Table 1. Prominent Clinical Features of Congenital Rubella Syndrome (12,22)
| Major |
|
Eye Cataract,
Glaucoma, Retinitis, and Micropthalmos
Ear Severe Bilateral Deafness Heart Patent Ductus Arteriosus(PDA), Pulmonary Stenosis(PS), Atrial Septal Defect (ASD), Ventricular Septal Defect(VSD), Valvular Aortic Stenosis(AS), and Pulmonary Hypertension(PS) |
|
Minor |
|
Thrombocytopenia Microcephaly Mental retardation Growth retardation Late Onset Diabetes Mellitus |
Table 2. Current Rubella Vaccines Unused in the World
|
Virus Strain |
Isolation of Progenitor Virus |
Cells Used for Production |
Manufacturer |
Country |
References |
|
RA27/3 |
1964 USA |
Human diploid cells |
Merck Sharp & Dohme Institute Butantan GlaxoSmithKline Aventis Pasteur Scalvo Berna Products Institute of Sera & Vaccines Institute of Immunology
Serum Institute Dong Shin Pharm |
USA Brazil Belgium, UK France Italy, Germany Switzerland Czech Croatia Sweden India Korea |
|
|
BRD2 |
1980 China |
Human diploid cells |
National Vaccine & Serum Institute |
China |
|
|
Matsuba |
1969 Japan |
Rabbit kidney cells |
Lanzhou Institute of Biological Products Chemo-Sero-Therapeutic Research Institute |
China Japan |
|
|
Takahashi |
1968 Japan |
Rabbit kidney cells |
Kitasato Institute |
Japan |
|
|
Matsura |
1966 Japan |
Quail embryo fibroblasts |
Research Foundation for Microbial Diseases of Osaka University |
Japan |
|
|
TO-336 |
1967 Japan |
Rabbit kidney cells |
Takeda Chemical Industries |
Japan |
Table 3. Target Groups for Rubella Vaccination (31)
Infants (12 months and more)
Older unvaccinated children and adolescents
College Students
Childcare personnel
Healthcare workers
Military personnel
Adult women before pregnancy
Adult seronegative women post partum
Adult men in contact with pregnant women
All of the above as part of a two-dose elimination strategy
Table 4. Results of Field Tests of Rubella Vaccines in Japan (32)
Clinical Reactions
Vaccine Strain
Potency log TCInD50/ 0.5 ml
Group (*1)
Vaccinees (N)
Fever
Rash
Arthralgia
Lymph-adenopathy
Serocon-version
Mean HI Titer (log2)
Virus Isolation from vaccineees
Antibody production by contacts (*2)
Matsuura
3.3
A
B
C
Total
69
72
184
325
0
0
3
3
0
0
0
0
0
0
2
2
0
0
2
2
91.3
98.6
97.8
96.6
5.7
5.5
4.7
5.1
-- (*3)
0/23
--
0/23
0/105
0/23
0/28
0/156
T0-336
3.1
A
B
C
Total
134
434
311
879
0
11
7
18
0
1
13
14
0
0
17
17
0
0
12
12
96.3
97.7
99.0
97.9
7.4
6.9
6.0
6.7
6/15
1/8
0/11
7/34
0/90
0/21
0/36
0/147
Matsuba
4.2
A
B
C
Total
17
108
655
780
0
0
4
4
0
0
5
5
0
0
3
3
0
0
9
9
100.0
100.0
100.0
100.0
6.7
6.6
5.7
5.8
2/7
--
--
2/7
0/33
--
0/75
0/108
Takahashi
3.3
A
B
C
Total
132
84
435
651
0
0
0
0
0
0
0
0
0
0
1
1
0
0
0
0
93.9
100.0
100.0
98.8
6.4
6.5
6.2
6.3
17/50
--
--
17/50
0/126
--
--
0/126
*1A: Closed group of infants (1-14 years old), B: Open group of infants (1-14 years old), C: Young women (15-21 years old).
*2. None developed illness by contact with any of four vaccines
*3. Not tested
Table 5. Hemagglutination-inhibiting Antibody Response Of Initially Seronegative Children And Adults Who Received RA27/3 Rubella Vaccine (31).
|
Group |
Seroconverting Total (%) |
HI Titer Range |
Mean |
|
Children |
153/153 (100%) |
8-1024 |
153 |
|
Adults |
98/99 (99%) |
<8-512 |
84 |
Table 6. Frequencies of Acute and Chronic Reactions to Rubella Vaccine (RA27/3) Or Placebo In Adult Women (33).
|
Reactions |
Placebo (N=275) |
Group (%) Vaccine (N=268) |
Odds Ratio (95% Cl) |
|
Acute |
|
|
|
|
Sore throat |
32 |
34 |
1.09 (0.75-1.59) |
|
Cervical Lymphadenopathy |
10 |
19 |
2.21 (1.31-3.76) |
|
Rash |
11 |
25 |
2.57 (1.58-4.21) |
|
Myalgia |
16 |
21 |
1.36 (0.88-2.10) |
|
Paresthesias |
7 |
7 |
1.09 (0.57-2.09) |
|
Arthralgia |
16 |
21 |
1.42 (0.92-2.19) |
|
Arthritis |
4 |
9 |
2.36 (1.13-4.92) |
|
Arthralgia or Arthritis |
20 |
30 |
1.73 (1.17-2.57) |
|
Chronic |
|
|
|
|
Myalgia |
9 |
15 |
1.68 (0.99-2.84) |
|
Paresthesias |
4 |
5 |
1.12 (0.50-2.50) |
|
Arthralgia or Arthritis |
15 |
22 |
1.58 (1.01-2.45) |
Table 7. Accidental Vaccination Before Pregnancy and During Early Pregnancy of Women
Outcome for Live Births
Study Location and Vaccines
Vaccinated Women (N)
Susceptible Mother Before Vaccination
Total Live-born
Live-born to Susceptible Mothers
Asymptomatic Infection
CRS
Detect
Products of
Conception
Positive for
Rubella
Theoretical
Risk of CRS Defect (%)
Reference
United States
Cendehill and
HPV-77
538
149
290
94
8
0
17/85
0-3.8
RA27/3
683
272
562
226
3
0
1/35
0-1.6
Germany
Cendehill
340
130
177
107
2
0
1/34
RA27/3
25
16
17
12
0
0
England
Cendehill
5
1
RA27/3
32
15
51
21
0
0
0/44
Unknown
17
7
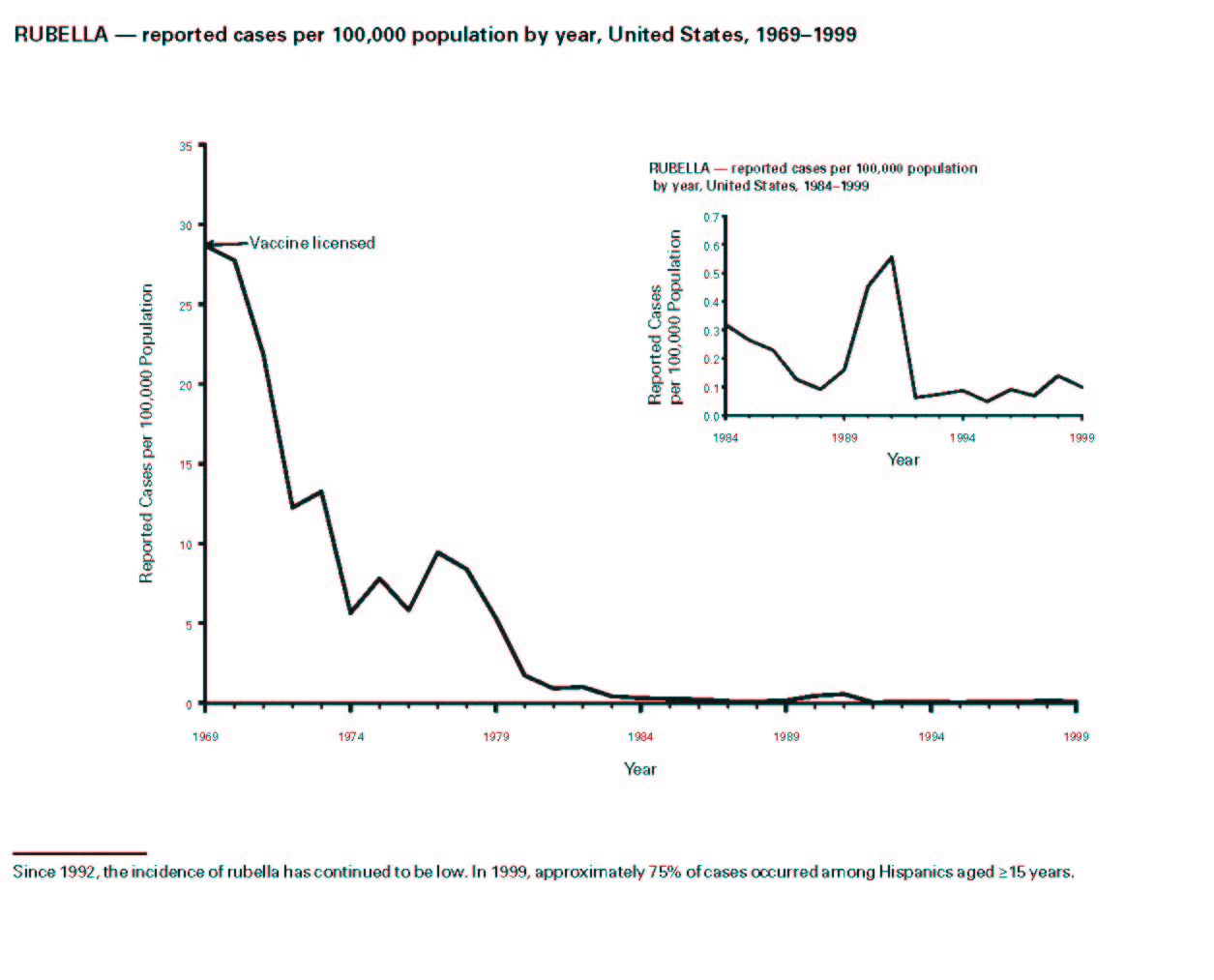
Figure 1. Rubella - reported cases per 100,000 population by year, United States, 1969-1999(40).

Figure 2. Number of reported rubella and congenital rubella syndrome cases by year - United States, 1980-1996(42).
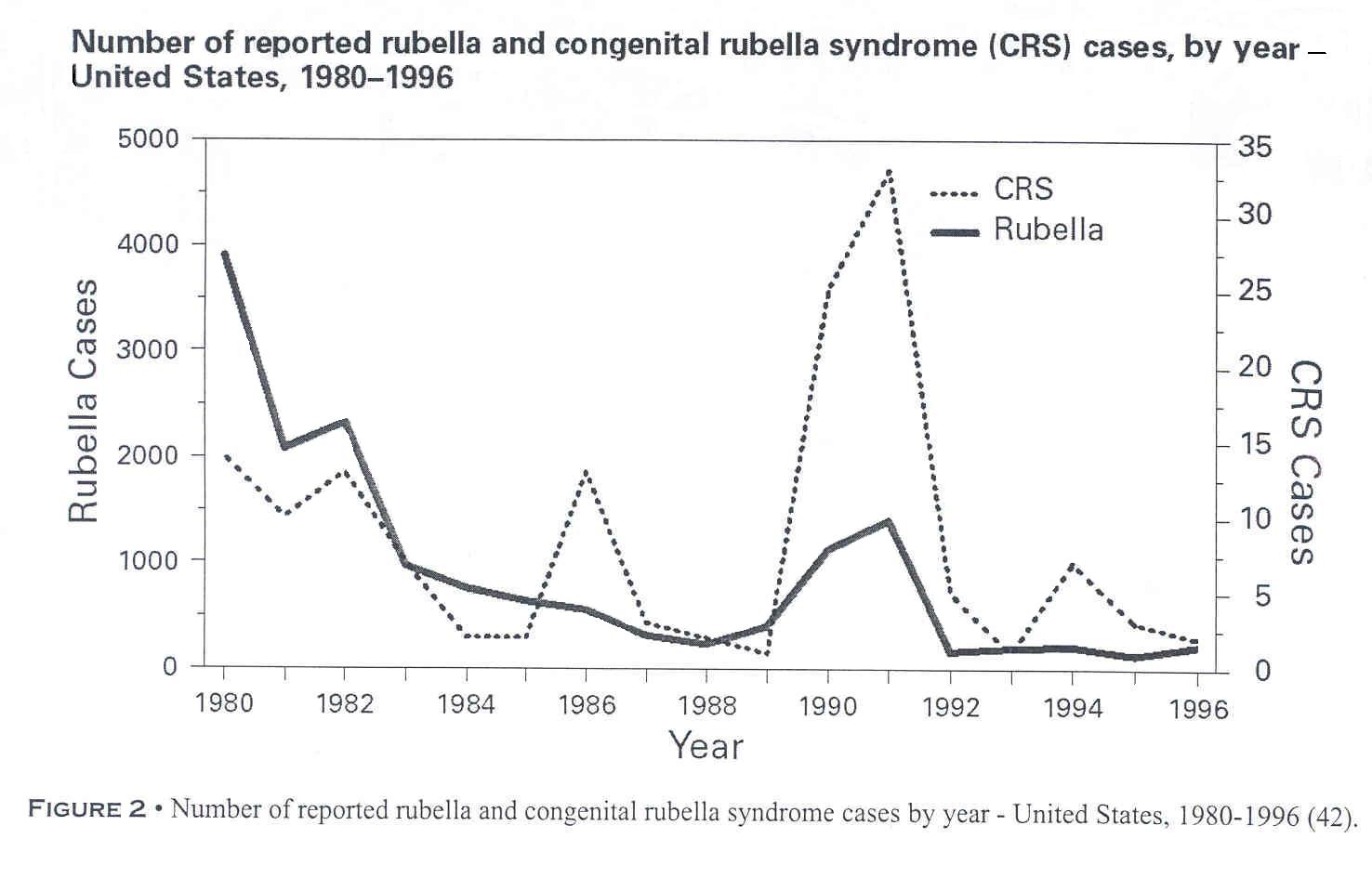
Figure 3. Weekly rubella cases reported per sentinel in Japan, 1982-2001(44).
Rubella cases reported from approximately 3000 sentinels(mainly pediatric hospitals and clinics) are plotted as weekly cases per sentinel.
Figure 4. Congenital rubella syndrome cases in Japan, 1978-2001(46).
Congenital rubella syndrome cases collected by questionnaire to major hospitals(approximately 1000), by searching in scientific meetings and reports and in addition of cases examined in the National Institute of Infectious Diseases. Dotted vertical line represents year of nationwide epidemic of rubella. In 1997, there was not a large but a small epidemic. Note the close correlation of peak in congenital rubella syndrome cases with the year of epidemic.