FIGURE LEGENDS
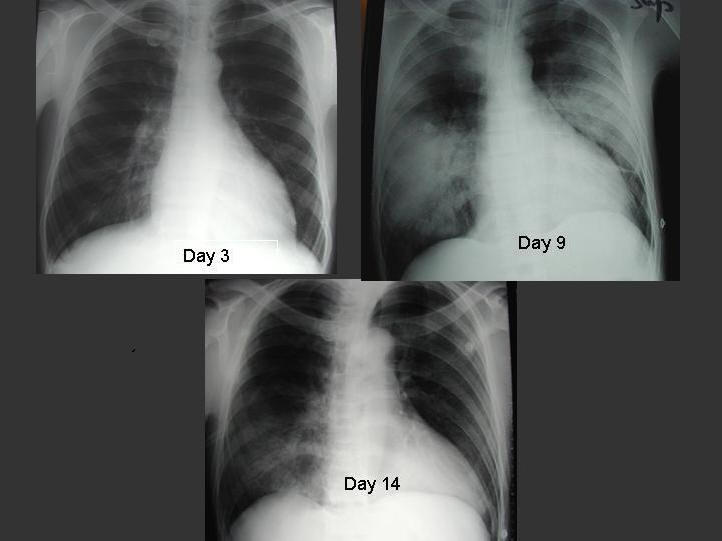
Figure 1. Serial chest radiographs of a 30 year-old male patient with SARS. Initial chest radiograph on day 3 of illness showed right lower zone infiltrate. He developed ARDS on day 9 requiring invasive ventilatory support. His condition improved following 3 pulses of 0.5g per day of methylprednisolone.

Figure 2. High resolution CT of thorax of a 32 year-old male patient showing bilateral ground-glass opacification with interlobular septal and intralobular interstitial thickening.
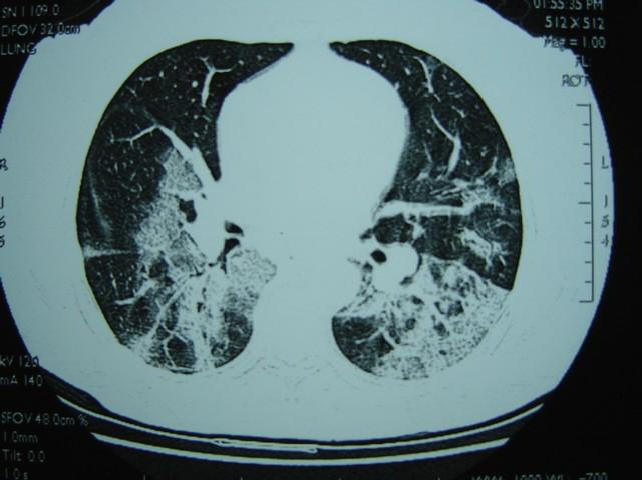
Table 1. Summary of Probable SARS Cases With Onset of Illness From November 1, 2002 to July 31, 2003 (85)
|
Areas |
Cumulative n |
Deaths n |
Case fatality ratio (%) |
Age (median range) yrs |
Health Care Workers n(%) |
|
Australia |
6 |
0 |
0 |
15(1-45) |
1 (16) |
|
Canada |
251 |
43 |
17 |
49 (1-98) |
109 (43) |
|
China |
5327 |
349 |
7 |
pending |
1002 (19) |
|
Hong Kong |
1755 |
299 |
17 |
40 (0-100) |
386 (22) |
|
Taiwan |
346 |
37 |
11 |
42 (0-93) |
68 (20) |
|
Malaysia |
5 |
2 |
40 |
30 (26-84) |
0 |
|
Philippines |
14 |
2 |
14 |
41 (29-73) |
4 (29) |
|
Singapore |
238 |
33 |
14 |
35 (1-90) |
97 (41) |
|
Thailand |
9 |
2 |
22 |
42 (2-79) |
1 (11) |
|
UK |
4 |
0 |
0 |
59 (28-74) |
0 |
|
USA |
29 |
0 |
0 |
33 (0-83) |
0 |
|
Vietnam |
63 |
5 |
8 |
43 (20-76) |
36 (57) |
|
Global |
8098 |
774 |
9.6 |
N/A |
1007 (21) |
Table 2. Clinical Features of SARS on Presentation (4,37,47,78)
|
Symptom |
% of patients with symptom |
|
Persistent fever > 38C |
99-100 |
|
Non-productive cough |
57-75 |
|
Myalgia |
45-61 |
|
Chills/rigor |
15-73 |
|
Headache |
20-56 |
|
Dyspnea |
40-42 |
|
Malaise |
31-45 |
|
Nausea and vomiting |
20-35 |
|
Diarrhea |
20-25 |
|
Sore throat |
13-25 |
|
Dizziness |
4.2-43 |
|
Sputum production |
4.9-29 |
|
Rhinorrhea |
2.1-23 |
|
Arthralgia |
10.4 |
Table 3. WHO Case Definitions of SARS in the Post-Outbreak Period (88)
Clinical case definition of SARS:
A person with a history of :
Fever ≥ 38C
AND
one or more symptoms of lower respiratory tract illness (cough, difficulty breathing, shortness of breath)
AND
Radiographic evidence of lung infiltrates consistent with pneumonia or Respiratory distress syndrome (RDS) OR autopsy findings consistent with the pathology of pneumonia or RDS without an identifiable cause.
AND
No alternative diagnosis can fully explain the illness.
Laboratory case definition of SARS:
A person with symptoms and signs that are clinically suggestive of SARS AND with positive laboratory findings for SARS CoV based on one or more of the following diagnostic criteria:
a) PCR positive for SARS CoV
PCR positive using a validated method from:
At least 2 different clinical specimens (eg nasopharyngeal aspirate or stool) OR
The same clinical specimen collected on 2 or more occasions during the course of the illness (eg sequential nasopharyngeal aspirates) OR
Two different assays or repeat PCR using a new RNA extract from the original clinical sample on each occasion of testing.
b) Seroconversion by ELIZA or IFA
Negative antibody test on acute serum followed by positive antibody test on convalescent phase serum tested in parallel OR
Fourfold or greater rise in antibody titre between acute and convalescent phase sera tested in parallel.
c) Virus isolation
Isolation in cell culture of SARS CoV from any specimen AND PCR confirmation using a validated method.
Table 4. CDC Updated Interim Case Definition for SARS (8).
Clinical criteria:
Early illness
· Presence of 2 or more of the following features: fever (might be subjective), chills, rigors, myalgia, headache, diarrhoea, sore throat, rhinorrhoea.
Mild to moderate respiratory illness
· Temp >100.4F or 38C) and at least one lower respiratory illness (eg cough, dyspnoea, difficulty breathing)
Severe respiratory illness
· Meets clinical criteria of mild to moderate respiratory illness, and
· One or more of the following findings:
radiographic evidence of pneumonia, or acute respiratory distress syndrome, or autopsy findings consistent with pneumonia, or acute respiratory distress syndrome without an identifiable cause.
Epidemiologic criteria:
Possible exposure to SARS CoV
At least one of the following exposures in the 10 days before onset of symptoms:
· Travel to a foreign or domestic location with documented or suspected recent transmission of SARS, or
· Close contact with a person with mild-to-moderate or severe respiratory illness and with history of travel in the 10 days before onset of symptoms to a foreign or domestic location with documented or suspected recent transmission of SARS CoV.
Likely exposure to SARS CoV
One of the following exposures in the 10 days before onset of symptoms:
· Close contact with a confirmed case of SARS CoV disease or
· Close contact with a person with mild-to-moderate or severe respiratory illness for whom a chain of transmission can be linked to a confirmed case of SARS CoV disease in the 10 days before onset of symptoms.
Laboratory criteria:
· Detection of serum antibody to SARS-CoV by a test validated by CDC (eg enzyme immunoassay), or
· Isolation in cell culture of of SARS-CoV from a clinical specimen, or
· Detection SARS-CoV RNA by reverse- transcriptase polymerase chain reaction (RT-PCR) test validated by CDC and with subsequent confirmation in a reference laboratory (eg CDC).
Exclusion criteria
A person may be excluded as a SARS report under investigation if any of the following applies:
· An alternative diagnosis can fully explain the illness.
· Absence of antibody to SARS CoV in a serum specimen obtained > 28 days after symptom onset.
· The case was reported on the basis of contact with a person who was excluded subsequently as a case of SARS CoV disease; then the reported case is also excluded provided other epidemiologic or laboratory criteria are absent.
SARS disease Classification:
· Probable case of SARS CoV disease: in a person who meets the clinical criteria for severe respiratory illness and the epidemiologic criteria for likely exposure to SARS-CoV.
· Confirmed case of SARS CoV disease: in a person who has a clinically compatible illness (ie early, mild-to-moderate, or severe) that is laboratory confirmed.
Table 5. Diagnostic Tests for SARS-CoV
|
RT-PCR |
Detection rate |
|
Nasophargyneal aspirate |
Conventional RTPCR (62): 32% Day 3, 68% Day 14 Second-generation with real-time quantitative RTPCR assay (64): 80% during first 3 days |
|
Stool (62) |
97% Day 14 |
|
Urine (62) |
42% Day 15 |
|
Real-time quantitative Serum SARS CoV RNA (30,54,55) |
80% Day 1, 75% Day 7, 45% Day 14 |
|
Serology (62) IgG seroconversion to SARS-CoV |
15% Day15 60% Day 21 >90% Day 28 |