tender, but become painful with localized swelling and increased warmth. Abscesses may form and rupture with pus draining. The adenolymphangitis attack is self-limiting and last for approx. 4-8 days. In males acute funiculitis or epididymo-orchitis may occur. Adenolymphangitis may be uni- or bi-lateral and also occur in patients with chronic manifestations (8, 18).
Hydrocele is the most common chronic manifestation of lymphatic filariasis. The onset might be silent or preceded by recurrent attacks of adenolymphangitis with funiculitis or epididymo-orchitis. Initially the size of the hydrocele fluctuates and small hydroceles may regress completely (3). With ultrasonography sub-clinical hydroceles are commonly found (52). However, over time there is progressive enlargement of the hydrocele leading to sometimes very large hydroceles and elephantiasis of the scrotum (Figure 2, Figure 3).
Lymphoedema progressing to elephantiasis most commonly affect the legs. However, arms, breast scrotum, penis and vulva may be affected. Initially edema following adenolymphangitis attacks is reversible, but over the years the edemas consolidate with thickening of the skin and loss of elasticity. Lymhoedema may occur in childhood. As the condition progresses elephantiasis develops with dermatosclerosis and papilomatosis (4, 36) (Figure 4 and Figure 5). Secondary bacterial and fungal infections are common at this stage and may lead to further progression of elephantiasis (33, 34) (Figure 6).
In late stage infections patients are often amicrofilaraemic and progression of elephantiaisis is considered to be mainly related to acute exacerbations of localized infections with especially streptococcal and staphylococcal infections (cellulites). This condition is commonly referred to as acute dermatolymphangitis.
Other Clinical Manifestations of Lymphatic Filariasis
A number of clinical conditions of rarer appearance are associated lymphatic filariasis. Chyluria, the presence of chyle in the urine follows rupture of dilated lymphatics into the kidney pelvis or ureteres or bladder. The urine becomes milky in appearance often with blood. This condition can lead to weight loss, hypo-proteinaemia and anaemia (53) (Figure 7).
Tropical pulmonary eosinophilia is due to immunological hyper-responsiveness to microfilariae in the lungs (24, 40). Microfilariae are absent from the peripheral blood, but can be found in lung tissues (7). Main symptoms are “asthmatic” paroxysms and extreme levels of eosinophilia and antifilarial antibodies. Lung function is impaired showing a mixed pattern of restrictive and obstructive changes. Tropical pulmonary eosinophilia may progress to interstitial fibrosis and permanent lung function reduction.
Species and Geographical Variations in Clinical Manifestations
In brugian and timorian filariasis hydrocele are less common and lymphoedma and elephantiasis is often confined to below the knees. Tropical pulmonary eosinophilia and chyluria is more commonly found in south and south East Asia than on the African South American continents.
Laboratory Diagnosis
Although some clinical symptoms (hydrocele, lymhoedema) are pathognomonic in endemic areas the diagnosis should be confirmed by laboratory methods.
Diagnosis of infection with lymphatic filariasis was for most of the 20th century dependent on demonstration of microfilariae (mf) in the peripheral blood (60). However blood samples from patients with clinical (acute or chronic) manifestations are often negative. There is no association between intensity of infection and severity of disease. A blood sample should be obtained at the time of the day when peak concentration of microfilariae is expected (e.g. for nocturnal periodic species, between 21.00 and 03.00 hours). Many different techniques have been used, however the counting chamber techniques is fast, cheap and quantitative (26). 100 μL of capillary blood is added to a tube with 0,9ml of 3% acetic acid. Using low power the microfilariae are counted in the counting chamber. Membrane filtration techniques offer high sensitivity but are expensive (monofilament filters). In areas where more than one species co-exist staining techniques (e.g. Giemsa or haematoxylin) are needed to differentiate different microfilariae species.
The diethylcarbamazine provocative day test can be used to provoke W. bancrofti and B. malayi to enter the peripheral blood during day time. 30-60 min after a single dose of DEC 2 mg/kg is the optimal time to examine a blood sample (27). The method is as sensitive as the examination of night blood for W. bancrofti. Because of the risk of severe Mazzoti reaction, the test is contraindicated in areas endemic for onchocerciasis.
Immunological and PCR based diagnosis are increasingly used. Specific IgG4 detecting immunodiagnostic tests are of particular value in brugian filariasis (20, 42). Sensitive and specific tests for detection of circulating filarial antigens (CFA) are now available for bancroftian filariasis. Two test principles are in use: an ELISA based test for detection of circulating filarial antigens in serum or plasma and an immunochromatographic card test that in few minutes gives a positive/negative answer (work with capillary blood) (45, 50). An improved point of care card test for CFA is now available. PCRassays for detection of microfilariae have been developed for both W. bancrofti and B. malayi. However, these tests are not more sensitive than microscopical examination of blood (6, 14, 44).
Ultrasonography can be used to detect adult live W. bancrofti parasites in dilated lymphatics in the scrotal area (filarial dance sign) and in breast tissues (1, 9, 10). The locations of adult live B. malayi parasites are not suitable for ultrasonographic detection.
Pathogenesis
Pathology in lymphatic filariasis is mainly caused by the adult worms, except for tropical pulmonary eosinophilia, which is caused by an intense inflammatory reaction to the microfilariae.
Adult worms of W. bancrofti cause local lymphangiectasia of the lymphatics (11) by an unknown mechanism that could involve secretory products from the parasite, increased resistance to flow, or the vigorous movements of the worm (58). Although sometimes eliciting no immune reaction at all, adult parasites can also provoke a local inflammatory response resulting in fibrosis (22), with reduced function of lymphatics or blockage of lymph flow. Abnormal lymphatic flow resulting from lymphatic damage is common in individuals from communities endemic for lymphatic filariasis, even when they have no clinical signs of lymphatic dysfunction (16, 17).
The pathogenesis of adenolymphangitis is not fully understood, but probably involves some of the following: bacterial infections (35); infective larvae (59); dead or dying adult parasites (8) or physical activity (43). The relative significance of each of these mechanisms has not been determined and may in fact vary in different epidemiological situations, different age groups, with parasite species or disease status.
The pathogenesis of hydrocele is associated with parasite induced lymphatic dysfunction of the spermatic cord leading to fluid accumulation in the tunica vaginalis. Lymphangiectasia in the spermatic cord has been detected in large proportions of the male population even without clinical hydrocele (8).
The exact pathogenesis of filarial lymphoedema remains unknown but involves adult parasite induced lymphatic dysfunction (dilatation) as well as fibrosis and obliteration of lymphatics, leading to increased tissue pressure and accumulation of protein rich fluid. Repeated adenolymphangitis episodes and bacterial super-infections most probably also play an important role for progression of filarial lymphoedema and elephantiasis (41). Progressive dermatosclerosis and papilomatosis is seen in the skin of the affected region (Figure 8a, Figure 8b).
The majority of individuals in endemic areas show immunological responses to filarial antigens. Microfilariae positive individuals have a preponderant T-Helper (Th) cell type 2 response while individuals with chronic manifestations tend to demonstrate a Th1 type response (25). Protective immunity has not been proved. Patients with tropical pulmonary eosinophilia are immunologically hyper-responsive with high levels of specific IgG and IgE antibodies (40).
SUSCEPTIBILITY IN VITRO AND IN VIVO
Although microfilariae and adult parasites of W. bancrofti and B. malayi can be maintained in culture media (48) they are not susceptible to any of the drugs used against filariae in vitro. Animal models have been developed although none of them accurately imitates the immunology and the pathology of filarial infection in humans. These include rodents (gerbils), feline, canine, ferret and primate models. Most animal models systems have been developed with B. malayi or B. pahangi. For W. bancrofti the only reported model is with the silverleaf monkey (23).
ANTIPARASITIC THERAPY
Drug of Choice
Currently there are three drugs used for treatment and control of lymphatic filariasis; diethylcarbamazine (DEC) (Hetrazan®), ivermectin (Mectizan®, Stromectol®) and albendazole (Zentel®) used either alone or in combinations (28). To date no drugs have been developed with a specific curative macrofilaricidal effect. Treatment therefore relies on microfilaricidal agents with the aim of reducing the number blood circulating microfilaria to a level where transmission can be interrupted. There is some evidence that DEC in varying dosages have limited macrofilaricidal effects based on ultrasonographic and histological studies (13, 31). Using ultrasonography no effect on adult parasites of W. bancrofti was found after treatment with ivermectin (11). Neither ivermectin nor albendazole are registered for treatment but are exclusively used for control of lymphatic filariasis (see below).
DEC is a piperazine derivative introduced in 1947 and is effective against microfilariae of W. bancrofti, B. malayi and B. timori. DEC has no direct lethal effect on microfilariae, but apparently modifies them so they are attacked and removed by the host immune system. DEC is administered orally and the recommended dose is 6 mg/kg daily, given in three divided doses for a total of 12 days. The microfilariae load decreases rapidly after treatment (>90%) and remain low for several months before it starts to increase again. Clearance of circulating filarial antigen is variable probably because only few adult parasites are killed (12). DEC has no effect on already established lymphatic damage or chronic manifestations, but repeated treatments reduce the number of adenolymphangitis episodes and halt development of chronic manifestations (28). Due to this long term effects of treatment, indications for treatment are wide and asymptomatic microfilaraemic or antigenaemic patients should be given treatment. DEC has a curative effect on tropical pulmonary eosinophilia. For mass treatment a single dose DEC of 6mg/kg given annually is used (see below).
Drug reactions are of two types: systemic and local. Systemic reactions may start within a few to 48 hours after commencing treatment and last 1-3 days. They include fever, malaise, headache, muscle pains and Haematuria. Local reactions usually begin 2-4 days after commencing treatment and may include localized pain and inflammation, adenitis and retrograde lymphangitis. Acute transient lymphoedema and hydrocele may be seen (23). Localized reactions are more severe in B. malayi infections than in W. bancrofti infections. DEC is contraindicated in persons co-infected with Onchocerca volvulus due to severe adverse reactions and even symptom progression. DEC is not recommended during pregnancy, unless there is strong indication for use (which rarely occurs).
Ivermectin is a macrocyclic lactone of the avermectin group of compounds. It is highly effective in clearing microfilariae of W. bancrofti and B. malayi (28). It can be given as a single oral dose of up to 400 μg/kg, although a dose of 150 μg/kg is being used for mass treatment (see below). Drug reactions are of the same type as for DEC but generally less pronounced. Ivermectin is contraindicated in pregnant women and children.
Albendazole is a benzimidazole derivative that is highly effective against intestinal nematodes. Only recently has it been shown to have an effect against microfilariae of Wuchereria and Brugia (19). The effect of albendazole alone is not better than either DEC or ivermectin alone, but albendazole seems to work synergistically with DEC or ivermectin leading to a sustained longer lasting effect (> 12 months) on microfilariae (35) of such combinations. Albendazole can be used in pregnancy, but it is not a relevant option for treatment of microfilaraemia.
Over the last 15-20 years research has been undertaken regarding endosymbiont bacteria (Wolbachia) of adult filarial worms and microfilariae. These endosymbionts can be eliminated by tetracyclines (doxycycline) and as they are important for the metabolism ot the adult worm and microfilariae this may become a new treatment avenue (55, 56). In one study patients were treated with 200 mg doxycycline daily for 8 weeks and this resulted in a 87% clearance of microfilariae and reduction in CFA indicated a reduction in live adult worms (57). However such long treatment schedules with a broad spectrum antibiotic would not be appropriate for large scale use due to problems with adherence, adverse ractions and antibiotic resistance development in bacteria.
Special Situations
Large Scale Treatments For Disease Control
Since the discovery of long term suppressive effects of DEC, ivermectin and combinations of these drugs with albendazole several countries have launched national control programmes. These are supported by the Mectizan Donation Programme (Merck & Co. Inc) and GlaxoSmithKline albendazole Donation Programme who donates ivermectin and albendazole free of charge to the Global Programme to Eliminate Lymphatic Filariasis GPELF). The GPELF was started in 2000.These programmes are based on a single dose of ivermectin (150 μg/kg ) and albendazole (400 mg) given once yearly to whole populations (except pregnant women and children < 3 years of age). In areas where O. volvulus and Loa Loa do not occur an alternative regimen consisting of a single dose of DEC (6 mg/kg) combined with albendazole (400 mg) once yearly can be used. Treatments have to be repeated yearly for a minimum of 5 years (to ensure natural deaths of adult worms). Details of these programmes can be found in a number of reports and reviews providing information on strategies, progress and challenges (21, 37, 38) and on the internet: www.filariasis.org. According to WHO, %3 out of 72 filariasis endemic countries have initiated MDA as part of Integrated Control of Neglected Tropical diseases. A few countries have stopped MDA and are in th surveillance phase towards elimination of the parasites (61).
Co-infections with Onchocerca volvulus and Loa Loa
In areas where infections with O. volvulus and Loa Loa occur together with lymphatic filariasis it is contraindicated to use DEC, due to severe adverse reactions induced in patients with these two infections.
AIDS
There are actually no data available yet neither on the course of disease or effects of treatment in patients with HIV or other immunodeficiency conditions. A study to elucidate whether immunoreactions in filariasis patients differ according to HIV status and possible immunomodulatory effects of DEC, treatment is ongoing in Northern Tanzania.
Alternative Therapy
There is no alternative therapy available.
ADJUNCTIVE THERAPY
Hydrocelectomy is widely used and different types of reconstructive surgery have been applied to lympoedema patients. During adenolymphangitis attacks with or without abscess formation antibiotics can be used as supportive therapy. Complex decongestive physiotherapy combined with rigoristic hygienic measures has been shown to reduce lymphoedema and prevent recurrent adenolymphangitis attacks (2).
ENDPOINTS FOR MONITORING THERAPY
As there is no antimicrobial agent available that kills adult filarial worms effectively microfilaraemia is used to monitor effect of treatment. Reduction in number of acute adenolymphangitis attacks and regression of early hydroceles and lymphoedema can also be used to monitor treatment.
VACCINES
There is no vaccine currently available for prevention or treatment of lymphatic filariasis.
PREVENTION OR INFECTION CONTROL MEASURES
The only way to prevent infection is to reduce exposure to infected mosquitoes. Control measures have been dealt with above under large scale treatments.
REFERENCES
1. Amaral F, Dreyer, G, Figueredo-Silva J, Noroes J, Cavalcnati A, Samico, SC, Santos A, Couthino A. Live adult worms detected by ultrasonography in human bancroftian filariasis. Am J Trop Med Hyg 1994; 50: 753-757. [PubMed]
2. Bernhard L, Bernhard P, Magnussen P. Management of patients with lymphoedema caused by filariasis in North-eastern Tanzania: alternative approaches. Physiotherapy 2003; 89: 743-749.
3. Bernhard P, Magnussen P Lemnge M. A randomized, double-blind placebo-controlled study with diethylcarbamazine for the treatment of hydrocele in an area of Tanzania endemic for lymphatic filariasis. Trans R Soc Trop Med Hyg 2001; 95: 534-536. [PubMed]
4. Burri H, Loutan L, Kumaraswami V, Vijaysekaran V. Skin changes in chronic lymphatic filariasis. Trans R Soc Trop Med Hyg 1996; 90: 671-674. [PubMed]
5. Defining the roles of vector control and xeno-monitoring in the Global Programme to Eliminate Lymphatic Filariasis 2002. World Health Organization, Geneva, WHO/CDS/CPE/PVC/2002.
6. Dissanayke S, Rocha A, Noroes J, Medeiros Z, Dreyer G, Piessens W. Evaluation of PCR-based methods for the diagnosis of infection in bancroftian filariasis. Trans Roy Soc Trop Med Hyg 2000; 94: 526-530. [PubMed]
7. Dreyer G, Dreyer P, Piessens WF. Extralymphatic disease due to bancroftian filariasis. Braz J Med Biol Res 1999; 32: 1467-1472. [PubMed]
8. Dreyer G, Medeiros Z, Netto MJ, Leal NC, Castro LG, Piessens F. Acute attacks in the extremities of persons living in an area endemic for bancroftian filariasis. Trans R Soc Trop Med Hyg 1999; 93: 413-417. [PubMed]
9. Dreyer G, Santos A, Noroes J, Amaral F, Addiss D. Ultrasonographic detection of living adult Wuchereria bancrofti using a 3.5 MHz transducer. Am J Trop Med Hyg 1998; 59: 399-403. [PubMed]
10. Dreyer G, Brandao CA, Amaral F, Medeiros Z, Addiss D. Detection of living adult Wuchereria bancrofti in the female breast. Mem Inst Oswaldo Cruz 1996; 91: 95-96. [PubMed]
11. Dreyer G, Addiss D, Noroes J, Amaral F, Rocha A, Coutinho. Ultrasonographic assessment of the adulticidal efficacy of repeat high-dose ivermectin in bancroftian filariasis. Trop Med Int Health 1996; 1: 427-432. [PubMed]
12. Eberhard AL, Hightower AW, Addiss D, Lammie PJ. Clearance of Wuchereria bancrofti antigen after treatment with diethylcarbamazine or ivermectin. Am J Trop Med Hyg 1997;57:483-486. [PubMed]
13. Figueredo S, Jungmann P, Noroes J, Piessens WF, Coutinho A, Brito C, Rocha A, Dreyer G. Histological evidence for adulticidal effect of low doses of diethylcarbamazine in bancroftian filariasis. Trans Roy Soc Trop med Hyg 1996;90: 192-194. [PubMed]
14. Fischer P, Supali T, Wibowo H, Bonow I, Williams SA. Detection of NA of nocturnally periodic Brugia malayi in night and day blood samples by polymerase chain reaction-ELISA-based methods using an internal control DNA. Am J Trop Med Hyg 2000; 62: 291-296. [PubMed]
15. Fox LM, Furness BW, Haser J, Brissau JM, Charles JL, Wilson SF, Addiss DG, Lammie PJ, Beach MJ. Ultrasonographic examination of Haitian children with lymphatic filariasis: a longitudinal assessment in the context of antifilarial drug treatment. Am J trop Med Hyg 2005; 72 (5); 642-648 [PubMed]
16. Freedman DO, Filho PJA, Besh S, Silva MCM, Braga C, Maciel A. Lymphoscintigraphic analysis of lymphatic abnormalities in symptomatic and asymptomatic human filariasis. J Infect Dis 1994; 70: 927-933.[PubMed]
17. Freedman DO, Filho PJA, Besh S, silva MC, Braga C, Maciel A, Furtado AF. Abnormal lymphatic function in presymptomatic bancroftian filariasis. J Infect Dis 1995; 171, 997-1001.[PubMed]
18. Gasarasi DB, Premji, ZG, Mujinja PGM, Mpembeni R. Acute adenolymphangitis due to bancroftian filariasis in Rufiji district, south east Tanzania. Acta Trop 2000; 75: 19-28. [PubMed]
19. Horton J. Albendazole: a broad spectrum anthelminthic for the treatment of individuals and populations. Curr Opin Infect Dis 2002; 15: 599-608. [PubMed]
20. Haarbrink M, Terhell A, Abadi K, Beers S, Asri M, Yazdanbakhsh M. IgG4 antibody assay in the detection of filariasis. Lancet 1995;346: 853-854. [PubMed]
21. Global Programme to eliminate Lymphatic Filariasis. Annual Report 2001. World Health Organization, Geneva, WHO/CDS/CPE/CEE/2002.28.
22. Jungmann P, Figueredo SJ, Dreyer G. Bancroftian lymphadenopathy: a histolopathological study of fifty-eight cases from northeastern Brazil. Am J Trop Med Hyg 1991; 45: 325-331. [PubMed]
23. Lymphatic filariasis 2000. Editor Nutman, T. B. Imperial College Press, London.
24. Magnussen P, Makunde WH, Simonsen PE, Meyrowitsch DW, Jakubowski K. Chronic pulmonary disorders in lymphatic endemic villages in Tanga region and Tanga town, Tanzania: Is tropical pulmonary eosinophilia an underlying cause? Trans Roy Soc Trop Med Hyg 1995;89:406-409. [PubMed]
25. Maizels RM, Sartono E, Kurniawan A, Partono F, Selkirk ME, Yazdanbakhsh M. T-cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today 1995; 11: 50-56. [PubMed]
26. McMahon JE, Marshall TFC, Vaughan JP, Abaru DE. Bancroftian filariasis: a comparison of microfilariae counting techniques using counting chamber, standard slide and membrane (nuclepore) filtration. Ann Trop Med Par 1979;73:457-464. [PubMed]
27. McMahon JE, Marshall TFC, Vaughan JP, Kolstrup N. Tanzania filariasis project: a provocative day test with diethylcarbamazine for the detection of microfilariae of nocturnal periodic Wuchereria bancrofti in the blood. Bull world Health Organ 1979; 57: 759-765 [PubMed]
28. Melrose WD. Chemotherapy for lymphatic filariasis: progress but not perfection. Expert Rev Anti-infect Ther 2003; 1: 571-577. [PubMed]
29. Meyrowitsch DW, Simonsen PE, Makunde WH. Bancroftian filariasis: analysis of infection and disease in five endemic communities of north-eastern Tanzania. Am J Trop Med Hyg 1995; 89: 653-663. [PubMed]
30. Michael E, Bundy DAP. Global mapping of lymphatic filariasis. Parasitology Today 1997; 13: 472-476. [PubMed] [PubMed]
31. Noroes J, Dreyer G, Santos A, Mendes VG, Medeiros Z, Addiss D. Assessment of the efficacy of diethylcarbamazine on adult Wuchereria bancrofti in vivo. Trans Roy Soc Trop Med Hyg 1997; 91: 78-81. [PubMed]
32. Noroes J, Addiss D, Santos A, Medeiros Z, Couthino A, Dreyer G. Ultrasonographic evidence of abnormal lymphatic vessels in young men with adult Wuchereria bancrofti infection in the scrotal area. J Urol 1996; 156: 409-412. [PubMed]
33. Olszewski WL, Jamal S, Manokran G, Pani S, Kumaraswami V, Kubicka U, Lukjomska B, Tripathi FM, Swoboda E, Meisel-Mikolajczyk F, Stelmach E, Zaleska M. Bacteriological studies of blood, tissue fluid, lymph and lymph nodes in patients with acute dermatolymphangioadenitis in course of filarial lymphoedema. Acta Trop 1999; 73: 217-224. [PubMed]
34. Olszewski WL, Jamal S, Manokran G, Pani S, Kumaraswami V, Kubicka U, Lukjomska B, Dworczynski A, Swoboda E, Meisel MF. Bacteriological studies of skin, lymph and lymph nodes in patients with filarial lymphoedema. Am J Trop Med Hyg 1997; 57: 7-15. [PubMed]
35. Olszewski WL. Episodic dermatolymphangioadenitis (DLA) in patients with lymphoedema of the lower extremities before and after administration of benzathine penicillin: a preliminary study. Lymphology 1996; 29: 126-131. [PubMed]
36. Olszewski WL, Jamal S, Manokran G, Lukomska B, Kubicka U. Skin changes in filarial and non-filarial lymphoedma of the lower extremities. Trop Med Par 1993; 44: 40-44. [PubMed]
37. Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis 2008; 2: e317. [PubMed]
38. Ottesen EA. The Global Programme to Eliminate Lymphatic Filariasis. Trop Med Int Health 2000; 5:591-594.[PubMed]
39. Ottesen EA, Ismail MM, Horton J. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitol today 1999; 15: 382-386. [PubMed]
40. Ottesen EA, Nutman TB. Tropical pulmonary eosinophilia. Annu Rev Med 1992; 43: 417-424. [PubMed]
41. Partono F. The spectrum of disease in lymphatic filariasis. Ciba Foundation symposium 1987; 127: 15-31. [PubMed]
42. Rahmah N, Anuar AK, Ariff RHT, Zurainee MN, A´shikin AN, Fadzillah A, Maimunah A, Haq JA. Use of antifilarial IgG4-ELISA to detect Brugia Malayi infection in an endemic area of Malaysia. Trop Med Int Health 1998; 3: 184-188. [PubMed]
43. Ramaiah KD, Ramu K, Kumar KN, Guyatt H. Epidemiology of acute filarial episodes caused by Wuchereria bancrofti infection in two rural villages in Tamil Nadu, south India. Trans Roy Soc Trop Med Hyg 1996; 90: 639-643. [PubMed]
44. Ramzy RMR, Farid HA, Kamal IH, Ibrahim GH, Morsey ZS, Faris R, Weil GJ, Williams SA, Gad AM. A polymerase chain reaction-based assay for detection of Wuchereria bancrofti in human blood and Culex pipiens. Tran Roy Soc Trop Med Hyg 1997; 91: 156-160.[PubMed]
45. Rocha A, Addiss D, Ribeiro M, Noroes J, Baliza M, Medeiros Z Dreyer G. Evaluation in Wuchereria bancrofti infection: infected persons with undetectable or ultra-low microfilarial densities. Trop Med Int Health 1996; 1: 859-864. [PubMed]
46. Shenoy RK, Suma TK, Kumaraswami V, Rahmah N, Dhananjayan G, Padma S, Abhilash G, Ramesh C. Preliminary findings from a cross-sectional study on lymphatic filariasis in children, in an area of India endemic for Brugia malayi infection. Ann Trop Med Par 2007;101;205-213. [PubMed]
47. Shenoy RK, Suma TK, Kumaraswami V, Padma S, Rahmah N, Abhilash G, Ramesh C. Doppler ultrasonography reveals adult-worm nests in the lymph vessels of children with brugian filariasis. Ann Trop Med Par 2007;101;173-180. [PubMed]
48. Simonsen PE. Wuchereria bancroftian Tanzania; immune reactions to the microfilarial surface, and the effect of diethylcarbamazine upon these reactions. Trans Roy Soc Trop Med Hyg 1985; 79: 852-858. [PubMed]
49. Simonsen PE, Meyrowitsch DW, Makunde WH, Magnussen P. Bancroftian filariasis: the pattern of microfilaraemia and clinical manifestations in three endemic communities of north-western Tanzania. Acta Trop 1995; 60: 179-187. [PubMed]
50. Simonsen PE, Dunyo SK. Comparative evaluation of three new tools for diagnosis of bancroftian filariasis based on detection of specific circulating antigens. Trans Roy Soc Trop Med Hyg 1999; 93: 278-282. [PubMed]
51. Simonsen PE, Meyrowitsch DW, Jaoko WG, Malecela MN, Mukoko D, Pedersen EM, Ouma JH, Rwegoshora RT, Masese N, Magnussen P, Estambale BE, Michael E. Bancroftian filariasis infection, disease, and specific antibody response patterns in a high and a low endemicity community in East Africa. Am J Trop Med Hyg 2002; 66: 550-559. [PubMed]
52. Simonsen PE, Bernhard P, Jaoko WG, Meyrowitsch DW, Malecela-Lazaro MN, Magnussen P, Michael E. Filaria dance sign and subclinical hydrocele in two East African communities with bancroftian filariasis. Trans R Soc Trop Med Hyg 2002; 96: 649-653. [PubMed]
53. Simonsen PE. Filariases in: Manson’s Tropical diseases, 21st Edition 2003. Eds. Cook GC, Zumla A, 1487-1502. WB Saunders Company Ltd., London.
54. Subramanian S, Stolk WA, Ramaiah KD, Plaisier AP, Krishnamoorthy K, Van Oortmarssen GJ, Dominic Amalraj D, Habbema JDF, Das PK. The dynamics of Wuchereria bancrofti infection: a model-based analysis of longitudinal data from Pondicherry, India. Parasitol 2004;128:467-482. [PubMed]
55. Supali T, Djuardi Y, Pfarr KM, Wibowo H, Taylor MJ, Hoerauf A, Houwing-Duistermaat JJ, Yazdanbakhsh M, Sartono E.. Doxycycline treatment of Brugia- malayi- infected persons reduced microfilaraemia and adverse reactions after dietylcarbamazine and albendazole treatment. Clin Infect Dis 2008;46:1385-93. [PubMed]
56. Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, placebo controlled trial. Lancet 2005; 365:2116-21. [PubMed]
57. Turner JD, Mand S, Debrah AY, Muehlfeld J, Pfarr K, McGarry HF, Adjei O, Taylor MJ, Hoerauf A. A randomized, double-blind clinical trial of a 3 week course of doxycycline plus albendazole and ivermectin for treatment of Wuchereria bancrofti infection. Clin Infect Dis 2006;42:1081-9. [PubMed]
58. von Lichtenberg F. The Wellcome Trust lecture. Inflammatory response to filarial connective tissue parasites. Parasitol 1987;94:S101-S122. [PubMed]
59. WHO. Lymphatic filariasis: the disease and its control. Fifth report of the WHO Expert Committee on Filariasis. World Health Organization Technical Report Series 1992: 1-71. [PubMed]
60. WHO. Lymphatic filariasis: diagnosis and pathogenesis. WHO expert committee on filariasis. Bull World Health Organ 1993;71:135-141. [PubMed]
61. WHO. Global Programme to eliminate lymphatic filariasis: progress report on mass drug administration 2010. Ekly Epidemiol Rec/ Health Section of the Secretariat of the League of Nations 2011:86:377-88. [PubMed]
Tables
None
What's New
Weil GJ, et al. Laboratory and Field Evaluation of a New Rapid Test for Detecting Wuchereria bancrofti Antigen in Human Blood. Am J Trop Med Hyg 2013:May 20 [EPub ahead of print].
