Bordetella pertussis (Whooping Cough) and other species
Authors: Jussi Mertsola, M.D., Qiushui He, M.D.
Microbiology
Bordetella pertussis, a small gram-negative coccobacillus, is the most important cause of pertussis. B. parapertussis causes similar, but often milder type of cough with posttussive vomiting. B. bronchiseptica and B. avium are important pathogens in wild and domestic animals. B. bronchiseptica and B. hinzii have been rarely found in immunocompromised patients (65). B. holmesiihas been originally isolated from blood cultures of young adults. Recently, it has been isolated from nasopharyngeal samples of school children with a cough (81). B. trematum is the most recently discovered species and has been isolated from wounds and ear infections in humans (76). Variants of B. bronchiseptica have been found in immunized children in Italy suggesting that there might be some "intermediate strains" of Bordetella which could cause significant illness in humans (69).
The whole genome sequencing of B. pertussis, B. parapertussis and B. bronchiseptica is being completed and showed that the B. pertussisgenome is significantly smaller than that of B. bronshiseptica and of B. parapertussis (http://www.sanger.ac.uk/Projects/Microbes/).
Epidemiology
It is estimated that there are 40 million cases of pertussis causing 360.000 deaths annually (3). In the prevaccine era in the United States pertussis was the leading cause of death from communicable disease among children less than 14 years of age (57). Pertussis is a highly contagious respiratory disease. Transmission of the disease is airborne and usually results from a contact of an infected coughing patient. In households the spread is 90-100%. In these settings even 50% of fully immunized persons develop subclinical or symptomatic infection.
Several studies from countries with high vaccination coverage have shown that pertussis has shifted to older age groups indicating that lifelong immunity from pertussis immunization and childhood pertussis does not exist (36, 73). When adults with cough longer than 4 weeks were tested in Australia, 26% had serologic findings indicating a recent pertussis infection (67). Nennig and associates studied adults with prolonged cough of 2 weeks or longer and found a prevalence of 12.4% (63). The incidence was estimated to be 176 per 100.000 persons a year. Adults as a source of transmission of pertussis in households is stressed by the findings in 16 families of the 23 infants who died in pertussis (80). Thirteen (81%) were exposed to others in their homes who had cough before the onset of cough in those children. Adults accounted for at least 46% of those contacts. In a prospective contact study in 122 households in Germany 15% of the sources were adults (79).
Recently, pertussis is increasing in incidence in countries despite high vaccination rates (1,6,24,25). One explanation for the increase might be the adaptation of B. pertussis bacteria to vaccine-induced immunity, because the antigenic divergence has been recently found between the B. pertussis vaccine strains and circulating strains (13,60,61).
Clinical Manifestations
The incubation period is 1-3 weeks. Rhinorrhea, lacrimation, and low grade fever occur in the catarrhal phase. After a few days, a nonproductive cough can lead to the paroxysmal phase. The cough characterized by a whoop occurs most commonly in children. Paroxysm of cough may cause cyanosis, vomiting and syncope. Pertussis is most severe in young infants. In an analysis of pertussis cases in 1989-1991 in the United States, 69% of infants < 6 months of age were hospitalized, 16% had pneumonia, 1.8% had seizures, 0.2% had encephalopathy and deaths occurred in 0.4% of infants (8). Although the disease is milder in older children even in the age-group of 5-9 years 1.4% had seizures and 1 out of 704 patients died. The risk of complications during pertussis is much higher than any risk attributed to the significant adverse effects of the whole-cell pertussis vaccine (18).
B. pertussis has a notable capacity to alternate between virulent and avirulent forms (21). It has been speculated that in the late stage of pertussis disease the B. pertussis bacteria may be in a avirulent phase which represents a pathogenetically dormant state in the situation when the host immune response has developed. Bacteria may use this as a strategy for immune evasion and persistence within the host. This is of a hypothetical concern if new acellular vaccines are to consist of antigens produced only during the virulent phase (21).
Classically B. pertussis infection is considered not to be an invasive disease but merely a local process on the epithelial surfaces. The organisms can, however, survive in vitro in HeLa and mouse kidney cells and also in vivo in alveolar macrophages in a mouse model (15, 22). Friedman and colleagues showed the long term survival of B. pertussis organisms in vitro in human macrophages (32). The important, but yet open, clinical question is, does this intracellular survival of B. pertussis exist during pertussis disease. Recently, intracellular Bordetellae were found in macrophages of bronchoalveolar lavage samples from 3 children with an HIV infection (10). The knowledge of intracellular survival of B. pertussis in humans would have clinical implications in development of vaccines and in use of other preventive and therapeutic strategies.
Laboratory Diagnosis
In unvaccinated infants the clinical diagnosis of pertussis is relatively easy. The knowledge of symptoms is critical because physical examination is generally uninformative. Mainly for the purpose of conducting clinical vaccine efficacy trials a case definition for pertussis was agreed upon in a WHO consensus conference in Geneva in 1992. This definition requires that a patient has laboratory evidence of B. pertussis infection and a paroxysmal cough for 21 days or longer. The laboratory confirmation of a household member could also be used as an indirect evidence of pertussis in a patient fulfilling the clinical criteria. Case determination is difficult, however, because clinical pertussis is highly variable disease and with the WHO criteria the diagnosis of several pertussis cases will be missed (16,41).
Because mild and atypical cases of pertussis are often encountered, laboratory diagnosis of pertussis is highly important. Culture has remained the gold standard of diagnosis. The specimens should be taken from the posterior nasopharynx, preferably by intranasal aspiration or by a swab. Calcium alginate swabs are better than dacron, rayon or cotton wool swabs (51) (Figure 1). The specimens should be plated immediately onto selective media, and the charcoal supplemented with 10% horse blood and 40 mg/l cephalexin is currently the medium of choice (45). Bordetellae are first identified by colony morphology and gram-stains. B. parapertussis grows faster than B. pertussis and is oxidase negative, whereas B. pertussis is oxidase positive. The two species are also identified by slide agglutination with antisera. In addition B. parapertussis shows urease activity and pigment formation on tyrosine agar.
The culture positivity is often highest during the first 2 weeks of illness, and cultures are seldom positive if cough has lasted more than 4 weeks. Usually the sensitivity of culture is less than 50% (better in unvaccinated infants) and false negative results are common. The direct fluorescent antibody test (DFA) from nasopharyngeal secretions is a rapid test but often lacks specificity.
Several polymerase chain reaction (PCR) assays have been developed for the rapid diagnosis of B. pertussis infection (58). Usually two types of samples, nasopharyngeal aspirates or nasopharyngeal swabs (taken as for cultures) have been used. PCR results are available in 1-2 days and with this method it is usually possible to detect about four times more positive samples when compared to culture (40). Also in situations when effective antimicrobial therapy is started several days before the specimen is collected, the patient is likely to be PCR positive but culture negative (27).
Enzyme immunoassay (EIA) for detection of IgM, IgA and IgG antibodies to B. pertussis is particularly useful in diagnosis during the late stage of the disease when other tests are negative (59,77). The strict criteria for the seropositivity often decrease the sensitivity to a level of 50-70%. Even with the use of various purified antigens (PT, FHA, PRN) and measurement of different antibody classes no single test is shown to be highly sensitive to be used alone. Although EIA has its limitations, it is shown to be practical aid in recognition of small local outbreaks in older children and adults (57). In immunized persons the laboratory confirmation of the diagnosis of pertussis is difficult, and combinations of culture, PCR and EIA serology should be used. Epidemiological data and laboratory confirmed contact patients often help in the diagnosis.
Pathogenesis
B. pertussis has many virulence factors. Several are important in adhesion of the bacteria to the respiratory epithelium whereas others have toxic properties (43). Filamentous hemagglutinin (FHA), pertactin (PRN) and fimbriae (FIM) are adhesion proteins which have been used as antigens in new acellular pertussis vaccines. In addition to these adhesions, other surface-associated proteins have been recently implicated in the pathogenesis of pertussis (30,31). Pertussis toxin (PT) is considered to play an essential role in this disease. It is a hexameric protein with an A- promoter and a pentameric B-oligomer. PT has a wide variety of biological activities. The A-promoter induces the ADP-ribosylation of Gi proteins which leads to a chronic activation of GTP second messenger system. This results in the intracellular activation of the cells finally paralyzing the normal functions of the target cells. Tracheal cytotoxin is a specific peptidoglycan fragment that causes ciliostasis and damage to the ciliated epithelial cells (43). The toxicity of this cytotoxin is mediated by intracellular production of interleukin-1 and nitric oxide (42). Also PT can induce nitric oxide via gamma interferon (68). It could be speculated that selective inhibitors of inducible nitric oxide synthase may have an unique therapeutic application in pertussis.
SUSCEPTIBILITY IN VITRO AND IN VIVO
Antimicrobial susceptibility testing of the fastidious species B. pertussis and B. parapertussis is not standardized (47). The methods employed have been widely divergent. Testing by broth dilution has usually resulted in higher MICs than testing by agar dilution (47). In addition to different broth media used, the inoculum has varied 100-fold and different sources of blood with different concentrations have been used. Other variables have been the incubation atmosphere and period of time used.
Erythromycin has been a drug of choice and there has been no need for routine susceptibility tests of B. pertussis since all the strains have been susceptible to erythromycin. In 1994 the first erythromycin resistant strain was isolated from a 2-month old infant in Yama, Arizona (56). Hoppe and Tschirner have recommended the use of Mueller-Hinton broth supplemented with 5% of horse blood for testing the activity of erythromycin (50). The erythromycin containing plates should be incubated for 48 h for B. parapertussis and 72 h for B. pertussis. Incubation should be done in ambient air. The E test has been evaluated as an alternative to the agar dilution testing (45). Standardization of test methods is especially needed if erythromycin resistant strains become more common and also for studying the new antimicrobial agents.
In vitro the new macrolides show similar activity against B. pertussis. Usually B. parapertussis is more resistant than B. pertussis against all these macrolides when tested in vitro conditions (Table 1) (55). Tuomanen and colleagues proposed that the bvg locus of B. pertussis, which determinates phase transition in these organisms, also controls the cell wall hydrolases (75). It is known that virulent strains of B. pertussis grow much more slowly than avirulent strains and might thus be more difficult to kill with antibiotics. It was shown, however, that penem antibiotics, known to rapidly kill even slowly growing organisms, demonstrated in vitro a more-than-twofold greater rate of killing of slowly growing virulent strain of B. pertussis compared with erythromycin (75).
Several quinolones are also very active against B. pertussis and B. parapertussis in vitro (Table 1). Other agents showing good in vitro activity against B. pertussis are piperacillin and mezlocillin, ceftazidime, cefotaxime and ceftriaxone (Table 1). The in vitro data on co-trimoxazole is difficult to interpret because of the large discrepancies in the test results (48).
ANTIMICROBIAL THERAPY
In older children, especially those previously immunized and in adults, the diagnosis is often difficult. Usually treatment should be initiated to prevent spread of the disease even when the patient has had cough for weeks. After 4-5 weeks about 5-10% of patients are still culture positive and 30-40% have positive PCR results (39). If treatment is started later than 2 weeks after the onset of symptoms, it often has no significant clinical effect on the patient’s symptoms.
Drug of Choice
Erythromycin for 14 days is the standard treatment recommend by the American Academy of Pediatrics (2), whereas in Canada 10 days treatment has been recommended (38). Halperin and colleagues showed that 7 days treatment with erythromycin estolate is as effective as 14 days treatment (38). It is of note, however, that the mean age of their patients was about 7 years and 88% of them had received at least the primary series of immunizations. The 7-day treatment of infants needs further studies. Erythromycin estolate is preferred with a dose of 40 to 50mg/kg/day (maximum, 2g/day). If erythromycin ethylsuccinate or stearate is used, the dose is 50-60 mg/kg/day (45). Erythromycin is usually given in four divided doses (57). Hoppe and colleagues treated 190 culture-positive children with 2-dose regimen of estolate or with 3-doses of ethylsuccinate per day and had relapse rates of only 2% and 1%, respectively (44). Most patients were treated during the first 2 weeks after onset of coughing. After completion of the treatment 82% and 75% of the parents, respectively, judged the clinical condition as cured or improved. Baraff showed that erythromycin therapy resulted in the elimination of B. pertussis from the nasopharynx in 2 to 7 days (mean 3.6 days) and in the study of Bergqvist none of the patients had positive cultures 5 days after initiation of the erythromycin treatment (5,7). Co-trimoxazole is considered as an alternative for those who do not tolerate erythromycin but the controlled clinical data supporting this therapy is limited (45).
New macrolides are superior to erythromycin in terms of absorption, acid stability, and tissue penetration. Their in vitro activity against B. pertussis is good (Table 1). One small study showed promising results with clarithromycin (10 mg/kg/day twice a day for 7 days) or azithromycin (10 mg/kg/day, once a day for 5 days)(4). Number of treated children were 9 and 8, respectively. Eradication rates at 7 days after treatment were 100% in both groups. Cultures were taken from some patients also 3-4 days after treatment and the eradication occurred in 5 of 8 and in 4 of 5 children, respectively. Although the results are encouraging, further studies are needed before these agents could be recommend for routine treatment of pertussis.
Based on in vitro results quinolones could also be an alternative for adults, but clinical data of their efficacy is lacking. Piperacillin and third generation cephalosporins could, in theory, be used as a combination therapy with erythromycin for those patients with severe secondary infections (usually pneumonia). In one retrospective analysis, all deaths due to severe pneumonia and septicaemia in hospitalized pertussis patients were caused by gram-negative organisms other than B. pertussis (33).
ADJUNCTIVE THERAPY
There is no convincing evidence of the efficacy of cough suppressants, salbutamol, antihistamines or corticosteroids in the treatment of pertussis (11,23,54). Hyperimmune serum was widely used and regarded as beneficial in the 1930s and 1950s but later studies showed little or no effect of this treatment in pertussis. More recent, but limited, data indicates that treatment with specific immunoglobulin with high antitoxin concentration has a beneficial effect in the treatment of this disease (35,52).
ENDPOINTS FOR MONITORING THERAPY
The follow-up cultures after therapy are usually not needed.
VACCINES
Whole-cell vaccines prepared from killed whole-cell B. pertussis bacteria have been used for decades. The local and systemic side effects are the major disadvantage of whole-cell vaccines and have earlier resulted in declining vaccination uptake in several countries such as Japan, Germany, Italy, Sweden, and the United Kingdom. Subsequently, large epidemics occurred in these countries. Therefore, extensive efforts have been made to develop less reactogenic acellular vaccines (14,18).
Acellular Pertussis Vaccines
Acellular vaccines are based on bacterial components and at present, four main components including PT, FHA, PRN, and FIM are considered to be suitable antigens (Table 2). All acellular vaccines for clinical evaluation contain inactivated PT in different concentrations and several vaccines have one or more of the other three components in varying amounts.
Acellular vaccines containing PT and FHA were first developed in Japan and have been extensively used there since 1981. The reportedreactogenicity of these vaccines is low, and their efficacy has already shown in Japan by the fact that pertussis has remained again "well-controlled" in that country after the introduction of the new vaccines.
Several controlled efficacy trials of acellular vaccines have been carried out in Germany, Italy, Senegal, and Sweden (43,66). The acellular vaccines used in these trials were from different manufacturers, and either a whole-cell DTP vaccine or a DT vaccine served as control. In most trials 3 doses were administered before 6 months of age. The results are summarized in Table 2. The efficacy trials have proved that acellular vaccines can prevent pertussis disease and give less adverse reactions than whole-cell vaccines. However, several important issues remain to be elucidated.
What are optimal antigens and their quantities in an acellular vaccine? PT is considered to be the most important protective antigen and is included in all the currently available vaccines. Without clinical efficacy trial the efficacy of the vaccine is difficult to predict because studies have not identified a correlation between the serum antibody levels and protection. In addition not even the amount of antigens in some vaccines correlate with mean antibody levels elicited by these antigens. Comparisons between the studies should be done with caution but the results indicate that addition of FHA, PRN, or FIM to the inactivated PT component conveyed modest additional efficacy (16,37,43). The peroxide-detoxified PT vaccine was clearly protective against what might be considered moderately severe pertussis but was less effective against mild disease (43,74). A significant benefit of FHA, PRN and especially FIM have been observed with respect to long-term protection against pertussis disease (64). The high level of antibodies to PRN, FIM or PT has been found to associate with lower likelihood of acquiring pertussis: the strongest correlation is with PRN and the weakest with PT (19,71). Since the antigenic divergence with respect to PRN and PT has been found between the B. pertussis vaccine strains and circulating strains (13,60,61), it remains to be shown if the acellular vaccines provide equal protection against B. pertussis representing vaccine or non-vaccine type strains.
How rapidly does pertussis immunity wane after immunization with different vaccines? It is not known if circulating antibodies are protective and even less is known about the role of cell-mediated immunity in protection against pertussis. B. pertussis remains localized to the respiratory tract throughout the course of the disease, suggesting that a potent local immune response may be highly effective in clearing infection (9). This, however, is difficult to measure in humans. Plotkin and Cadoz suggest that there are likely to be multiple protective factors rather than a single protective correlate (66). To eradiate the organism, the best vaccine should provide long lasting protection not only against disease but also against both colonization and infection.
Do adolescent and adult need booster immunizations? Pertussis has been increasingly reported in adolescents and adults (63,67,72). Although generally milder in adolescents and adults, pertussis can cause substantial, prolonged illness with associated economical costs. Further, the role of older children, adolescents and adults in transmission of B. pertussis to non-immunized and partially immunized infants has been well documented. Therefore, a strategy that universal adolescent booster vaccination combined with a programme targeted at adults most likely to have contact with very young babies including healthcare and childcare workers, parents and close family contacts should be considered, as recommended by an international consensus group on pertussis immunization (12).
What are the mechanisms of the local reaction often seen after the booster immunizations? The reactions tend to be more frequent after a booster dose when the child has been immunized with an acellular vaccine, but less so if the primary immunizations were done with a whole-cell vaccine.
Is there interference with reactogenicity and immunogenicity when the vaccines are used in combination? Combined vaccines including antigens against diphtheria, tetanus, B. pertussis, Haemophilus influenzae type b, hepatitis B and inactivated polio vaccines have been developed. At present, the effect of the combination of antigens in the same dose of vaccine on reactogenicity and immune responses are poorly understood. It has been discovered that antibody response against H. influenzae type b is reduced in some combined acellular vaccines (28,29). The combined vaccines will, however, induce immunologic memory and although it is well-documented that DTaP/Hib vaccines elicit lower anti-Hib titers than separate vaccines, such combinations are likely to be effective in reducing the incidence of invasive H. influenzae type b disease.
From an economical and a practical point of view the new acellular vaccines in comparison with whole-cell vaccines should not be too expensive. This is crucial for developing countries.
General
Acellular pertussis vaccines are highly efficacious against pertussis disease. Several developed countries, like Germany and Italy have recently taken acellular vaccines into their primary immunization programs. In the United States, acellular pertussis vaccines have been licensed by the Food and Drug Administration for the five-dose series (14). Recent efficacy trials indicate that at least some whole-cell pertussis vaccines are as effective as the new acellular vaccines (Table 2). Therefore, some countries may continue to use the potent whole-cell vaccines. Also in these situations, acellular vaccines are needed for continuous booster immunizations, which is important for elimination of pertussis in older population.
PREVENTION
Chemoprophylaxis and Control Measures
There are several reports showing wide spread of pertussis in families, schools, nursing homes and hospitals. Erythromycin prophylaxis (same dose and duration as in treatment) is recommended in Canada and in the USA to all household and other close contacts of pertussis patients irrespective of their immunization status (2,62). A contact is defined as a person living in the same household, attending the same day-care centre or sharing the same air space for more than 1 hour (62). Health care workers who have used appropriate protective measures, such as masks, were not to be considered contacts. It has been difficult to assess the real benefits of erythromycin prophylaxis because early treatment of index cases also reduce the spread of pertussis effectively. The beneficial effect of early treatment of the index case in the household was clearly shown during an acellular pertussis vaccine efficacy trial in Mainz, Germany. Early use of erythromycin, for treatment and/or for prophylaxis has been shown to reduce the spread (78). A study of Steketee and associates during an outbreak showed that during early use of erythromycin the infectivity rate was 16% compared with 75% during the late use of this antibiotic (70). A retrospective cohort study of de Serres showed that when prophylaxis was used before the onset of secondary case the attack rate was 4% in families compared to 35% if the family already had a secondary case (26). Granström showed that if a mother has pertussis at the time of labor, she can safely be allowed to nurse her infant if the mother and the baby are given erythromycin (34). In this cases the father and siblings at home should also receive the prophylaxis.
The patient with pertussis should be placed in respiratory isolation for 5 days after initiation of erythromycin, or until 3 weeks after the onset of paroxysmal cough is passed if antimicrobial therapy is not given (2). Asymptomatic adult contacts who are on prophylaxis can return to work (78).
Christie and associates implemented a vigorous 15-point control plan to reduce spread of pertussis in hospitals (20). This included a program of early diagnosis, treatment and prevention for employees, the immediate isolation of all patients with respiratory illnesses that might prove to be pertussis, the wearing of masks by patients, by visitors and personnel in the test referral center where pertussis cultures were performed, the restriction of patient travel and visitors, and restrictions in the employees’ child care service. It must be kept in mind that the new epidemiological data indicates that pertussis is common, but often unrecognized, in adults also in the population outside the hospital and therefore the control plans have to be practical (17). Wide use of erythromycin has problems and one small analysis indicated that only less than 50% of healthcare workers in prophylaxis completed the 14-day course (17). The general value and the practicability of the wide use of prophylaxis in the community is questionable. It is important to increase awareness of pertussis disease in adults and school-children in the population, to start treatment early already in the catarrhal phase of pertussis and to give prophylaxis to those of high risk if infected (infants, other high-risk patients).
During outbreak conditions it is important to stress the importance of proper immunizations in children. The recommend series should be completed. Children who are less than 7-year old and have received a third dose 6 months or more before exposure, or a fourth dose 3 years or more before exposure, could be given a booster dose (57). In the future, acellular vaccines may be used also in older age-groups to decrease and prevent the endemic circulation of B. pertussis.
REFERENCES
1. Andrews R, Herceq A, Roberts C. pertussis notifications in Australia. Commun Dis Intell 1997;21:145-148. [PubMed]
2. American Academy of Pediatrics.pertussis. In: Pickering LK, ed. 2000 Red Book: Report on the Committee on Infectious Diseases. 25th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2000:435-448.
3. Anonymous. pertussis vaccines. The International Vaccine Institute Newsletter 1996;2:3-8.
4. Aoyama T, Sunakawa K, Iwata S, Takeuchi Y, Fujii R. Efficacy of short-term treatment of pertussis with clarithromycin and azithromycin. J Pediatr 1996;129:761-764.[PubMed]
5. Baraff LJ, Wilkins J, Wehrle PF. The role of antibiotics, immunizations, and adenoviruses in pertussis. Pediatrics 1978;61:224-230. [PubMed]
6. Bass JW, Wittler RR. Return of epidemic pertussis in the United States. Pediatric Infect Dis J 1994;13:343-345. [PubMed]
7. Bergquist SO, Bernander S, Dahnsjö H, Sundelöf B. Erythromycin in the treatment of pertussis: a study of bacteriologic and clinical effects. Pediatr Infect Dis J 1987;6:458-461. [PubMed]
8. Black S. Epidemiology of pertussis. Pediatr Infect Dis J 1997;16:S85-S89. [PubMed]
9. Brennan MJ, Shahin RD. pertussis antigens that abrogate bacterial adherence and elicit immunity. Am J Respir Crit Care Med 1996;154:S145-S149. [PubMed]
10. Bromberg K, Tannis G, Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun 1991;59:4715-4719. [PubMed]
11. Broomhall J, Herxheimer A. Treatment of whooping cough: the facts. Arch Dis Child 1984;59:185-187. [PubMed]
12. Campins-Marti M, Cheng HK, Forsyth K, et al. Recommendations are needed for adolescent and adult pertussis immunization: rationale and strategies for consideration. Vaccine 2001;20:641-646. [PubMed]
13. Cassiday P, Sanden G, Heuvelman K, Mooi F, Bisgard KM, Popovic T. Polymorphism in Bordetella pertussis pertactin and pertussis toxin virulence factors in the United States, 1935- 1999. J Infect Dis 2000;182:1402-1408. [PubMed]
14. CDC, General recommendations on Immunization:Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP).MMWR 2002;51:1-36. [PubMed]
15. Cheers C, Gray DF. Macrophage behaviour during the complaisant phase of murine pertussis. Immunology 1969;17:875-887. [PubMed]
16. Cherry JD. Comparative efficacy of acellular pertussis vaccines: an analysis of recent trials. Pediatr Infect Dis J 1997;16:S90-S96. [PubMed]
17. Cherry JD. Nosocomial pertussis in the nineties. Infect Control Hosp Epidemiol 1995;16:553-555. [PubMed]
18. Cherry JD, Brunell PA, Golden GS, Karzon DT. Report of the task force on pertussis and pertussis immunization-1988. Pediatrics 1988;81:S939-S984.
19. Cherry JD, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illness. Vaccine 1998;16:1901-1906.[PubMed]
20. Christie CD, Glover AM, Willke MJ, Marx ML, Reising SF, Hutchinson NM. Containment of pertussis in the regional pediatric hospital during the greater Cincinnati epidemic of 1993. Infect Control Hosp Epidemiol 1995;16:556-563. [PubMed]
21. Coote JG. Antigenic switching and pathogenicity: environmental effects on virulence gene expression in Bordetella pertussis. J Gen Microbiol 1991;137:2493-2503.[PubMed]
22. Crawford JG, Fishel GW. Growth of Bordetella pertussis in tissue culture. J Bacteriol 1959;77:465-474.
23. Danzon A, Lacroix J, Infante-Rivard C, Chicoine L. A double-blind clinical trial on diphenhydramine in pertussis. Acta Paediatr Scand 1988;77:614-615. [PubMed]
24. De Melker HE, Conyn-van Spaendonck MA, Rümke HC, van Wijngaarden JK, Mooi FR, Schellekens JF. pertussis in the Netherlands: an outbreak despite high levels of immunization with whole cell vaccine. Emerg Infect Dis 1997;3:175-178. [PubMed]
25. De Serres G, Boulianne N, Duval B. Field effectiveness of erythromycin prophylaxis to prevent pertussis within families. Pediatr Infect Dis J 1995;14:969-975.[PubMed]
26. DeSerres G, Boulianne N, Douville Fradet M, Duval B. pertussis in Quebec: ongoing epidemic since the late 1980s. Can Commun Dis Rep 1995;15:45-48. [PubMed]
27. Edelman K, Nikkari S, Ruuskanen O, He Q, Viljanen M, Mertsola J. Detection of Bordetella pertussis by polymerase chain resaction and culture in the nasopharynx of erythromycin- treated infants with pertussis. Pediatr Infect Dis J 1996;15:54-57. [PubMed]
28. Edwards KM, Decker MD. Combination vaccines consisting of acellular pertussis vaccines. Pediatr Infect Dis J 1997;16:S97-S102. [PubMed]
29. Eskola J, Olander RM, Hovi T, Litmanen L, Peltola S, Kayhty H. Randomised trial of the effect of co-administration with acellular pertussis DTP vaccine on immunogenicity of Haemophilus influenzae type b conjugate vaccine. Lancet 1996;348:1688-1692. [PubMed]
30. Fernandez RC, Weiss AA. Serum resistance in Bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol Lett 1998;163:57-63. [PubMed]
31. Finn TM, Amsbaugh DF. Vag8, a Bordetella pertussis bvg-regulated protein. Infect Immun 1998;66:3985-3989. [PubMed]
32. Friedman RL, Nordensson K, Wilson L, Akporiaye ET, Yocum DE. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun 1992;60:4578-4585. [PubMed]
33. Gan VN, Murphy TV. pertussis in Hospitalized children. Am J Dis Child 1990;144:1130- 1134. [PubMed]
34. Granström G, Sterner G, Nord CE, Granström M. Use of erythromycin to prevent pertussis in newborns of mothers with pertussis. J Infect Dis 1987;155:1210-1214.[PubMed]
35. Granström M, Olinder-Nielsen AM, Holmblad P, Mark A, Hanngren K. Specific immunoglobulin for treatment of whooping cough. Lancet 1991;338:1230-1233.[PubMed]
36. Grimprel E, Begue P, Anjak I, Njamkepo E, Francois P, Guiso N. Long-term human serum antibody responses after immunization with whole-cell pertussis vaccine in France. Clin Diagn Lab Immunol 1996;3:93-97. [PubMed]
37. Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A placebo-controlled trial of a two and five component acellular and a US licensed whole-cellpertussis vaccine. N Engl J Med 1996;334:349-355. [PubMed]
38. Halperin SA, Bortolussi R, Langley JM, Miller B, Eastwood BJ. Seven days erythromycin estolate is as effective as fourteen days for the treatment of Bordetellapertussis infections. Pediatrics 1997;100:65-71. [PubMed]
39. He Q, Schmidt-Schläpfer G, Just M, et al. Impact of polymerase chain reaction on clinical pertussis research: Finnish and Swiss experiences. J Infect Dis 1996;174:1288-1295. [PubMed]
40. He Q, Viljanen MK, Nikkari S, Lyytikäinen R, Mertsola J. Outcomes of Bordetella pertussis infection in different age-groups of an immunized population. J Infect Dis 1994;170:873-877. [PubMed]
41. Heininger U, Cherry JD, Eckhardt T, Lorenz C, Christenson P, Stehr K. Clinical and laboratory diagnosis of pertussis in the regions of a large vaccine efficacy trial in Germany. Pediatr Infect Dis J 1993;12:504-509. [PubMed]
42. Heiss LN, Lancaster JR, Corbett JA, Goldman WE. Epithelial autotoxity of nitric oxide: Role in the respitory cytopathology of pertussis. Proc Natl Acad Sci USA 1994;91:267-270. [PubMed]
43. Hewlett EL. pertussis: current concepts of pathogenesis and prevention. Pediatr Infect Dis J 1997;16:S78-S84. [PubMed]
44. Hoppe JE, and The Erythromycin study group. Comparison of erythromycin estolate and erythromycin ethylsuccinate for treatment of pertussis. Pediatr Infect Dis J 1992;11:189-193. [PubMed]
45. Hoppe JE. Update on epidemiology, diagnosis, and treatment of pertussis. Eur J Clin Microbiol Infect Dis 1996;15:189-193. [PubMed]
46. Hoppe JE, Eichhorn A. Activity of new macrolides against Bordetella pertussis and Bordetella parapertussis. Eur J Clin Microbiol Infect Dis 1989;8:653-654.[PubMed]
47. Hoppe JE, Haug A. Antimicrobial susceptibility of Bordetella pertussis (part I). Infection 1988;16:126-130. [PubMed]
48. Hoppe JE, Haug A. Treatment and prevention of pertussis by antimicrobial agents (part II). Infection 1988;16-148-152. [PubMed]
49. Hoppe JE, Simon CG. In vitro susceptibilities of Bordetella pertussis and Bordetella parapertussis to seven fluoroquinolones. Antimicrob Agents and Chemother 1990;34:2287- 2288. [PubMed]
50. Hoppe JE, Tschirner T. Comparison of media for agar dilution susceptibility testing of Bordetella pertussis and Bordetella parapertussis. Eur J Clin Microbiol Infect Dis 1995;14:775-779. [PubMed]
51. Hoppe JE, Weiss A. Recovery of Bordetella pertussis from four kinds of swabs. Eur J Clin Microbiol 1987; 6: 203-205. [PubMed]
52. Ichimaru T, Ohara Y, Hojo M, Miyazaki S, Harano K, Totoki T. Treatment of severe pertussis by administration of specific gamma globulin with high titers anti-toxin antibody. Acta Paediatr 1993;82:1076-1078. [PubMed]
53. Kerr JR, Preston NW. Current pharmocotherapy of pertussis. Expert Opin Pharmacother 2001;8:1275-1282 [PubMed]
54. Krantz I, Norrby SR, Trollfors B. Salbutamol vs. placebo for treatment of pertussis. Pediatr Infect Dis J 1985;4:638-640. [PubMed]
55. Kurzynski TA, Boehm DM, Rott-Petri JA, Schell RF, Allison PE. Antimicrobial susceptibilities of Bordetella species isolated in a multicenter pertussis surveillance project. Antimicrob Agents Chemother 1988;32:137-140. [PubMed]
56. Lewis K, Saubolle MA, Tenover FC, Rudinsky MF, Barbour SD, Cherry JD. pertussis caused by an erythromycin-resistant strain of Bordetella pertussis. Pediatr Infect Dis J 1995;14:388- 91. [PubMed]
57. Long SS. Bordetella pertussis (pertussis) and other species. In: Long SS, Pickering LK, Prober CG, eds. Principles and practice of pediatric infectious diseases. New York:Churchil Livingstone, 1997:976-986.
58. Meade BD, Bollen A. Recommendations for use of the polymerase chain reaction in the diagnosis of Bordetella pertussis infections. J Med Microbiol 1994;41:51-55.[PubMed]
59. Mertsola J, Ruuskanen O, Eerola E, Viljanen MK. Intrafamilial spread of pertussis. J Pediatr 1983;103:359-363. [PubMed]
60. Mooi FR, van Oirschot H, Heuvelman K, van der Heide HG, Gaastra W, Willems RJ. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin andpertussis toxin in the Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect Immun 1998;66:670-675. [PubMed]
61. Mooi FR, He Q, Van Oirschot H, Mertsola J. Variation in the Bordetella pertussis virulence factors pertussis toxin and pertactin in vaccine strains and clinical isolates in Finland. Infect Immun 1999;67:3133-3134. [PubMed]
62. National Advisary Committee on Immunization, Advisory Committee on Epidemiology and Canadian Pediatric Society. Management of people exposed to pertussisand control of pertussis outbreaks. Can Med Assoc J 1990;143:751-753. [PubMed]
63. Nennig ME, Shinefield HR, Edwards KM, Black SB, Fireman BH. Prevalence and incidence of adult pertussis in an urban population. JAMA 1996;275:1672-1674.[PubMed]
64. Olin P. Commentary: the best acellular pertussis vaccines are multicomponent. Pediatr Infect Dis J 1997;16:517-519.
65. Parton R. New perspectives on Bordetella pathogenicity. J Med Microbiol 1996;44:233-235. [PubMed]
66. Plotkin SA, Cadoz M. The acellular pertussis vaccine trials: an interpretation. Pediatr Infect Dis J 1997;16:508-517. [PubMed]
67. Robertson PW, Goldberg H, Jarvie BH, Smith DD, Whybin LR. Bordetella pertussis infection. Med J Aust 1987;146:522-525. [PubMed]
68. Sakurai S, Kamachi K, Konda T, Miyajima N, Kohase M, Yamamoto S. Nitric oxide induction by pertussis toxin in mouse spleen cells via gamma interferon. Infect Immun 1996;64:1309-1313. [PubMed]
69. Stefanelli P, Mastrantonio P, Hausman SZ, Giuliano M, Burns DL. Molecular Characterization of two Bordetella bronchiseptica strains isolated from children with coughs. J Clin Microbiol 1997;35:1550-1555. [PubMed]
70. Steketee RW, Wassilak SG, Adkins WN, Burstyn DG, Manclark CR, Berg J, Hopfensperger D, Schell WL, Davis JP. Evidence for a high attack rate and efficacy of erythromycin prophylaxis in a pertussis outbreak in a facility for the developmentally disabled. J Infect Dis 1988;157:434-440. [PubMed]
71. Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998;16:1907-1916. [PubMed]
72. Strebel P, Nordin J, Edwards K, et al. Population-based incidence of pertussis among adolescents and adults, Minnesota, 1995-1996. J Infect Dis 2001;183:1353-1359.[PubMed]
73. Thomas MG. Epidemiology of pertussis. Rev Infect Dis 1989;11:255-262. [PubMed]
74. Trollfors B, Taranger J, Lagergard T, Lind L, Sundh V, Zackrisson G, Lowe CU, Blackwelder W, Robbins JB. A placebo-controlled trial of a pertussis-toxoid vaccine. N Engl J Med 1995;333:1045-1050. [PubMed]
75. Tuomanen E, Schwartz J, Sande S. The vir locus affects the response of Bordetella pertussis to antibiotics: phenotypic tolerance and control of autolysis. J Infect Dis 1990;162:560-563. [PubMed]
76. Vandamme P, Heyndrickx M, Vancanneyt M, et al. Bordetella trematum sp.nov., isolated from wounds and ear infections in humans, and reassessment of Alcaligenes denitrificans Ruger and Tan 1983. Int J Syst Bacteriol 1996;46:849-858. [PubMed]
77. Viljanen MK, Ruuskanen O, Granberg C, Salmi TT. Serological diagnosis of pertussis: IGM, IgA and IgG antibodies against Bordetella pertussis measured by enzyme-linked immunosorbent assay (ELISA). Scand J Infect Dis 1982;14:117-122. [PubMed]
78. Weber DJ, Rutala WA. Management of healthcare workers exposed to pertussis. Infect Control Hosp Epidemiol 1994;15:411-415. [PubMed]
79. Wirsing von König CH, Postels-Multani S, Bock HL, Schmitt HJ. pertussis in adults: frequency of transmission after household exposure. Lancet 1995;346:1326-1329.[PubMed]
80. Wortis N, Strebel PM, Wharton M, Bardenheier B, Hardy IR. pertussis deaths: Report of 23 cases in the United States, 1992 and 1993. Pediatrics 1996;97:607-612.[PubMed]
81. Yih WK, Silva EA, Ida J, Harrington N, Lett SM, George H. Bordetella holmesii-like organisms isolated from Massachusetts patients with pertussis-like symptoms. Emerg Infect Dis 1999;5:441-443. [PubMed]
Tables
Table 1. Antimicrobial Susceptibility (µg/ml) of Bordetella pertussis and B. parapertussis
| Antimicrobial Agent | B. pertussis | B. parapertussis | ||
|---|---|---|---|---|
| MIC(range) | MIC90 | MIC(range) | MIC90 | |
| Macrolidesa | ||||
| Azithromycin | 0.015-0.03 | 0.015 | 0.125-0.25 | 0.125 |
| Clarithromycin | =0.008-0.03 | 0.03 | 0.25 | 0.25 |
| Dirithromycin | 0.03 | 0.03 | 0.125 | 0.125 |
| Erythromycin | =0.008-0.03 | 0.03 | 0.25 | 0.25 |
| Josamycin | 0.06-0.0.5 | 0.06 | 1 | 1 |
| Roxithromycin | =0.008-0.03 | 0.03 | 0.25 | 0.25 |
| Miscellanousb, c | ||||
| Ciprofloxacin | 0.03-0.25 | 0.06 | 0.06 | 0.06 |
| Enoxacin | 0.5 | 0.5 | 0.5 | 0.5 |
| Fleroxacin | 0.06-0.125 | 0.125 | 0.25 | 0.25 |
| Gemifloxacin | 0.008 | 0.003-0.125 | ||
| Levofloxacin | 0.06 | 0.06 | ||
| Lomefloxacin | 0.125-0.25 | 0.25 | 0.25 | 0.25 |
| Moxifloxacin | 0.03 | 0.06 | ||
| Norfloxacin | 0.25 | |||
| Ofloxacin | 0.125-0.5 | 0.125 | 0.125 | 0.125 |
| Pefloxacin | 0.25-0.5 | 0.5 | 0.25-0.5 | 0.5 |
| Temafloxacin | 0.06 | 0.06 | 0.06 | 0.06 |
| Trovafloxacin | 0.06 | 0.125 | ||
| Penicillin G | =0.1-5 | |||
| Ampicillin | 0.063->32 | 0.24 | ||
| Amoxicillin | 0.5 | |||
| Piperacillin | =0.003-0.006 | |||
| Cephalexin | 12.5-125 | |||
| Cefaclor | 12->64 | |||
| Cefuroxime | 1.5-6 | 5.3 | ||
| Ceftazidime | 0.16-0.5 | 0.29 | ||
| Ceftriaxone | 0.06-0.125 | 0.1 | ||
| Cefotaxime | 0.16-0.78 | 0.3 | ||
| Aztreonam | 4 | |||
| Co-trimoxazole | 0.006/0.125-32/640 | |||
| Clindamycin | 0.25->8 | |||
| Doxicycline | =0.06->2 | |||
| Gentamycin | 0.063-16 | |||
| Imipenem | =0.06->8 | 0.05 | ||
| Rifampicin | =0.006->2 | 0.125 | ||
| Tetracycline | =0.1-16 | 0.25 | ||
Modified from aref. 46, bcref. 47, 49, 53.
Table 2. Results from pertussis vaccine efficacy trials
| Manufacturer | Componentsa regimen (months) | Vaccination (95% CI)b | Site | Efficacy |
|---|---|---|---|---|
| Amvax | PT | 3, 5, 12 | Göteborg | 71 (63-78) |
| Pasteur Merieux | PT, FHA | 2, 4, 6 | Senegal | 86 (71-93) |
| Pasteur Merieux | Whole cell | 2, 4, 6 | Senegal | 96 (87-94) |
| Connaught (US)-Biken | PT, FHA | 2, 4, 6 | Munich | 96 (78-99)c |
| Behringwerke | Whole cell | 2, 4, 6 | Munich | 97 (79-100)d |
| SmithKline Beecham | PT, FHA | 2, 4, 6 | Stockholm | 59 (51-66) |
| Connaught (Canada) | PT, FHA, PRN, FIM | 2, 4, 6 | Stockholm | 85 (81-89) |
| Connaught (Canada) | Whole cell | 2, 4, 6 | Stockholm | 48 (37-58) |
| SmithKline Beecham | PT, FHA, PRN | 2, 4, 6 | Rome | 84 (76-90) |
| Chiron Biocine | PTe , FHA, PRN | 2, 4, 6 | Rome | 84 (76-90) |
| Connaught (Canada) | Whole cell | 2, 4, 6 | Rome | 36 (14-52) |
| SmithKline Beecham | PT, FHA, PRN | 3, 4, 5 | Mainz | 89 (77-95) |
| Behringwerke | Whole cell | 3, 4, 5 | Mainz | 97 (83-100) |
| Lederle-Takeda | PT, FHA, PRN, FIM | 3, 4, 5, 15-18 | Erlangen | 82 (73-87)d |
| Lederle | Whole cell | 3, 4, 5, 15-18 | Erlangen | 91 (85-94)d |
Modified from ref. 43
apertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin (PRN), fimbriae (FIM)
bUsing the WHO case definition unless otherwise noted.
cUsing the WHO clinical case definition and positive culture.
dUsing a modified WHO case definition
eGenetically toxoided PT.
Figure 1. Proper Technique for obtaining a nasopharyngeal specimen for the isolation of Bordetella pertussis.
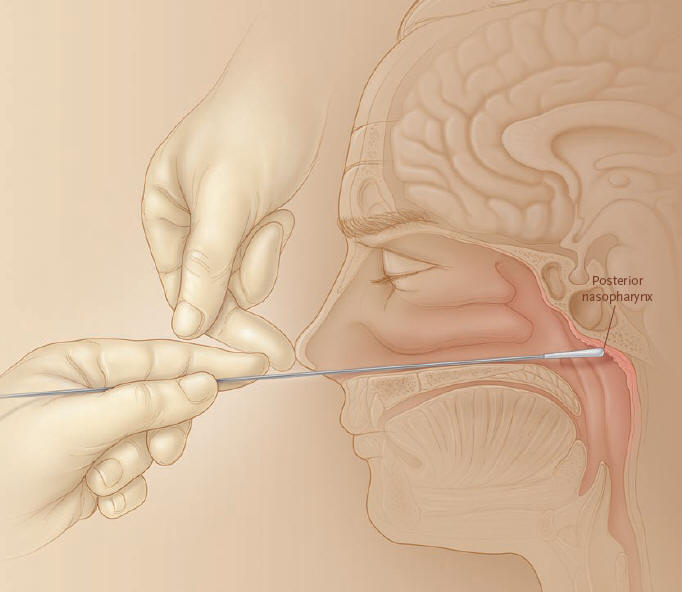
Dacron or calcium alginate swabs are recommended to obtain culture specimens.
Dacron swabs are appropriate if PCR testing will be performed.
What's New
Mooi FR, van Loo IH, et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence.Emerg Infect Dis. 2009 Aug;15:1206-13.
Devasia RA, Jones TF, Compliance with Azithromycin Versus Erythromycin in the Setting of a Pertussis Outbreak. Am J Med Sci. 2009 Mar;337:176-8.
Guided Medline Search For:
Reviews
Adhikari P, Mietzner T. Cell Mediated Immunity. 2008.
Guided Medline Search For Recent Reviews
History
Cherry JD. Historical Perspective on Pertussis and Use of Vaccines To Prevent It. 100 years of pertussis (the cough of 100 days). Microbe 2007;2:139-144.
Lee J. Development of the Complement-Fixation Test: Jules Bordet, 2011.
Lee J. Jules Bordet and the Discovery of Bordetella pertussis, 2011.
Shapiro-Shapin CG. The Pertussis Vaccine: Triumph of Collaboration in a Small Town in Michigan. Emerg Infect Dis. 2010 Aug;16:1273-78.

