Varicella-Zoster Virus
Authors: Authors: Nicholas L. Baird, PhD, Jacqueline L. Bowlin, MS, Maria A. Nagel, M.D., Don Gilden, M.D.
First Edition (2003): Ann M Arvin, M.D. and Jason G Newland, M.D.
Virology
Varicella-zoster virus (VZV) is an exclusively human, highly neurotropic alphaherpesvirus. Replication is restricted to cells of human or simian origin. VZ virions are 180 to 200 nm in diameter. The major components of VZV particles include a core of double-stranded DNA and an icosahedral nucleocapsid consisting of 162 capsomere proteins surrounded by a tegument and lipid envelope (6). The DNA genome contains approximately 125,000 base pairs and encodes at least 70 distinct gene products. Both a unique long region and unique short region are flanked by internal repeat regions, although VZV lacks a terminal region with flanking inverted repeats at the leftward end of the genome. The virus particle is highly temperature-sensitive and depends upon the envelope for infectivity. VZV spreads as cell-free virus to susceptible hosts, but within the infected host, virus spreads cell-to-cell. VZV isolated from clinical samples shows no significant phenotypic differences. While complete DNA sequence analysis of over 23 VZV samples has identified five distinct geographically isolated variations (clades), most naturally occurring mutations involve single nucleotide polymorphisms that do not alter virus growth characteristics (15). Similarly, mutations within VZV vaccine DNA do not drastically alter virus gene expression and only slightly affect the overall growth rate in tissue culture (59).
VZV is highly contagious. While VZV DNA can be found on surfaces such as door knobs, toys, air-conditioning filters and tables in rooms where children or adults have active varicella or zoster, enveloped VZV is highly sensitive to desiccation such that infection is typically acquired through inhalation of aerosolized virus.
Epidemiology
VZV is a ubiquitous human pathogen with a worldwide geographic distribution. The annual incidence of varicella in the United States (U.S.) was equivalent to the annual birth rate (4 million) before varicella vaccine was introduced in 1995, and the prevalence of anti-VZV IgG antibodies indicates that virtually 99% of adults are infected (63).
Primary VZV infection causes varicella (chickenpox), and virus reactivation from cranial nerve ganglia or dorsal root ganglia produces zoster (shingles). Humans are the only reservoir for VZV, and anyone with varicella or zoster can transmit disease to seronegative individuals, often by the respiratory route. Varicella is primarily a childhood disease that occurs worldwide. Zoster occurs primarily in the elderly as cell-mediated immunity to VZV declines with age, as well as in individuals whose immune system is compromised by human immunodeficiency virus (HIV) infection or by treatment with corticosteroids or immunosuppressive drugs.
Studies using varied methods and conducted in different populations in the U.S., Canada, South America, Europe, Asia and Australia revealed a median zoster incidence of 4-4.5 per 1000 person-years (136). Unlike varicella, which occurs most often in the winter and early spring, zoster is not seasonal because it originates from reactivation of latent virus. Zoster can occur at any age, but the incidence increases dramatically after middle-age. Furthermore, the age-adjusted incidence of zoster appears to be increasing (70, 137). In Olmsted County, MN, zoster rates increased 28% over 5 years, from 3.2 per 1000 person-years in 1996-1997 to 4.1 in 2000-2001, an increase not explained by changes in age distribution or the prevalence of immunocompromised persons (88). Similar increases over variable time periods have been seen in most, but not all, analyses conducted in the U.S., Canada, Europe, Asia and Australia, but the reasons for these increases are unknown. All studies show a striking increase of zoster with age, particularly after age 50 years. The mean age at onset of zoster among adults (age 22 years and older) is 59.4 years, with 68% of cases occurring in those 50 years and older. Age-adjusted rates are higher in women than in men [3.9 vs. 3.2 per 1000 person-years, respectively (p <0.0001)]. Based upon these age-specific rates, ~1 million new episodes of zoster have been estimated to occur in the U.S. yearly, with a lifetime risk of ~30%. Children or adolescents who acquired primary VZV infection in utero or in the first year of life are 20.9 times more likely to develop zoster before age 20 (8, 17, 50). Recurrent zoster has 3 to 5% lifetime risk to 6.2% over 8 years (137), and is associated with decreased immune competency. Among individuals with HIV, the recurrence rate is 13 to 26%. Since the risk of zoster increases with age, and the U.S. population over age 65 is expected to reach 72 million in 2030, zoster and serious neurologic and ocular complications of zoster will continue to be a significant health care burden.
ANTIVIRALS
Pharmacokinetics Overview
Oral antivirals to treat VZV infection or reactivation (varicella or zoster, respectively) include acyclovir, valacyclovir, and famciclovir; acyclovir is also licensed for intravenous treatment of VZV infections (Table 1). Oral acyclovir has a low bioavalability (15-30% absorption) and achieves plasma concentrations of 1.6 mg/L after treatment with 800 mg 5 times daily. Oral valacyclovir has a bioavailability of >50% (113) and achieves peak plasma concentrations of 5.65 mg/L (71, 130) after treatment with 1 g 3 times daily. Oral famciclovir (the pro-drug of penciclovir) has a bioavailability of 77% (23, 110) and achieves peak plasma concentrations of 3.3 mg/L after treatment with 500 mg 3 times daily. Oral penciclovir is not used due to low bioavailablity (5%). Intravenous acyclovir at doses of 10-15 mg/kg every 8 hours for 10 to 14 days achieves plasma concentrations of 7-20 mg/L.
Successful treatment of VZV CNS infections (e.g., meningoencephalitis, myelitis, vasculopathy) requires antivirals to cross the blood/-brain barrier. Levels of acyclovir in CSF after treatment with acyclovir or valacyclovir are 25-50% of that in plasma (112, 124). It is unknown whether penciclovir (the active drug of famciclovir) crosses the blood-brain barrier.
Mechanisms of Action
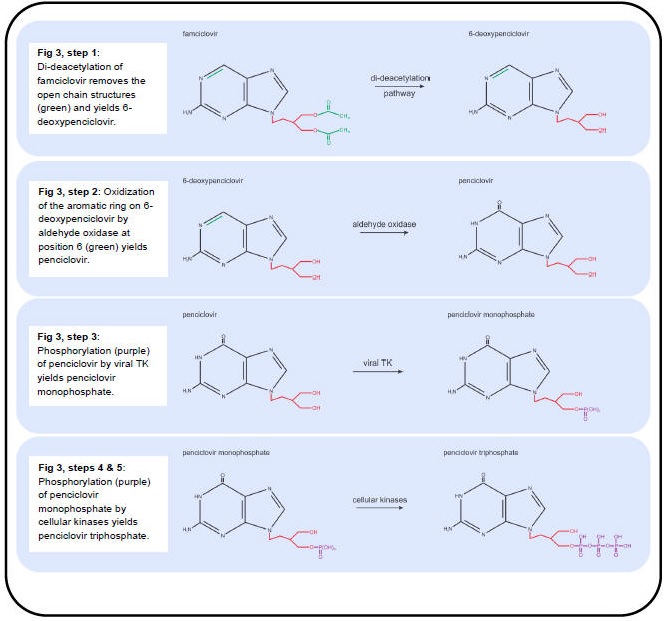
The anti-VZV drugs in use (acyclovir, valacyclovir and famciclovir) effectively inhibit viral DNA replication by targeting the viral DNA polymerase. Valacyclovir is an L-valyl ester pro-drug that is rapidly converted to acyclovir in the liver by hepatic and plasma esterases (Figure 2, step 1) (96). Famciclovir is the pro-drug of penciclovir and is rapidly modified in the intestine and liver in a two-step process. First, famciclovir is di-deacetylated to form 6-deoxypenciclovir (Figure 3, Step 1) (123) and then further modified by aldehyde oxidase to form penciclovir (Figure 3, step 2) (22). The active compounds (acyclovir and penciclovir) are both partial-guanosine analogues, i.e., they lack the ribose (Figure 1, blue), and instead have open chain structures (Figure 2 and Figure 3). Upon incorporation into the viral DNA, both act as competitive inhibitors of the viral DNA polymerase. Without the ribose molecule, the analogues lack the 3’ hydroxyl group required for DNA elongation. Although penciclovir lacks a ribose, it does possess a hydroxyl group that can support minimal chain extension (Figure 3), but a deceleration and eventual termination of viral DNA elongation does occur (9).
The partial-guanosine analogues have a two-layered specificity for VZV-infected cells. First, the non-phosphorylated compounds require the viral thymidine kinase (TK, VZV protein 36) to convert them into deoxynucleotide monophosphate (dNMP) - analogues (deoxynucleotide monophosphate) (42). Unlike cellular TKs, viral TK does not have a high specificity for substrates and therefore accepts several nucleotide analogues (90). The dNMP-analogues are converted to dNTP-analogues by cellular kinases and are incorporated into DNA by the viral DNA polymerase (9). The second layer of specificity is that the cellular DNA polymerase rarely accepts dNTP-analogues such as acyclovir- and penciclovir- triphosphate for incorporation into host DNA due to its high substrate specificity, whereas the viral polymerase is much less specific (32,41).
PRIMARY VZV INFECTION - VARICELLA
VZV infection is restricted to humans and is highly contagious. During primary exposure, VZV infects Langerhans and plasmacytoid dendritic cells of the upper respiratory mucosa and nasopharyngeal region, with subsequent transfer of virus by these antigen-presenting cells to CD4 T-cells, which in turn transmit virus to dermal endothelial cells, leading to varicella (chickenpox). During primary infection, VZV infects ganglionic neurons most likely hematogenously as supported by the finding that ganglionic infection with simian varicella virus, a closely related neurotropic alphaherpesvirus, precedes rash (73). Varicella is clinically diagnosed by the presence of vesicular rash on an erythematous base.
Healthy Individuals
In healthy children under the age of 12, varicella is usually self-limited, and treatment is usually limited to antihistamines and acetaminophen for relief of pruritus and fever, respectively. Clinical benefit from acyclovir is only modest and does not reduce the risk of complications (27); thus, the American Academy of Pediatrics (AAP) does not recommend routine antiviral treatment of uncomplicated varicella in otherwise healthy children (3). However, an oral antiviral agent (valacyclovir, famciclovir or acyclovir) should be given to immunocompromised children or children who are taking long-term corticosteroids. Healthy adults who develop varicella are often sicker than children and are at greater risk of VZV pneumonitis (21). Thus, adults with varicella are treated with an oral antiviral agent.
Varicella Pneumonia
Pneumonia is a serious life-threatening complication of varicella and is more common in adults and in immunocompromised hosts. The incidence in adults is approximately 1 in 400 cases (78). The overall mortality ranges from 10% to 30% (108, 117), and is increased with tobacco use (33,38), pregnancy (37) and immunosuppression. Treatment is with intravenous acyclovir (108). Whether corticosteroids confer additional benefit over antivirals alone is unclear (1, 95).
Immunosuppressed Individuals
Patients receiving immunosuppressive therapy and HIV-infected individuals are at greater risk for disseminated varicella. Treatment for varicella in high-risk patients is intravenous acyclovir, 10-15 mg/kg every 8 hours for 7-10 days or longer, depending on the clinical response and extent of immunodeficiency. In milder cases, antiviral therapy can be continued until no new lesions have appeared for 48 hours. HIV-infected children with mild disease and a high CD4 count should be treated immediately withan oral antiviral agent. If the child does not improve quickly, intravenous acyclovir should be started. Treatment should be for 1-2 weeks, depending on the clinical response (44). As in varicella pneumonia, additional benefits of corticosteroids over antivirals alone in immunosuppressed individuals remain unclear (1, 95).
Pregnancy, In Utero, and in Neonates
Pregnancy is associated with an increased risk of severe varicella (107) and VZV pneumonitis. The incidence of varicella is 1 to 5 cases per 10,000 pregnancies (114). Morbidity is greatest with third trimester infection (24). Mortality can be as high as 45% in untreated pregnant women (55, 107), but decreases to 14% with acyclovir treatment (16). Up to 20% of VZV infections in pregnancy can be complicated by pneumonia. There are no well-controlled studies on the teratogenicity of acyclovir; the AAP does not recommend treatment of uncomplicated varicella during pregnancy. Pregnant women with complicated varicella (e.g., pneumonia) should receive intravenous acyclovir at 10-15 mg/kg every 8 hours for 7 days and possibly longer.
Prophylaxis is recommended for pregnant women who have been exposed to varicella or zoster. VariZIG, a purified human immunoglobulin, should be administered within 10 days of exposure at a dose of 125 units/10 mg body weight intramuscularly, with a maximum dose of 625 units. If VariZIG is not given within 10 days of exposure, a single dose of intravenous immunoglobulin (IVIG) at 400 mg/kg can be given; alternatively, the patient can be observed and if varicella develops, antiviral agents are given.
The risk of congenital varicella after maternal primary VZV infection is approximately 0.4% when infection occurs during the first trimester and 2% with second trimester varicella (34, 53, 80, 93). Polymerase chain reaction (PCR) testing of fetal blood or amniotic fluid for VZV DNA, in conjunction with ultrasonography to detect fetal abnormalities, helps in deciding about antiviral therapy.
Neonatal varicella may occur if a mother contracts varicella during the last 3 weeks of pregnancy. The severity of intrauterine acquired neonatal varicella is closely associated with the time of onset of maternal infection. Mortality is 20-30% (107). Approximately 20% of neonates born to mothers with varicella 5 days before or 2 days after delivery develop disseminated varicella (77, 107). Thus, neonates should receive either VariZIG or IVIG. The dose for VariZIG is 125 units/10 kg body weight, with a maximum dose of 625 units, given intravenously or intramuscularly. The minimum dose is 125 units. The dose of IVIG is 400 mg/kg. Intravenous acyclovir, 500 mg/m2 every 8 hours or 10 mg/kg every 8 hours, should be administered only if the neonate develops varicella rash.
VZV REACTIVATION - ZOSTER AND OTHER NEUROLOGICAL AND OCULAR COMPLICATIONS
With advancing age or immunosuppression, cell-mediated immunity to VZV declines and virus reactivates to cause herpes zoster (shingles) which is often complicated by chronic pain (postherpetic neuralgia), cranial nerve palsies, zoster paresis, meningoencephalitis and cerebellitis, myelopathy, vasculopathy and multiple ocular disorders. VZV reactivation also produces chronic radicular pain without rash (zoster sine herpete).
Pathology and Pathogenesis
The cardinal pathological features of zoster are characterized by inflammation and hemorrhagic necrosis with associated neuritis, localized leptomeningitis, unilateral segmental poliomyelitis and degeneration of related motor and sensory roots (57). Demyelination is seen in areas with mononuclear cell (MNC) infiltration and microglial proliferation. Intranuclear inclusions, viral antigen and herpesvirus particles are found in acutely infected ganglia.
Zoster
VZV reactivation (zoster) is usually manifest by a painful dermatomal distribution vesicular eruption on an erythematous base as well as unpleasant sensations (dysesthesias) produced by a light touch (allodynia). Rash and pain usually develop within a few days of each other, although pain can precede rash by weeks to months (46). Because VZV becomes latent in ganglia along the entire neuraxis, zoster can develop anywhere on the body. Zoster in an otherwise healthy young person may be the first manifestation of HIV infection (119). Varicella in infancy also predisposes to zoster in early adulthood.
Zoster is associated with optic neuritis and ophthalmoplegia. Involvement of the maxillary and mandibular distribution of the trigeminal nerve can produce osteonecrosis and spontaneous tooth exfoliation. Geniculate zoster causes weakness or paralysis of ipsilateral facial muscles. Facial palsy and vesicles in the external auditory canal or on the tympanic membrane (zoster oticus) or on the ipsilateral anterior two-thirds of the tongue or hard palate constitutes the Ramsay Hunt syndrome (RHS). Ramsay Hunt syndrome is frequently associated with tinnitus, hearing loss, nausea, vomiting, vertigo and nystagmus, indicating involvement of cranial nerve VIII within the bony facial canal. Zoster is also followed by involvement of cranial nerves IX, X, XI and XII.
Zoster paresis (weakness) may present with arm weakness or diaphragmatic paralysis after cervical distribution rash, leg weakness after lumbar or sacral distribution rash, or urinary retention after sacral distribution rash. Magnetic resonance imaging (MRI) of patients with zoster paresis reveals involvement of both anterior and posterior roots at spinal levels corresponding to clinical deficit. Rarely, clinical deficit in cervical zoster paresis extends to the brachial plexus, confirmed by both electrodiagnostic testing and MRI. Thoracic zoster has been associated with abdominal muscle weakness, resulting in abdominal hernia.
Postherpetic Neuralgia
Dermatomal-distribution pain persisting for more than 3 months after zoster is operationally defined as postherpetic neuralgia (PHN). Age is the most important factor in predicting its development. Among persons older than 50 and 80 years, the incidence of PHN in zoster patients is 18% and 33%, respectively. Overall, 80% of PHN occurs among persons 50 years and older. In addition, more than 40% of zoster patients >60 years of age experience chronic pain. Analysis of ganglia from an early case of postherpetic neuralgia of 2.5 months’ duration revealed diffuse and focal infiltration by chronic inflammatory cells (111), an observation confirmed by Watson et al., (126) who found prominent collections of lymphocytes in ganglia from a patient with postherpetic neuralgia of 2 years’ duration. The inflammatory response in ganglia raised the possibility of prolonged viral infection. Further evidence that postherpetic neuralgia may be produced by low-level ganglionitis has come from the detection of VZV DNA and proteins in blood MNCs of many patients with postherpetic neuralgia and from the favorable response of some postherpetic neuralgia patients to antiviral treatment.
Treatment of Herpes Zoster, Acute Pain and PHN
The optimal approach to the management of herpes zoster is analgesia for pain and antivirals to speed healing of rash and shorten the duration of acute pain.
Oral acyclovir, famciclovir and valacyclovir have similar clinical efficacy in herpes zoster in immunocompetent patients if started within 72 hours of rash. Current guidelines recommend treating zoster with famciclovir 500 mg 3 times daily, valacyclovir 1 g 3 times daily or acyclovir 800 mg 5 times daily. While the dosing of acyclovir requires treatment 5 times a day, it is considerably cheaper than valacyclovir or famciclovir. Table 1 lists treatments for zoster and its neurological complications; the choice of drug may depend on dosing and drug price.
Immunocompromised patients should be treated with valacyclovir (1 g 3 times daily for 7 days); if rash does not resolve within a few days, these patients may need intravenous acyclovir (10-15 mg/kg 3 times daily for 5-7 days).
In contrast, zoster during pregnancy does not pose any risks for intrauterine infection (34) and should only be treated with acyclovir in cases of severe disease (107).
Randomized placebo-controlled studies have shown that oral acyclovir reduces new lesion formation and significantly accelerates rash healing in normal hosts with herpes zoster (60, 133). In the largest study, the time to full crusting of skin lesions was reduced by more than 36 hours by the use of acyclovir (134). Pain during the acute phase was also improved, but conflicting results were obtained regarding the effect of acyclovir on the incidence of PHN. A meta-analysis of 4 placebo-controlled trials showed that acyclovir, when given within 48 to 72 hours of the onset of rash, accelerated the resolution of pain by all of the measures employed, with benefits more evident in patients 50 years or older (133). Another meta-analysis that included one additional trial also demonstrated that acyclovir treatment reduced PHN by 46% at 6 months (60). Increasing the duration of acyclovir therapy beyond 7 days provides no additional benefit (132).
In a randomized, double-blind study of 1141 immunocompetent adults of age ≥50 years with herpes zoster that compared the safety and efficacy of valacyclovir to acyclovir, valacyclovir significantly accelerated resolution of zoster-associated pain (median pain durations were 38 and 44 days, respectively, vs. 51 days for acyclovir) (11). Valacyclovir significantly reduced the duration of PHN and also decreased the proportion of patients with pain persisting for 6 months (19.3 vs. 25.7%). However, there were no differences in rates of resolution of skin lesions or quality-of-life measures. Adverse events were similar. A later study confirmed the benefits of valacyclovir over acyclovir irrespective of whether treatment was started 48 to 72 hours after rash onset (135).
In a placebo-controlled trial of 429 immunocompetent adults with herpes zoster treated within 72 hours of rash (120), famciclovir accelerated lesion healing and reduced the duration of viral shedding. The median duration of PHN was reduced by approximately 2 months. In another randomized, double-blind study, the efficacy and tolerability of famciclovir (250 mg, 500 mg and 750 mg 3 times daily) were compared to that of oral acyclovir (800 mg 5 times daily). A total of 545 immunocompetent adults were treated within 72 hours of rash. Famciclovir was as effective as acyclovir at all dose levels for cutaneous lesion healing, duration of VZV shedding and time to loss of pain (25). At all doses, famciclovir was superior to acyclovir when given within 48 hours of rash. A more recent study that compared famciclovir, 500 mg 3 times daily with valacyclovir, 1 g 3 times daily revealed no differences in rash healing, resolution of pain, loss of PHN or safety profiles (121).
Overall, clinical trials demonstrate that treatment of zoster with acyclovir, famciclovir or valacyclovir reduces the duration of viral shedding and new lesion formation and accelerates rash healing. Antiviral therapy may also reduce PHN by inhibiting viral replication and limiting neural damage, and thus should be instituted without delay. Antiviral therapy is recommended for all immunocompetent patients’> 50 years of age, moderate or severe pain/rash or with ophthalmic-distribution zoster. For patients who present >72 hours after rash, the benefit of antiviral therapy is unclear, but treatment should be considered in elderly patients, particularly those with severe pain, continued new vesicle formation or neurological complications (29).
Administration of corticosteroids during acute zoster has been shown to be efficacious in large double-blind studies (36, 132). Studies that used prednisolone and oral acyclovir revealed that pain was reduced during the first few days, but had no effect on the incidence, duration or severity of PHN (36, 132). A placebo-controlled study of treatment with acyclovir and prednisone showed that combination therapy significantly shortened the duration of rash and increased quality of life, but did not affect resolution of pain during a 6-month period (131). Corticosteroids should be considered for patients with moderate to severe pain and for patients with neurological complications of zoster (29). Systemic corticosteroids are usually prescribed for acute retinal necrosis but their effectiveness is unknown. Topical corticosteroids aid resolution of episcleritis, scleritis or iritis in patients with ophthalmic-distribution zoster (94).
PHN is difficult to manage and no universal treatment exists. The same medications used to treat zoster pain are also used for PHN. These include opioid analgesics (97, 101), tricyclic antidepressants (75, 102, 127, 129), antiepileptics such as gabapentin (99, 100), pregabalin (28, 103) and divalproex sodium (65). First-line therapies include tricyclic antidepressants, gabapentin and pregabalin and topical lidocaine patches. Opioids, tramadol, capsaicin cream and capsaicin 8% patches are second- or third-line therapies (Table 2).
Clinical trials of opioid agonists to treat neuropathic pain have been analyzed by meta-analyses (30, 31). While short-term studies provide only equivocal evidence regarding the efficacy of opioids in reducing pain, intermediate-term studies demonstrate significant efficacy of opioids over placebo. Two studies have shown the efficacy of oxycodone and morphine (MS contin or methadone) (97, 125).
Tricyclic antidepressants have been evaluated in PHN (14, 75, 102, 127). In a randomized double-blind trial, amitriptyline and nortriptyline had similar analgesic effects (129). In a randomized, double-blind trial comparing desipramine, amitriptyline, and fluoxetine, desipramine provided the best outcome, with satisfactory relief in 80% of treated patients (102). Since amitriptyline has significant side effects, especially in elderly patients, nortriptyline is preferable. Desipramine is more sedative that nortriptyline (29).
Antiepileptics such as gabapentin (10, 99, 100) and pregabalin (28, 40, 103, 122) provide significant relief of neuropathic pain. Both drugs reduce severity of pain and sleep interference associated with PHN and improve mood and quality of life (28, 40, 99, 100, 123). A single dose of gabapentin at 900 mg also reduces acute pain and allodynia (10). Both drugs are FDA-approved for PHN. Pregabalin might be preferable to gabapentin since it can be titrated to an effective dose more rapidly (29). Divalproex sodium provides significant pain relief with minimal adverse reaction (65); however, clinical experience to treat PHN is limited.
Topical lidocaine patches or capsaicin (128) provides some relief. Medical interventions are often used in combination, or sequentially, because no single approach is beneficial. The benefits of sympathetic blockade, intravenous lidocaine or cryotherapy have not been convincingly demonstrated.
A newer potentially promising treatment for PHN is percutaneous peripheral nerve field stimulation. Rare reports indicate its effectiveness for refractory PHN (115). Subjects became pain-free with minimal to no medication needed after ophthalmic, cervical and thoracic distribution PHN.
VZV Meningoencephalitis, Meningoradiculitis and Cerebellitis
All of these neurological complications of VZV reactivation can occur after zoster, but may also occur in the absence of zoster rash, as demonstrated by reports of VZV meningitis (52), meningoradiculitis (51) and cerebellitis (79) in which diagnosis was confirmed by the detection of VZV DNA and anti-VZV antibody in CSF. Treatment is intravenous acyclovir (Table 1).
VZV Myelopathy
VZV myelopathy can present as a self-limiting, monophasic spastic paraparesis, with or without sensory features and sphincter problems. This so-called post-infectious myelitis usually occurs in immunocompetent patients, days to weeks after acute varicella or zoster. VZV myelitis also occurs in the absence of zoster rash. Its pathogenesis is unknown. The cerebrospinal fluid (CSF) usually contains a mild mononuclear pleocytosis, with a normal or slightly elevated protein. Steroids are used to treat these patients, although some improve spontaneously. VZV can also invade the spinal cord. In such instances, VZV myelopathy presents as an insidious, progressive and sometimes fatal myelitis, mostly in immunocompromised individuals, such as patients with AIDS. MRI reveals longitudinal serpiginous enhancing lesions. Diagnosis is confirmed by the presence of VZV DNA or anti-VZV IgG or both in CSF (45). Pathological and virological analyses of the spinal cord from fatal cases have revealed frank invasion of VZV in the parenchyma (64) and, in some instances, spread of virus to adjacent nerve roots. Early diagnosis and aggressive treatment with intravenous acyclovir have been helpful, even in immunocompromised patients (26). Rarely, VZV myelitis recurs, even in immunocompetent patients (45). VZV can also produce spinal cord infarction identified by diffusion-weighted MRI and confirmed virologically (91). Thus, VZV vasculopathy (see below) can cause stroke in the spinal cord as well as in the brain.
VZV Vasculopathy
VZV vasculopathy is caused by productive infection of cerebral arteries, resulting in transient ischemic attacks and ischemic and hemorrhagic stroke. While the exact incidence is unknown, it is most likely more common than previously believed, given recent studies which showed that there is a 30% increased risk of stroke within a year after zoster (62) and a 4.5-fold increased risk with ophthalmic-distribution zoster (68).
A study of 30 subjects with virologically confirmed VZV vasculopathy revealed rash in 63%, CSF pleocytosis in 67% and imaging abnormalities in 97% (82). Angiography revealed abnormalities in 70% of subjects. Large and small arteries were involved in 50%, small arteries in 37%, and large arteries in only 13% of 30 subjects. The CSF of 30% of subjects contained VZV DNA; in contrast 93% had anti-VZV IgG antibody in CSF with a reduced serum/CSF ratio of anti-VZV IgG that confirmed intrathecal synthesis of anti-VZV IgG. Thus, detection of anti-VZV IgG antibody in CSF is the best test to diagnose VZV vasculopathy (83). VZV vasculopathy is treated with intravenous acyclovir. Importantly, diagnosis of this treatable cause of stroke is often missed because there is no history of zoster rash in one-third of subjects, the CSF is normal in one-third of subjects, there is an average 4.2-month delay from zoster to neurological symptoms and signs, and VZV DNA is often not present in CSF.
When VZV reactivates from cranial nerve ganglia, it most likely spreads transaxonally to the outermost layer of the artery wall (adventitia), a notion supported by detection of VZV infection in adventitia in early VZV vasculopathy (86). VZV infection of cerebral arteries is associated with a thickened intima composed of myofibroblasts, which can potentially lead to ischemia, a disrupted internal elastic lamina and a paucity of smooth muscle cells resulting in aneurysm formation and hemorrhage (86). Furthermore, inflammatory cells (primarily CD4 and CD8 T cells and CD68 macrophages) are present predominantly in the adventitia and to a lesser degree in the luminal surface of the thickened intima (87). In early VZV vasculopathy, there is a striking number of neutrophils in the adventitia. A remarkable finding in VZV-infected arteries is the association of inflammation with a thickened intima, supporting findings in the cardiovascular and pulmonary vascular fields that inflammation is intimately involved in vascular remodeling.
Multifocal VZV Vasculopathy with Temporal Artery Infection and Giant Cell Arteritis
Recently, three subjects with a novel variant of multifocal VZV vasculopathy with temporal artery infection were described (74, 85, 106). All three patients presented with ischemic optic neuropathy, in one instance followed by acute retinal necrosis, and VZV infection of the ipsilateral temporal artery was confirmed in all three patients. Importantly, these patients experienced symptoms, signs and laboratory abnormalities characteristic of giant cell arteritis (GCA), a vasculitis of unclear etiology that is treated with corticosteroids. However, histopathological exam of their temporal arteries was negative for GCA. These cases illustrate that patients with suspected GCA, but whose arteries are pathologically negative for GCA, may have multifocal VZV vasculopathy with temporal artery infection. It is essential to differentiate GCA from multifocal VZV vasculopathy because treatment with corticosteroids for presumed GCA may potentiate VZV infection and lead to loss of vision. In contrast, patients with multifocal VZV vasculopathy require immediate antiviral (intravenous acyclovir) treatment.
To further address the incidence of VZV infection in GCA biopsy-negative patients, 24 temporal arteries from patients with clinically suspect GCA, but biopsy-negative, were examined by immunohistochemistry for the presence of VZV antigen. Remarkably, 5 (21%) contained VZV (81). All five subjects whose temporal arteries contained VZV antigen presented with clinical and laboratory features of GCA and early visual disturbances. Thirteen normal temporal arteries did not contain VZV antigen. Detection of VZV antigen and VZV DNA in another GCA-negative temporal artery in multiple regions (skip areas) as well as in skeletal muscle adjacent to the infected artery led to additional pathological analysis of sections contiguous with those containing VZV antigen. Remarkably, inflammation involving the arterial media and abundant multinucleated giant cells were seen, resulting in a change in pathological diagnosis from GCA-negative to GCA-positive (84). Overall, multifocal VZV vasculopathy with temporal artery infection can present with the full spectrum of clinical features and laboratory abnormalities characteristically seen in GCA. The role of VZV as the major cause of GCA is under intense study (48).
Ocular Disease
VZV infection produces acute retinal necrosis (ARN) or progressive outer retinal necrosis (PORN). VZV is the most common cause of PORN, although herpes simplex virus (HSV) and cytomegalovirus can also cause this disease. Most cases are seen in AIDS patients with CD4 T-cell counts less than 10 cells/µl of blood as well as in other immunosuppressed individuals. PORN may be preceded by retrobulbar optic neuritis and aseptic meningitis (39), central retinal artery occlusion or ophthalmic-distribution zoster (76), and may occur together with multifocal vasculopathy or myelitis. PORN patients treated with ganciclovir alone or in combination with foscarnet had a better final visual acuity than those treated with acyclovir or foscarnet alone. Like all neurological disorders caused by VZV, ocular disease caused by VZV can also occur in the absence of rash.
Zoster Sine Herpete: Radicular Pain in the Absence of Rash
Zoster sine herpete was first described in a report of multiple patients with dermatomal distribution radicular pain in areas distinct from pain with rash in zoster (67). The first two virologically confirmed cases of zoster sine herpete were verified by detection of VZV DNA in CSF (47). A third case of thoracic-distribution zoster sine herpete, in which electromyography of paraspinal muscles demonstrated frequent fibrillation potentials restricted to chronically painful thoracic root segments, was confirmed by detection of VZV DNA in blood MNCs and anti-VZV IgG antibody in CSF (4). Blumenthal et al., (13) recently described a patient with zoster sine herpete whose CSF did not contain amplifiable VZV DNA but did contain anti-VZV IgG with reduced serum/CSF ratios of anti-VZV IgG indicative of intrathecal synthesis. Perhaps the most compelling evidence that persistent radicular pain without rash can be caused by chronic active VZV ganglionitis came from clinical, pathological and virological analysis of two cases. In the first, a trigeminal ganglionic mass was removed from an immunocompetent adult who had experienced relentless trigeminal-distribution pain for more than a year; pathological and virological analyses of the ganglionic mass revealed active VZV ganglionitis (58). In the second case, pathological and virological analysis of the trigeminal ganglia at autopsy of a subject who experienced chronic trigeminal-distribution pain for months before death revealed active VZV ganglionitis (12).
Diagnostic Tests
Examination of CSF and serum is necessary in patients with neurological disease caused by VZV in the absence of rash. Routine cell count can be helpful, since a mild lymphocytic pleocytosis is characteristically found in VZV vasculopathy, myelitis and meningoencephalitis. Furthermore, increased red blood cells and neutrophils may also be seen when VZV infects the nervous system. In the absence of rash, CSF should be examined for VZV DNA by PCR and for anti-VZV IgG and IgM. Importantly, many cases of VZV vasculopathy are protracted and VZV DNA is only found ~30% of the time (82). The detection of anti-VZV IgG antibody in CSF with intrathecal synthesis is superior to detection of VZV DNA in CSF to diagnose VZV vasculopathy (83), recurrent myelopathy and brainstem encephalitis produced by VZV (56).
VACCINES
Vaccination to Prevent Varicella
In 1995, live attenuated varicella vaccine derived from the Oka strain of VZV (VZVOka) (116) was licensed for administration to healthy children in the U.S. (5, 7). Vaccine prevents varicella in 71-100% children and prevents severe disease in 95-100% (109). By the end of 2005, over 47 million doses of varicella vaccine were distributed (20). The implementation of universal childhood vaccination led to a decline of varicella by 90% and mortality by 66% (89). Varicella-related hospitalization across the U.S. has also decreased by 66% (49, 98). Two live, attenuated VZV-containing vaccines are now available in the U.S. to prevent varicella: a single-antigen varicella vaccine (Varivax®, Merck & Co., Inc.) and a combination vaccine for measles, mumps, rubella and varicella (MMRV, ProQuad®, Merck & Co., Inc.). Varivax® contains a minimum 3.13 log10 PFUs (plaque-forming units) of attenuated virus. Studies with early versions of combination vaccines revealed that the immune response to VZV was diminished when given in combination rather than separately, possibly due to interaction between VZV and MMR components. Overall, ProQuad® uses a higher dose of VZV as compared to Varivax® to overcome interaction with MMR, each dose containing a minimum of 3.99 log10 PFUs.
In 1995, the Advisory Committee on Immunization Practices (ACIP) recommended one dose of varicella vaccine for children aged 12 months to 12 years, but two doses, 4-8 weeks apart, for those aged ≥13 years (19). However, despite vaccine effectiveness of 85% with the single-dose regimen, varicella outbreaks have occurred in highly vaccinated school populations (118). Moreover, a 2-dose regimen administered 3 months apart was more effective than a single injection over a 10-year observation period (66). Thus, in June 2006, ACIP adopted new recommendations that include a routine 2-dose vaccination program for children and for all susceptible adolescents and adults (18). Children should receive the first dose at age 12-15 months and the second at age 4-6 years. A second dose should be given to children, adolescents and adults who previously received 1 dose. All healthy persons aged > 13 years without evidence of immunity should also be vaccinated with 2 doses, 4-8 weeks apart.
In general, live vaccines (including varicella vaccines) should not be administered to patients with primary or acquired immunodeficiency (e.g., cancer, leukemia/lymphoma, HIV+ individuals or those taking immunosuppressive therapy). Six cases of disseminated VZVOka in immunocompromised patients were reported during 10 years of global post-marketing surveillance (43). Varivax® should be considered for HIV-infected children with age-specific CD4 T lymphocyte percentages of 15-24% and for adolescents and adults with CD4 T lymphocyte counts ≥200 cells/µL (18). For patients with leukemia, lymphoma or other malignancies, vaccination can be considered if disease is in remission and if chemotherapy has been terminated for at least 3 months (18). However, their immune status should be evaluated before deciding to vaccinate. Guidelines on varicella vaccines are detailed in ACIP recommendations (18). Alternative regimens recommended in other countries focus on targeted vaccination of high-risk individuals and their contacts (72, 105).
Live attenuated varicella vaccine becomes latent in ganglia. DNA sequencing has shown that zoster (20, 43), as well as VZV meningitis and multifocal VZV vasculopathy (104) can develop after reactivation of vaccine strain VZV.
The safety profile of varicella vaccine was studied from 1995-2005 and showed that it is generally safe and well tolerated (20, 43). Rash may develop after immunization and is usually mild; only 1% of 5054 cases met the regulatory definition of “serious” (43). Vaccinated individuals with rash may infect susceptible household contacts. Vaccine virus is inhibited by acyclovir.
Prevention of VZV Reactivation
Zostavax® (highly potent attenuated VZV) is indicated for prevention of zoster in individuals age 60 and older. Zoster vaccine increases CD4 and CD8 T cells (effector and memory), and the half-life of the boost in T-cell immunity to VZV is at least 5 years. Zoster vaccine also boosts VZV-specific immunity in adults with a history of zoster or with chronic illness. In the U.S., the Center for Disease Control and Prevention Advisory Committee on Immunization Practices recommends zoster vaccine for all persons over age 60 years. By 2008, three years after zoster vaccine was licensed and recommended by the Advisory Committee on Immunization Practices for persons age 60 and older, less than 7% of the age group in the U.S. was vaccinated (70). This was due to a combination of lack of patient awareness regarding the availability of a vaccine, physicians’ uncertainty about the duration of protection and different cost-sharing plans for immunization. Zoster vaccine should be universally administered to all individuals over age 60.
The Shingles Prevention Study (SPS) of the licensed zoster vaccine was a placebo-controlled, double-blind study of more than 38,000 adults over the age of 60. All subjects were monitored for zoster. Endpoints included the burden of illness due to zoster and zoster-associated pain as well as the incidence of PHN. Subjects received a single dose of Zostavax® (n = 19,270) or placebo (n = 19,276). Racial distribution in both vaccination groups was similar: Caucasian (95%), African-American (2%), Hispanic (1%) and other (1%). Gender distribution was 59% male and 41% female in both groups. The most common side effects were redness, pain, itching, swelling, warmth or bruising at the injection site and sometimes headache. Varicella-like rashes at the injection site were more common in recipients of zoster vaccine than in placebo recipients (0.1% vs. 6.4%; p < 0.05). After a mean follow-up of 3 years, Zostavax® reduced the incidence of zoster by 51%. Subjects in the immunization group who developed zoster reported significantly less pain and discomfort than those in the placebo group and PHN was less frequent (an overall 61% lower burden of pain). While the vaccine group had a significantly greater risk of a serious adverse event (1.9% vs. 1.3%) and experienced more adverse events at the injection site (48.3% vs. 16.6%) than the placebo group during the first 42 days after vaccination, no significant long-term serious adverse vaccine-related events were seen.
Like varicella vaccine, zoster vaccine should not be administered to immunocompromised individuals. Patients whose leukemia/lymphoma is in remission and who have not received chemotherapy or radiation for at least 3 months can be considered to receive zoster vaccine; physicians should carefully assess the immune status of recipients on a case-by-case basis. Guidelines for use of Zostavax® are published (54).
ACYCLOVIR PROPHYLAXIS
VZV reactivation in organ transplant recipients is common. Any immunosuppressed organ transplant recipient who develops a VZV-like rash should receive intravenous acyclovir until >2 days after lesions have crusted. Long-term acyclovir prophylaxis to prevent recurrent VZV reactivation is not routinely recommended by the Centers for Disease Control, except for patients who require long-term immunosuppressive therapy.
A retrospective study of VZV-seropositive subjects undergoing allogeneic or autologous hematopoietic stem cell transplantation revealed benefit from oral prophylaxis with acyclovir, 800 mg twice daily for one year after transplantation (35). Longer prophylaxis should be considered for patients requiring continuing immunosuppression (e.g., graft-versus-host disease). After one-year acyclovir prophylaxis following hematopoietic cell transplantation, there was no rebound disease caused by VZV after drug discontinuation.
For solid organ transplant recipients, no randomized clinical trials have studied the efficacy of antiviral prophylaxis to prevent VZV reactivation. Patients with a history of VZV infection are typically given antiviral prophylaxis for the first 3-6 months after transplantation and during periods of intense immunosuppression (e.g., treatment of graft rejection) or other "stresses" (e.g., concomitant infection or surgery) (2).
In AIDS patients, recurrent or relapsing zoster is frequent. After the second or third recurrence, lifelong antiviral therapy may be necessary. Such long-term prophylaxis has been effective, although acyclovir-resistant VZV has emerged (60, 69, 92). Foscarnet is used to treat acyclovir-resistant VZV. Given the potential problem of viral drug resistance and lack of placebo-controlled studies demonstrating the efficacy of long-term acyclovir prophylaxis in HIV/AIDS patients, acyclovir prophylaxis should not be routine, but should be decided individually (44). Finally, while all the above studies of prophylaxis used acyclovir, prophylaxis with famciclovir or valacyclovir is likely to be equally effective.
rEFERENCES
1. Adhami N, Arabi Y, Raees A, Al-Shimemeri A, Ur-Rahman M, Memish ZA. Effect of corticosteroids on adult varicella pneumonia: cohort study and literature review. Respirology 2006; 11:437-41. [PubMed]
2. Alexander BD, Fishman JA. Prophylaxis of infections in solid organ transplantation. UpToDate, www.uptodate.com, Topic 1408 Version 8.0 May, 2012.
3. American Academy of Pediatrics. Varicella-zoster infections. In: Pickering LK, eds. Red book: 2006 report of the Committee on Infectious Diseases, 27th ed. Elk Grove Village, IL.: Academy of Pediatrics, 2006:711-25.
4. Amlie-Lefon C, Mackin GA, Ferguson M, Wright RR, Mahalingam R, Gilden DH. Another case of virologically confirmed zoster sine herpete with electrophysiologic correlation. J NeuroVirol 1996; 2:136-8. [PubMed]
5. Arvin AM. Varicella vaccine--the first six years. N Engl J Med 2001; 344:1007-9. [PubMed]
6. Avin AM, Arvin AM, Gilden D. VZV. In: Knipe DM, Howley PM, eds. Fields Virology, 5th Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2013:2015-57.
7. Arvin AM, Gershon AA. Live attenuated varicella vaccine. Annu Rev Microbiol 1996; 50:59-100. [PubMed]
8. Baba K, Yabuuchi H, Takahashi M, Ogra PL. Increased incidence of herpes zoster in normal children infected with varicella zoster virus during infancy: community-based follow-up study. J Pediatr 1986; 108:372-7. [PubMed]
9. Balfour HH, Jr., Antiviral drugs. N Engl J Med 1999; 340:1255-68. [PubMed]
10. Berry JD, Petersen KL. A single dose of gabapentin reduces acute pain and allodynia in patients with herpes zoster. Neurology 2005; 65:444-7. [PubMed]
11. Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother 1995; 39:1546-53. [PubMed]
12. Birlea N, Nagel MA, Khmeleva N, Choe A, Kleinschmidt-DeMasters B, Hevner R, Boyer P, Lear-Kaul KC, Bos N, Wellish M, Cohrs RJ, Gilden, D. VZV trigeminal ganglioneuritis without rash. Neurology 2014, in press. [PubMed]
13. Blumenthal DT, Shacham-Shmueli E, Bokstein F, Schmid DS, Cohrs RJ, Nagel MA, Mahalingam R, Gilden D. Zoster sine herpete: virological verification by detection of anti-VZV IgG antibody in CSF. Neurology 2011; 76:484-5. [PubMed]
14. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 1997; 13:327-31. [PubMed]
15. Breuer J, Grose C, Norberg P, Tipples G, Schmid DS. A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry 24-25 July 2008. J Gen Virol 2010; 91:821-8. [PubMed]
16. Broussard RC, Payne DK, George RB. Treatment with acyclovir of varicella pneumonia in pregnancy. Chest 1991; 99:1045-7 [PubMed]
17. Brunell PA, Kotchmar GS, Jr. Zoster in infancy: failure to maintain virus latency following intrauterine infection. J Pediatr 1981; 98:71-3. [PubMed]
18. CDC. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1-40. [PubMed]
19. CDC. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. MMWR Recomm Rep 1996; 45:1-36. [PubMed]
20. Chaves SS, Haber P, Walton K, Wise RP, Izurieta HS, Schmid DS, Seward JF. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis 2008; 197 Suppl 2:S170-7. [PubMed]
21. Choo PW, Donahue JG, Manson JE, Platt R. The epidemiology of varicella and its complications. J Infect Dis 1995; 172:706-12. [PubMed]
22. Clarke SE, Harrell AW, Chenery RJ. Role of aldehyde oxidase in the in vitro conversion of famciclovir to penciclovir in human liver. Drug Metab Dispos 1995; 23:251-4. [PubMed]
23. Crumpacker C. The pharmacological profile of famciclovir. Semin Dermatol 1996; 15:14-26. [PubMed]
24. Daley AJ, Thorpe S, Garland SM. Varicella and the pregnant woman: prevention and management. Aust N Z J Obstet Gynaecol 2008; 48:26-33. [PubMed]
25. Degreef H, Famciclovir Herpes Zoster Clinical Study Group.Famciclovir, a new oral antiherpes drug: results of the first controlled clinical study demonstrating its efficacy and safety in the treatment of uncomplicated herpes zoster in immunocompetent patients. Int J Antimicrob Agents 1994; 4:241-6. [PubMed]
26. de Silva, SM, Mark AS, Gilden DH, Mahalingam R, Balish M, Sandbrink F, Houff S. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology 1996; 47:929-31. [PubMed]
27. Dunkle LM, Arvin AM, Whitley RJ, Rotbart HA, Feder HM, Jr., Feldman S, Gershon AA, Levy ML, Hayden GF, McGuirt PV, Harris J, Balfour HH. A controlled trial of acyclovir for chickenpox in normal children. N Engl J Med 1991; 325:1539-44. [PubMed]
28. Dworkin RH, Corbin AE, Young JP, Jr., Sharma U, LaMoreaux L, Bockbrader H, Garofalo, EA, Poole RM. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2003; 60:1274-83. [PubMed]
29. Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW, Nurmikko TJ, Oaklander AL, Oxman MN, Pavan-Langston D, Petersen KL, Rowbotham MC, Schmader KE, Stacey BR, Tyring SK, van Wijck AJ, Wallace MS, Wassilew SW, Whitley RJ. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44 Suppl 1:S1-26. [PubMed]
30. Eisenberg E, McNicol E, Carr DB. Opioids for neuropathic pain. Cochrane Database Syst Rev 2006; 3:CD006146. [PubMed]
31. Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA 2005; 293:3043-52. [PubMed]
32. Elion GB, Furman PA, Fyfe JA, de Miranda P, Beauchamp L, Schaeffer HJ. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci USA 1977; 74:5716-20. [PubMed]
33. Ellis ME, Neal KR, Webb AK. Is smoking a risk factor for pneumonia in adults with chickenpox? Br Med J (Clin Res Ed) 1987; 294:1002. [PubMed]
34. Enders G, Miller E, Cradock-Watson J, Bolley I, Ridehalgh M. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet 1994; 343:1548-51. [PubMed]
35. Erard V, Guthrie KA, Varley C, Heugel J, Wald A, Flowers ME, Corey L, Boeckh M. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood 2007; 110:3071-7. [PubMed]
36. Esmann V, Geil JP, Kroon S, Fogh H, Peterslund NA, Petersen CS, Ronne-Rasmussen JO, Danielsen L. Prednisolone does not prevent post-herpetic neuralgia. Lancet 1987; 2:126-9. [PubMed]
37. Esmonde TF, Herdman G, Anderson G. Chickenpox pneumonia: an association with pregnancy. Thorax 1989; 44:812-5. [PubMed]
38. Fairley CK, Miller E. Varicella-zoster virus epidemiology--a changing scene? J Infect Dis 1996; 174 Suppl 3:S314-9. [PubMed]
39. Franco-Paredes C, Bellehemeur T, Merchant A, Sanghi P, DiazGranados C, Rimland D. Aseptic meningitis and optic neuritis preceding varicella zoster progressive outer retinal necrosis in a patient with AIDS. AIDS 2002; 16:1045-9. [PubMed]
40. Freyhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005; 115:254-63. [PubMed]
41. Furman PA, de Miranda P, St Clair MH, Elion GB. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob Agents Chemother 1981;20:518-24. [PubMed]
42. Fyfe JA, Keller PM, Furman PA, Miller RL, Elion GB. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl) guanine. J Biol Chem 1978; 253:8721-7. [PubMed]
43. Galea SA, Sweet A, Beninger P, Steinberg SP, Larussa PS, Gershon AA, Sharrar RG. The safety profile of varicella vaccine: a 10-year review. J Infect Dis 2008; 197 Suppl 2:S165-9. [PubMed]
44. Gershon AA. Prevention and treatment of VZV infections in patients with HIV. Herpes 2001; 8:32-6. [PubMed]
45. Gilden DH, Beinlich BR, Rubinstien EM, Stommel E, Swenson R, Rubinstein D, Mahalingam R. Varicella zoster virus myelitis: an expanding spectrum. Neurology 1994; 44:1818-23. [PubMed]
46. Gilden DH, Dueland AN, Cohrs R, Martin JR, Kleinschmidt-DeMasters BK, Mahalingam R. Preherpetic neuralgia. Neurology 1991; 41:1215-8. [PubMed]
47. Gilden DH, Wright RR, Schneck SA, Gwaltney JM, Jr, Mahalingam R. Zoster sine herpete, a clinical variant. Ann Neurol 1994; 35:530-3. [PubMed]
48.Gilden D, White T, Khmeleva N, Heintzman A, Choe A, Boyer PJ, Grose C, Carpenter JE, Rempel A, Bos N, Kandasamy B, Lear-Kaul K, Homes DB, Bennett JL, Cohrs RJ, Mahalingram R, Mandava N, Eberhart CG, Bockelman B, Poppiti RJ, Tamhankar MA, Fogt F, Amato M, Wood E, Durairaj V, Rasmussen S, Petursdottir V, Pollak L, Mendlovic S, Chatelain D, Keyvani K, Brueck W, Nagel MA. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015 Feb 18. [Epub ahead of print] [PubMed]
49. Grose C. Varicella vaccination of children in the United States: assessment after the first decade 1995-2005. J Clin Virol 2005; 33:89-95; discussion 6-8. [PubMed]
50. Guess HA, Broughton DD, Melton LJ, 3rd., Kurland LT. Epidemiology of herpes zoster in children and adolescents: a population-based study. Pediatrics 1985; 76:512-17. [PubMed]
51. Gunson RN, Aitken C, Gilden D. A woman with acute headache and sacral dermatomal numbness. J Clin Virol 2011; 50:191-3. [PubMed]
52. Habib AA, Gilden D, Schmid DS, Safdieh JE. Varicella zoster virus meningitis with hypoglycorrhachia in the absence of rash and in an immunocompetent woman. J NeuroVirol 2009; 15:206-8. [PubMed]
53. Harger JH, Ernest JM, Thurnau GR, Moawad A, Momirova V, Landon MB, Paul R, Miodovnik M, Dombrowski M, Sibai B, Van Dorsten P. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Dis 2002; 185:422-7. [PubMed]
54. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of Herpes Zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1-30. [PubMed]
55. Harris RE, Rhoades ER. Varicella pneumonia complicating pregnancy. Report of a case and review of literature. Obstet Gynecol 1965; 25:734-40. [PubMed]
56. Haug A, Mahalingam R, Cohrs RJ, Schmid DS, Corboy JR, Gilden D. Recurrent polymorphonuclear pleocytosis with increased red blood cells caused by varicella zoster virus infection of the central nervous system. J Neurol Sci 2010; 292:85-8. [PubMed]
57. Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain 1900; 23:353-523. [PubMed]
58. Hevner R, Vilela M, Rostomily R, Cohrs R, Mahalingam R, Wellish M, Gilden DH. An unusual cause of trigeminal-distribution pain and tumor. Lancet Neurol 2003; 2:567-71. [PubMed]
59. Hoover SE, Cohrs RJ, Rangel ZG, Gilden DH, Munson P, Cohen JI. Downregulation of varicella-zoster virus (VZV) immediate-early ORF62 transcription by VZV ORF63 correlates with virus replication in vitro and with latency. J Virol 2006; 80:3459-68. [PubMed]
60. Hoppenjans WB, Bibler MR, Orme RL, Solinger AM. Prolonged cutaneous herpes zoster in acquired immunodeficiency syndrome. Arch Dermatol 1990; 126:1048-50. [PubMed]
61. Jackson JL, Gibbons R, Meyer G, Inouye L. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med 1997; 157:909-12. [PubMed]
62. Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke 2010; 40:3443-8. [PubMed]
63. Kilgore PE, Kruszon-Moran D, Seward JF, Jumaan A, Van Loon FP, Forghani B, McQuillan GM, Wharton M, Fehrs LJ, Cossen CK, Hadler SC. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol 2003; 70:S111-8. [PubMed]
64. Kleinschmidt-DeMasters BK, Gilden DH. Varicella zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med 2001; 125:770-80 [PubMed]
65. Kochar DK, Garg P, Bumb RA, Kochar SK, Mehta RD, Beniwal R, Rawat N. Divalproex sodium in the management of post-herpetic neuralgia: a randomized double-blind placebo-controlled study. Qjm 2005; 98:29-34. [PubMed]
66. Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, Reisinger K, Kim LL, Lupinacci L, Hartzel J, Chan I. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J 2004; 23:132-7. [PubMed]
67. Lewis GW. Zoster sine herpete. Br Med J 1958; 2:418-21. [PubMed]
68. Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology 2010; 74:792-7. [PubMed]
69. Linnemann CC, Jr., Biron KK, Hoppenjans WG, Solinger AM. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. Aids 1990; 4:577-9. [PubMed]
70.Lu PJ, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years and older, in the U.S., 2008. Am J Prev Med 2011; 40:e1-6. [PubMed]
71.Lycke J, Malmeström C, Ståhle L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother 2003; 47:2438-41. [PubMed]
72.Macartney KK, Burgess, MA. Varicella vaccination in Australia and New Zealand. J Infect Dis 2008; 197 Suppl 2:S191-5. [PubMed]
73. Mahalingam R, Wellish M, Soike K, White T, Kleinschmidt-DeMasters BK, Gilden DH. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology 2001; 279:339-42. [PubMed]
74. Mathias M, Nagel MA, Khmeleva N, Boyer PJ, Choe A, Durairaj VD, Bennett JL, Mandava N, Gilden D. VZV multifocal vasculopathy with ischemic optic neuropathy, acute retinal necrosis and temporal artery infection in the absence of zoster rash. J Neurol Sci 2013; 325:180-2. [PubMed]
75. Max MB, Schafer SC, Culnane M, Smoller B, Dubner R, Gracely RH. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology 1988; 38:1427-32. [PubMed]
76. Menerath JM, Gerard M, Laurichesse H, Goldschmidt P, Peigue-Lafeuille H, Rozenberg F, Beytout J. Bilateral acute retinal necrosis in a patient with acquired immunodeficiency syndrome. J Fr Ophtalmol 1995; 18:625-33. [PubMed]
77. Meyers JD. Congenital varicella in term infants: risk reconsidered. J Infect Dis 1974;129:215-7. [PubMed]
78. Mohsen AH, McKendrick M. Varicella pneumonia in adults. Eur Respir J 2003; 21:886-91. [PubMed]
79. Moses H, Nagel MA, Gilden DH. Acute cerebellar ataxia in a 41 year old woman. Lancet Neurol 2006; 5:984-8. [PubMed]
80. Mouly F, Mirlesse V, Meritet JF, Rozenberg F, Poissonier MH, Lebon P, Daffos F. Prenatal diagnosis of fetal varicella-zoster virus infection with polymerase chain reaction of amniotic fluid in 107 cases. Am J Obstet Gynecol 1997;177:894-8. [PubMed]
81. Nagel MA, Bennett JL, Khmeleva N, Choe A, Rempel A, Boyer PJ, Gilden D. Multifocal VZV vasculopathy with temporal artery infection mimics giant cell arteritis. Neurology 2013; 80:2017-2. [PubMed]
82. Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, Safdieh JE, Kamenkovich E, Ostrow LW, Levy M, Greenberg B, Russman AN, Katzan I, Gardner CJ, Häusler M, Nau R, Saraya T, Wada H, Goto H, de Martino M, Ueno M, Brown WD, Terborg C, Gilden DH. The varicella zoster virus vasculopathies: clinical, CSF, imaging and virological features. Neurology 2008; 70:853-60. [PubMed]
83. Nagel MA, Forghani B, Mahalingam R, Wellish MC, Cohrs RJ, Russman AN, Katzan I, Lin R, Gardner CJ, Gilden DH. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology 2007; 68:1069-73. [PubMed]
84. Nagel MA, Khmeleva N, Boyer PJ, Choe A, Bert R, Gilden D. VZV in the temporal artery of a patient with GCA. J Neurol Sci 2013; 335:229-30. [PubMed]
85. Nagel MA, Russman AN, Feit H, Traktinskiy I, Khmeleva N, Schmid DS, Skarf B, Gilden D. VZV ischemic optic neuropathy and subclinical temporal artery infection without rash. Neurology 2013; 80:220-22. [PubMed]
86. Nagel MA, Traktinskiy I, Azarkh Y, Kleinschmidt-DeMasters B, Hedley-Whyte T, Russman A, VanEgmond EM, Stenmark K, Frid M, Mahalingam R, Wellish M, Choe A, Cordery-Cotter R, Cohrs RJ, Gilden D. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology 2011; 77:364-70. [PubMed]
87. Nagel MA, Traktinskiy I, Stenmark KR, Frid MG, Choe A, Gilden D. Varicella zoster virus vasculopathy: Immune characteristics of virus-infected arteries. Neurology 2013; 80:62-8. [PubMed]
88. Naveen KN, Tophakane RS, Hanumanthayya K, Pv B, Pai VV. A study of HIV seropositivity with various clinical manifestation of herpes zoster among patients from Karnataka, India. Dermatol Online J 2011; 17:3. [PubMed]
89. Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med 2005; 352:450-8. [PubMed]
90. Nicholson KG. Properties of antiviral agents. 1. Lancet 1984; 2:503-6. [PubMed]
91. Orme HT, Smith AG, Nagel MA, Bert RJ, Mickelson TS, Gilden DH. VZV spinal cord infarction identified by diffusion-weighted magnetic resonance imaging (DWI). Neurology 2007; 69:398-400. [PubMed]
92. Pahwa S, Biron K, Lim W, Swenson P, Kaplan MH, Sadick N, Pahwa R. Continuous varicella-zoster infection associated with acyclovir resistance in a child with AIDS. JAMA 1988; 260:2879-82. [PubMed]
93.Pastuszak AL, Levy M, Schick B, Zuber C, Feldkamp M, Gladstone J, Bar-Levy F, Jackson E, Donnenfeld A, Meschino W, Gideon Koren. Outcome after maternal varicella infection in the first 20 weeks of pregnancy. N Engl J Med 1994; 330:901-5. [PubMed]
94. Pavan-Langston D. Opthalmic zoster. In: Arvin AM, Gershon AA, eds. Varicella-zoster virus: virology and clinical management. Cambridge, UK: Cambridge University Press, 2000:276-98. [PubMed]
95.Popara M, Pendle S, Sacks L, Smego RA, Jr., Mer M. Varicella pneumonia in patients with HIV/AIDS. Int J Infect Dis 2002; 6:6-8. [PubMed]
96. Pouplin T, Pouplin JN, Van Toi P, Lindegardh N, Rogier van Doorn H, Hien TT, Farrar J, Török ME, Chau TT. Valacyclovir for herpes simplex encephalitis. Antimicrob Agents Chemother 2011; 55:3624-6. [PubMed]
97. Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, Royall RM, Max MB. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2002; 59:1015-21. [PubMed]
98. Reynolds MA, Watson BM, Plott-Adams KK, Jumaan AO, Galil K, Maupin TJ, Zhang JX, Seward JF. Epidemiology of varicella hospitalizations in the United States, 1995-2005. J Infect Dis 2008; 197 Suppl 2:S120-6. [PubMed]
99. Rice AS, Maton S. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain 2001; 94:215-24. [PubMed]
100. Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA 1998; 280:1837-42. [PubMed]
100. Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA 1998; 280:1837-42. [PubMed]
101. Rowbotham MC, Reisner-Keller LA, Fields HL. Both intravenous lidocaine and morphine reduce the pain of postherpetic neuralgia. Neurology 1991; 41:1024-8. [PubMed]
102. Rowbotham MC, Reisner LA, Davies PS, Fields HL. Treatment response in antidepressant-naive postherpetic neuralgia patients: double-blind, randomized trial. J Pain 2005; 6:741-6. [PubMed]
103. Sabatowski R, Galvez R, Cherry DA, Jacquot F, Vincent E, Maisonobe P, Versavel M. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 2004; 109:26-35. [PubMed]
104. Sabry S, Hauk PJ, Jing H, Su HC, Stence NV, Mirsky DM, Nagel MA, Abbott JK, Dragone LL, Armstrong-Wells J, Curtis DJ, Cohrs R, Schmid SD, Gilden D, Gelfand EW. Vaccine strain varicella zoster-induced CNS vasculopathy as the presenting feature of DOCK8 deficiency. J Allergy Clin Immunol, 2014, in press. [PubMed]
105. Sadzot-Delvaux C, Rentier B, Wutzler P, Asano Y, Suga S, Yoshikawa T, Plotkin SA. Varicella vaccination in Japan, South Korea, and Europe. J Infect Dis 2008; 197 Suppl 2:S185-90. [PubMed]
106. Salazar R, Russman AN, Nagel MA, Cohrs RJ, Mahalingam R, Schmid DS, Kleinschmidt-DeMasters BK, VanEgmond EM, Gilden D. VZV ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol 2011; 68:517-20. [PubMed]
107. Sauerbrei A, Wutzler P. Herpes simplex and varicella-zoster virus infections during pregnancy: current concepts of prevention, diagnosis and therapy. Part 2: Varicella-zoster virus infections. Med Microbiol Immunol 2007; 196:95-102. [PubMed]
108. Schlossberg D, Littman M. Varicella pneumonia. Arch Intern Med 1988; 148:1630-2. [PubMed]
109. Seward JF, Marin M, Vazquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis 2008; 197 Suppl 2:S82-9. [PubMed]
110. Simpson D, Lyseng-Williamson KA. Famciclovir: a review of its use in herpes zoster and genital and orolabial herpes. Drugs 2006; 66:2397-416. [PubMed]
111. Smith FP. Pathological studies of spinal nerve ganglia in relation to intractable intercostal pain. Surg Neurol 1978; 10:50-3. [PubMed]
112. Smith JP, Weller S, Johnson B, Nicotera J, Luther JM, Haas DW. Pharmacokinetics of acyclovir and its metabolites in cerebrospinal fluid and systemic circulation after administration of high-dose valacyclovir in subjects with normal and impaired renal function. Antimicrob Agents Chemother 2010; 54:1146-51. [PubMed]
113. Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother 1995; 39:2759-64. [PubMed]
114. Stagno S, Whitley RJ. Herpesvirus infections of pregnancy. Part II: Herpes simplex virus and varicella-zoster virus infections. N Engl J Med 1985; 313:1327-30. [PubMed]
115. Surjya PU, Shiv PR, Mishra S, Bhatnagar S. Successful treatment of an intractable postherpetic neuralgia (PHN) using peripheral nerve field stimulation (PNFS). Am J Hosp Palliat Care 2010; 27:59-62. [PubMed]
116.Takahashi M, Asano Y, Kamiya H, Baba K, Ozaki T, Otsuka T, Yamanishi K. Development of varicella vaccine. J Infect Dis 2008; 197 Suppl 2:S41-4. [PubMed]
117.Triebwasser JH, Harris RE, Bryant RE, Rhoades ER. Varicella pneumonia in adults. Report of seven cases and a review of literature. Medicine (Baltimore) 1967; 46:409-23.
118.Tugwell BD, Lee LE, Gillette H, Lorber EM, Hedberg K, Cieslak PR. Chickenpox outbreak in a highly vaccinated school population. Pediatrics 2004; 113:455-9. [PubMed]
119.Tyndall MW, Nasio J, Agoki E, Malisa W, Ronald AR, Ndinya-Achola JO, Plummer FA. Herpes zoster as the initial presentation of human immunodeficiency virus type 1 infection in Kenya. Clin Infect Dis 1995; 21:1035-7. [PubMed]
120. Tyring S, Barbarash RA, Nahlik JE, Cunningham A, Marley J, Heng M, Jones T, Rea T, Boon R, Saltzman R. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med 1995; 123:89-96. [PubMed]
121. Tyring SK, Beutner KR, Tucker BA, Anderson WC, Crooks RJ. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med 2000; 9:863-9. [PubMed]
122. van Seventer R, Feister HA, Young JP, Jr., Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin 2006; 22:375-84. [PubMed]
123. Vere Hodge RA, Sutton D, Boyd MR, Harnden MR, Jarvest RL. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 [9-(4-hydroxy-3-hydroxymethylbut-l-yl) guanine; penciclovir]. Antimicrob Agents Chemother 1989;33:1765-73. [PubMed]
124.Wagstaff AJ, Faulds D, Goa KL. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1994; 47:153-205. [PubMed]
125. Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology 1998; 50:1837-41. [PubMed]
126. Watson CP, Deck JH, Morshead C, Van der Kooy D, Evans RJ. Postherpetic neuralgia: further post-mortem studies of cases with and without pain. Pain 1991; 44:105-17. [PubMed]
127. Watson CP, Evans RJ, Reed K, Merskey H, Goldsmith L, Warsh J. Amitriptyline versus placebo in postherpetic neuralgia. Neurology 1982; 32:671-3. [PubMed]
128. Watson CP, Evans RJ, Watt VR. Post-herpetic neuralgia and topical capsaicin. Pain 1988; 33:333-40. [PubMed]
129. Watson CP, Vernich L, Chipman M, Reed K. Nortriptyline versus amitriptyline in postherpetic neuralgia: a randomized trial. Neurology 1998; 51:1166-71. [PubMed]
130. Weller S, Blum MR, Doucette M, Burnette T, Cederberg DM, de Miranda P, Smiley ML. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin Pharmacol Ther 1993; 54:595-605. [PubMed]
131.Whitley RJ, Weiss H, Gnann JW, Jr., Tyring S, Mertz GJ, Pappas PG, Schleupner CJ, Hayden F, Wolf J, Soong SJ. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med 1996; 125:376-83. [PubMed]
132. Wood MJ, Johnson RW, McKendrick MW, Taylor J, Mandal BK, Crooks J. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med 1994; 330:896-900.[PubMed]
133. Wood MJ, Kay R, Dworkin RH, Soong SJ, Whitley RJ. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis 1996; 22:341-7. [PubMed]
134. Wood MJ, Ogan PH, McKendrick MW, Care CD, McGill JI, Webb EM. Efficacy of oral acyclovir treatment of acute herpes zoster. Am J Med 1988; 85:79-83. [PubMed]
135. Wood MJ, Shukla S, Fiddian AP, Crooks RJ. Treatment of acute herpes zoster: effect of early (< 48 h) versus late (48-72 h) therapy with acyclovir and valaciclovir on prolonged pain. J Infect Dis 1998; 178 Suppl 1:S81-4.[PubMed]
136. Yawn BP, Gilden DYawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology 2013; 81:928-30. [PubMed] . The global epidemiology of herpes zoster. Neurology 2013; 81:928-30. [PubMed]
137. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341-9. [PubMed]
Table 1. Antiviral Drugs Used For VZV Infection.
| Medication | Dosage (Pediatric) | Dosage (Adult) | Most common adverse effects | Approximate price for complete dosing1 |
|---|---|---|---|---|
| Oral formulations: | ||||
| acyclovir | > 2 years old; 20 mg/kg 4x/day; max. 3.2 g/day; 5 days2 | 800 mg 5x/day; 7-10 days2 | malaise, headache, nausea, vomiting | 35-800 mg tabs; $70 |
| valacyclovir | 2-18 years old; 20 mg/kg 3x/day; max. 1g 3x/day; 5 days2 | 1 g 3x/day; 7 days2 | headache, nausea, abdominal pain | 21-1 g tabs; $220 |
| famciclovir | not established | 500 mg 3x/day3; 7 days2 | headache, nausea, fatigue, diarrhea | 21-500 mg tabs; $184 |
| Intravenous formulations: | ||||
| acyclovir | 250 mg/m2 every 8h; 5-7 days2 | 10-15 mg/kg every 8h; 5-7 days2 | phlebitis, acute renal failure, nausea, vomiting, rash | |
| foscarnet4 | not established | 180 mg/kg/day in 2-3 divided doses; 7-14 days2 | fever, headache, renal dysfunction, electrolyte abnormalities, nausea, vomiting diarrhea, anemia, granulocytopenia | |
12013 cash price from a Denver, Colorado pharmacy.
2Durations listed represent general guidelines only, with exact duration depending on immune status and clinical response. Longer durations are required for immunocompromised patients and/or for treatment of disseminated infection, encephalitis or pneumonitis.
3Famciclovir dose is 250 mg 3x/day in Europe.
4Foscarnet is not licensed for treatment of varicella or zoster, but can be used to treat infection with VZV strains that are resistant to acyclovir or other nucleoside analogues. Dosage listed was established for treatment of cytomegalovirus retinitis in HIV-infected patients.
Table 2. Adjunctive Therapy For Postherpetic Neuralgia (29).
| Medication | Beginning dosage | Titration | Maximum dosage | Most common adverse effects |
|---|---|---|---|---|
| Tricyclic antidepressants: | ||||
| nortriptyline | 25 mg at bedtime | increase by 25 mg/day every 2-3 days as tolerated | usual effective dose 75 mg at bedtime or 75 mg 2x/day; maximum 150 mg/day | sedation, dry mouth, blurred vision, weight gain, urinary retention |
| Anti-epileptics: | ||||
| gabapentin | 300 mg at bedtime | increase to 300 mg 2x daily on day 2 and 300 mg 3x daily on day 3, then increase by 100-300 mg 3x/day every 2-3 days as tolerated | usual effective dose 600-1200 mg 3x/day; no additional benefit for dose >1800 mg per day | somnolence, dizziness, peripheral edema, ataxia |
| pregabalin | 75 mg 2x/day daily or 50 mg 3x/day | can titrate to 300 mg/day within 1 week as tolerated; for patients without sufficient pain relief at 300 mg/day, can further titrate to 600 mg/day after 2-4 weeks as tolerated | 600 mg per day in 2-3 divided doses | somnolence, dizziness, peripheral edema, ataxia, headache, weight gain |
| Analgesics: | ||||
| oxycodone | 2.5-5 mg every 4 h as needed | increase 50-100%/day, every 2 days as tolerated | usual effective dose 10-30 mg every 4 h; consider pain specialist evaluation if dosages >120 mg per day | nausea, vomiting, constipation, somnolence, dizziness |
| tramadol | 25-50 mg 2x/day | increase 50-100 mg/day in divided doses every 2 days as tolerated | 100 mg 4x/day | nausea, vomiting, constipation, somnolence, dizziness, seizures, postural hypotension |
Figure 1. Metabolic Processing of Antivirals Used to Treat Varicella and Zoster, Part I.
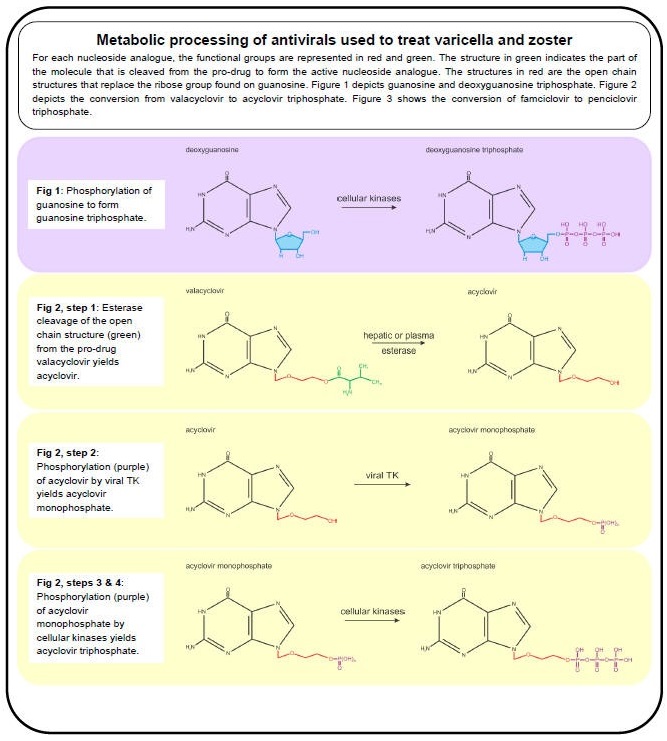
Figure 2. Metabolic Processing of Antivirals Used to Treat Varicella and Zoster, Part II.
Gershon AA. Strokes and Infection with Varicella Zoster Virus. Clin Infect Dis 2014;58:69-71
Guided medline search for:
Muenchhoff M, et al. Sex Differences in Pediatric Infectious Diseases. J Infect Dis 2014;209:S120-6.
Assi M, James S, Kimberlin D. Varicella Zoster Virus in Transplant Recipients
Adhikari P, Mietzner T. Cell Mediated Immunity.
Kimberlin DW, et al. Guidance on management of asymptomatic neonates born to women with active genital herpes lesions. Pediatrics 2013;131:e635-46.
Guided medline search for Recent reviews
Groce C. Pangaea and the Out-of-Africa Model of Varicella-Zoster Virus Evolution and Phylogeography. J of Virology 2012;86:9558-9565.
Guided Medline Search for Historical aspects
Table of Contents
- Virology
- Epidemiology
- Antivirals
- Primary VZV Infection - Varicella
- VZV Reactivation - Zoster and Other Neurological and Ocular Complications
- Pathology and Pathogenesis
- Zoster
- Postherpetic Neuralgia
- Treatment of Herpes Zoster, Acute Pain, and PHN
- VZV Meningoencephalitis, Meningoradiculitis, and Cerebellitis
- VZV Myelopathy
- VZV Vasculopathy
- Multifocal VZV Vasculopathy with Temporary Artery Infection and Giant Cell Arteritis
- Ocular Disease
- Zoster Sine Herpete
- Diagnostic Tests
- Vaccines
- Acyclovir Prophylaxis
