Human Pegivirus (GB Virus C/hepatitis G virus)
Authors: Jinhua Xiang, M.D.
Second Edition, 2002: Kendo Kiyosawa,M.D.
Virology
Human Pegivirus (also called GBV-C virus or hepatitis G virus-HGV) was discovered in 1995. The name comes from the initials (GB) of a surgeon whose serum caused transmissible hepatitis in new world primate animal models (21). It was thought to be a possible cause of non-A, non-B hepatitis until hepatitis C virus (HCV) was discovered; however, testing serum from G.B. did not reveal HCV and epidemiological studies did not identify associations between the virus and acute or chronic liver disease (21). Based on its genome structure and RNA sequence homology, HPgV is themost closely related human virus to HCV (21).Although not associated with hepatitis or other acute diseases, the virus is associated with non-Hodgkin's lymphoma (NHL) (3, 4, 7, 21). Since HPgV does not cause hepatitis, and there are no data to suggest that the surgeon "GB" was infected with this virus, the name of both GBV-C and HGV is erroneous. In part due to these facts, the virus was assigned to the genus "Pegivirus" within the Flaviviridae family in 2012 to represent persistent "G" viruses (1). Primate pegiviruses were renamed as Pegivirus A, and specific viruses are named after their host species. Thus, this virus is commonly called human Pegivirus or HPgV (1, 21).
HPgv is a positive single-stranded RNA virus with a genome of approximately 9400 nucleotides (reviewed in (21) and (17)). Like HCV, the genome has a 5'-nontranslated region (5'ntr), followed by a long open reading frame and a 3'-nontranslated region (3'ntr). The predicted viral polyprotein contains 2 structural envelope glycoproteins (E1 and E2) at the amino-terminal end, followed by nonstructural proteins (NS2, NS3, NS4, NS5) at the carboxy-terminal end. The presence and characterization of a core protein that forms a nucleocapsid has not been identified (7, 21).The 5'-ntr contains an internal ribosome entry site that is capable of directing CAP-independent translation of the polyprotein. Seven geographically related HPgV genotypes have been characterized (9, 18).Although there is mixing, genotype 1 is predominant in West Africa, genotype 2 in North America and Europe, genotype 3 and 7 are most common in Asia, genotype 4 in Southeast Asia, genotype 5 is primarily in South Africa, genotype 6 in Indonesia, and genotype 7 is found in China (9, 18, 21).Compared to HCV, the genetic diversity of HPgV is markedly lower. Only 11–14% difference of sequences in the polyprotein coding region occurs between genotypes (21). In contrast, 30% nucleotide divergence is required to be classified as a different genotype in HCV, and 15% difference defines sub-genotype (e.g. HCV genotype 1a vs. 1b) (19). HPgV has a similar rate of base substitutions each site per year (0.4 to 0.8x10-3) as HCV; however, and unlike HCV, HPgV does not contain hypervariable regions(16, 25).
Epidemiology
HPgV infection is transmitted by sexual, parenteral, and vertical (mother to child) mechanisms (17). The infection is prevalent worldwide and is thought to infect between one sixth and one third of the world's population (23). In economically developed countries, HPgV RNA is detected in 1% to 4% healthy blood donors, and the prevalence is higher among those with risk of parenteral exposure including blood and blood products, hemodialysis, or among intravenous drug users (23). Between 10% and 25% of hepatitis C infected individuals, 14% to 36% of drug users, and 16% to 42% of HIV-infected people have HPgV infection in cross-sectional studies (21). Although HPgV viremia persists in an estimated 25% of infected people, the majority of those infected clear viremia over a period of 2 years and coincidentally develop antibody against the E2 envelope glycoprotein (17, 21). In general, antibodies to HPgV E2 are not detected during viremia, but develop after clearance. Thus detection of E2 antibody is indicative of prior infection. Coexisting viremia and E2 antibodies are uncommon in blood donors (< 1%), and slightly more common in HIV-infected subjects (range 1% to 5%) (22).
Clinical Manifestations
When GBV-C/HGV (now HPgV) was discovered in 1995 it was thought to be an etiological agent for non-A to E hepatitis. However, well controlled, prospective studies failed to identify an association between infection and acute or chronic hepatitis (reviewed in (2, 17, 21, 22). Due to this lack of association with known acute disease, the US Food and Drug Administration decided not to screen the blood and related blood products for HPgV in human use (2, 21). Recently, an association between HPgV and NHL has been reported, and this is thought to possibly relate to reduced immune surveillance in HPgV-infected individuals (7, 11, 23).
GBV-C Association with other diseases
HCV Co-Infection: Several studiesfound an association between prolonged survival in HIV-infected individuals co-infected with HPgV compared to those with HIV mono-infection (26, 29, 33). Although some groups did not find this association, a study of transfusion-related HPgV infection in HIV-infected individuals confirmed improved survival following acquisition of HPgV infection (27) providing strong support of a causal relationship between HPgV infection and prolonged survival in HIV-infected people. In addition to prolonged survival, HPgV viremia is associated with slowerCD4+ T cell decline, lower HIV viral load and delayed progression to AIDS in some studies (17, 22). Furthermore,a large study of HIV-infected women in Thailand found that vertical transmission of HPgV from HIV-infected women was associated with an 87% reduction in maternal-fetal HIV transmission (RH 0.13; 95% C.I. 0.03, 0.54) (24).
Considerable research has investigated potential mechanisms by which HPgV might improve outcome in HIV-infected individuals. Both direct antiviral effects of HPgV (15, 30), and anti-inflammatory effects of persistent infection in HIV-infected individuals have been identified (12) and (reviewed in 5). This anti-inflammatory effect may influence other diseases, and a recent report found that people with Ebola virus infection had improved survival is they had HPgV infection (13). Figure 1 illustrates potential mechanisms by which HPgV contributes to prolonged survival in HIV-infection.
HIV Co-Infection: GBV-C/HGV/HPgV co-infection with HCV has been studied extensively in acute HCV, chronic HCV with and without cirrhosis, and in liver transplant patients (2, 14, 28). HPgV does not influence HCV viral load or the severity of liver disease compared to HCV monoinfection (14). Furthermore, pre-exisiting HPgV or HPgV acquisition during liver transplant does not influence the outcome of transplantation or recurrent of hepatitis (10, 28). Interestingly, HCV levels drop immediately post-transplant yet HPgV levels do not, indicating that the liver is not the primary target of HPgV infection. The most successful cell culture system utilizes primary peripheral blood mononuclear cell cultures, and considerable evidence supports the hematopoietic and lymphoreticular systems as the major target of HPgV in vivo (reviewed in 8).
Laboratory Diagnosis
Due to the lack of association with acute disease with the exception of NHL, no commercial diagnostic tests are available for HPgV infection.Detection of viral RNA by nucleic acid amplification is available as a special request from some commercial laboratories, though none are approved by the U.S. Food and Drug Association. Nucleic acid amplification usually amplifies highly conserved nucleotide sequences in the HPgV 5'-ntr and 3'-ntr regions (20). Two enzyme linked immunosorbent assays (ELISA) were widely used to detect antibodies to HPgV E2 protein; however, neither is currently available. These assays were the basis for the comments above stating that most people develop anti-E2 antibody during viral clearance. Using these methods, anti-E2 antibodies were found more frequently in healthy blood donors than viremia, suggesting that the majority of healthy blood donors clear HPgV viremia (8, 17, 21). Unfortunately, no other validated antibody assays are currently available.
Pathogenesis
Since discovery, numerous studies examined potential associations between HPgV and human diseases. Because the infection is so prevalent, associations studies require careful consideration of control populations. As noted above, HPgV is not associated with acute or chronic liver disease, hepatocellular carcinoma, cryoglobulinemia, lichen planus and Sjögren's syndrome (17). The best association between HPgV and disease to date is that with NHL, where the odds ratio of HPgV infection prior to development of NHL versus no HPgV infection was 3.43 (7). Although there are reports suggesting potential associations between HPgV viremia and multiple sclerosis and chronic fatigue syndrome, these remain to be validated.
SUSCEPTIBILITY IN VITRO AND IN VIVO
GBV-C/HPgV appears to be a lymphotropic virus, and does not replicate in the liver. The lymphocyte progenitor cells may be the primary permissive cell for GBV-C/HPgV replication. Based on ex vivo and in vivo studies, the virus replication in cell culture systems has proven inefficient and inconsistent. Some of the limitations of GBV-C/HPgV replication systems include the fact that GBV-C/HPgV replicates best in primary PBMCs, including CD4+, CD8+,CD19+, and B lymphocytes (8). Unfortunately, there is considerable donor and isolate variability, and a small percentage of PBMCs appear to support replication. Long term viral passage has not been described.
Bukh et al. have proofed that GBV-C/HPgV was susceptible in chimpanzees (6). They found GBV-C/HPgV viremia at week 11 and lasted for 4 months after inoculation. The liver functions tests in serial serum samples did not show any abnormal chemical and transaminaseenzyme levels in all experimental chimpanzees. Up to now, other animals have been attempted for the susceptibility to GBV-C/HPgV without success.
ANTIVIRAL THERAPY
The sensitivity of HPgV to alpha interferon therapy has been studied in HCV-HPgV co-infected individuals treated for HCV infection. The rate of HPgV clearance was similar to that of HCV-infection, indicating that HPgV is cleared by alpha interferon, and suggesting that HPgV and HCV have shared modes of immune evasion to natural antiviral cytokine responses of humans (reviewed in (5, 23, 32). However, in co-infected individuals, there was no correlation between HCV clearance and HPgV clearance, suggesting that host immune polymorphisms in the IL-28b gene that are associated with HCV clearance do not provide protection against HPgV (23).
VACCINES
No vaccines for HPgV have been developed, though the presence of antibody to E2 protein provides partial protection against reinfection (5). Thus, vaccines that elicit E2 antibodies should in theory be somewhat protective against infection. The relatively recent confirmation that HPgV infection is associated with NHL raises the question of whether blood donors should be screened for HPgV infection and/or the need for a HPgV vaccine.
PREVENTION
HPgV is transmitted by the same modes as HIV and hepatitis B virus (21). Consequently, preventive measures for HPgV are similar to those used for these two viruses.
REFERENCES
1.Adams MJ, King AMQ, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Arch Virol 2013;158:2023-30, 2013. [PubMed]
2.Alter HJ. G-pers creepers, where'd you get those papers? A reassessment of the literature on the hepatitis G virus, Transfusion 1997;37: 569–572.[PubMed]
3. Alter HJ, Nakatsuji Y, Melpolder J, Wages , Wesley R, Shih JW, Kim JP. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747-754.[PubMed]
4. Alter MJ, Gallagher M, Morris TT, Moyer LA, Meeks EL, Krawczynski K Kim JP, Margolis HS. Acute non-A-E hepatitis in the United States and the role of hepatitis G virus infection. N Engl J Med 1997;336:741-746.[PubMed]
5. Bhattarai N, Stapleton JT. GB virus C: The Good Boy Virus? Trends in Microbiol,2012;20:123-130.[PubMed]
6. Bukh J, Kim JP, Govidarajan S, Apgar CL, Foung SK, Wages J, Yun AJ, Shapiro M, Emerson SU, Purcell RH. Experimental infection of chimpanzees with hepatitis G virus and genetic analysis of the virus. J Infect Dis 1998;177:855-862. [PubMed]
7. Chang CM, Stapleton JT, Klinzman D, McLinden JH, Purdue MP, Katki HA, Engels EA. GBV-C infection and risk of NHL among U.S. adults. Cancer Res 2014;74:5553-5560.[PubMed]
8. Chivero ET, Stapleton JT.Tropism of human Pegivirus (formerly known as GB virus C/Hepatitis G virus) and host immunomodulation: insights into a highly successful viral infection.J. Gen. Virol. In press, 2015.[PubMed]
9.Feng Y, Zhao W, Feng Y, Dai J, Li Z, Zhang X, Liu L, Bai J, Zhang H, Lu L, Xia X. A novel genotype of GB virus C: its identification and predominance among injecting drug users in Yunnan, China. PLoS One 2011;5:321151.[PubMed]
10. Fried MW, Khudyakov YE, Smallwood GA, Cong M, Nichols B, Diaz E,et al. Hepatitis G virus co-infection in liver transplantation recipientswith chronic hepatitis C and nonviral chronic liver disease. Hepatology1997;25:1271–5.[PubMed]
11.Krajden M, Yu A, Braybrook H, Lai AS, Mak A, Chow R, Cook D, Tellier R, Petric M, Gascoyne RD, Connors JM, Brooks-Wilson AR, Gallagher RP, Spinelli JJ. GBV-C/hepatitis G virus infection and non-Hodgkin lymphoma: a case control study.Int J Cancer, 2010;126:2885-2892.[PubMed]
12. Lanteri M, Vahidnia F, Tan S, Stapleton JT, Norris PJ, Heitman J, Deng X, Keating S, Brambilla D, Busch MP, Custer B.Down-regulation of cytokines and chemokines by GB virus C after transfusion-transmission in HIV+ blood recipients. J Infect Dis, 2015;211:1585-1596.[PubMed]
13. Lauck M, Bailey AL, Andersen KG, Goldberg TL, Sabeti PC, O'Connor DH. GB virus C coinfections in west African Ebola patients. J Virol, 2015;89:2425-2429.[PubMed]
14. McHutchison JG, Nainan OV, Alter MJ, Sedghi-Vaziri A, Determer J, Collins M, Kolberg J. Hepatitis C and G co-infectetion: Response to interferon therapy and quantitative changes in serum HGV-RNA. Hepatology 1997;26:1322-1327.[PubMed]
15. Mohr EJ, Stapleton JT.GB virus type C interactions with HIV: The role of envelope glycoproteins. J Viral Hep, 2009;16:757-768. [PubMed]
16. Nakao H, Okamoto H, Fukuda M, Tsuda F, Mitsui T, Masuko K, Iizawa H, Miyakawa Y, Mayumi M. Mutation rate of GB virus C/hepatitis G virus over the entire genome and in subgenomic regions. Virology 1997;233:43-50.[PubMed]
17. Polgreen P, Xiang J,Stapleton JT.GB Virus C – HIV Co-Infection.Microbes and Infection.2003;5:1255-1261.[PubMed]
18. Smith DB, Basaras M, Frost S, Haydon D, Cuceanu N, Prescott L, Kamenka C, Millband D, Sathar MA, Simmonds P. Phylogenetic analysis of GBV-C/hepatitis G virus. J Gen Virol, 81:769-80, 2000.[PubMed]
19. Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P.Expanded classification of hepatitis C virus into 7 genotypes and 66 subtypes, updated criteria and assignment web resource.Hepatology, 2014;59:318-327.[PubMed]
20.Souza IE, Allen JB, Xiang J, Klinzman D, Diaz R, ZhangS, Chaloner K, Zdunek D, Hess G, Williams CF, Benning L, Stapleton JT.Optimal testing for GB Virus C viremia: Effect of primer selection on estimates of GBV-C prevalence and response to antiretroviral therapy. J. Clin. Microbiol. 2006;44:3105-3113.[PubMed]
21. Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. The GB viruses: A Review and proposed re-classification as Pegiviruses. J Gen Virol, 2011;92:233-246.[PubMed]
22.Stapleton JT, Williams CF, Xiang J.GB Virus Type C: a Beneficial Infection?J Clin Micro,2004;42: 3915–3919. [PubMed]
23. Stapleton JT, Xiang J, McLinden JH, Bhattarai N, Chivero ET, Klinzman D, Kaufman TM, Chang Q. A novel T cell evasion mechanism in persistent RNA virus infection. Trans Am Clin Climatol Assn. 2014;125:14-26.[PubMed]
24. Supapol WB, Remis RS, Raboud J, Millson M, Tappero J, Kaul R, Kulkarni P, McConnell MS, Mock PA, Culnane M, McNicholl J, Roongpisuthipong A, Chotpitayasunondh T, Shaffer N, Butera S. Reduced mother-to-child transmission of HIV associated with infant but not maternal GB virus C infection. J Infect Dis, 2008;197:1369-1377.[PubMed]
25. Tanaka E, Alter HJ, Nakatsuji Y, Shih WK, Kim JP, Matsumoto A, Kobayashi M, Kiyosawa K. Effect of hepatitis G virus infection on chronic hepatitis C. Ann Intern Med,1996;125:740-743.[PubMed]
26. Tillmann H, Heiken H, Knapik-Botor A, Heringlake S, Ockenga J, Wilber JC, Goergen B, Detmer J, McMorrow M, Stoll M, Schmid RE, Manns MP. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med 2001;345:715-724.[PubMed]
27. Vahidnia F, Petersen M, Stapleton JT, Rutherford GW, Busch M, Custer B.Acquisition of GB virus type C and lower mortality in patients with advanced HIV disease. Clin Infect Dis, 2012;55:1012-9.[PubMed]
28. Vargas HE, Laskus T, Radkowski M, Poutous A, Wang LF, Lee R, et al. Hepatitis G virus co-infection in hepatitis C virus-infected liver transplant recipients. Transplantation, 1997;64:786–8.[PubMed]
29. Williams CF, Klinzman D, Yamashita T, Xiang J, Polgreen PM, Rinaldo C, Liu C, Phair J, Margolick JB, Zdunek D, Hess G, Stapleton JT. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med 2004;350:981-90.[PubMed]
30. Xiang J, George SL, Wunschmann S, Chang Q, Klinzman D, Stapleton, JT. GB virus C infection inhibits HIV-1 replication by increasing RANTES, MIP-1a, MIP-1b, and SDF-1. Lancet, 2004;363:2040–2046.[PubMed]
31.Xiang, JH, Klinzman D, McLinden J,Schmidt WN, LaBrecque DR, Gish R,Stapleton JT. Characterization of Hepatitis G Virus (GB-C Virus) Particles: Evidence for a Nucleocapsid and Expression ofSequences Upstream of the E1 Protein.J Virol. 1998;72:2738-2744.[PubMed]
32. Xiang J, Martinez-Smith C, Gale Jr. M, Chang Q, LaBrecque DR,Schmidt WN,Stapleton JT.GB Virus type C NS5A sequence polymorphisms:Association with interferon susceptibility and inhibition of PKR-mediated eIF-2 phosphorylation. J Interferon Cytokine Res, 2005;25:261-270.[PubMed]
33. Xiang J, Wunschmann S, Diekema D, Klinzman D, Patrick KD, George SL, Stapleton JT. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med, 2001;345:707-714.[PubMed]
Tables
None
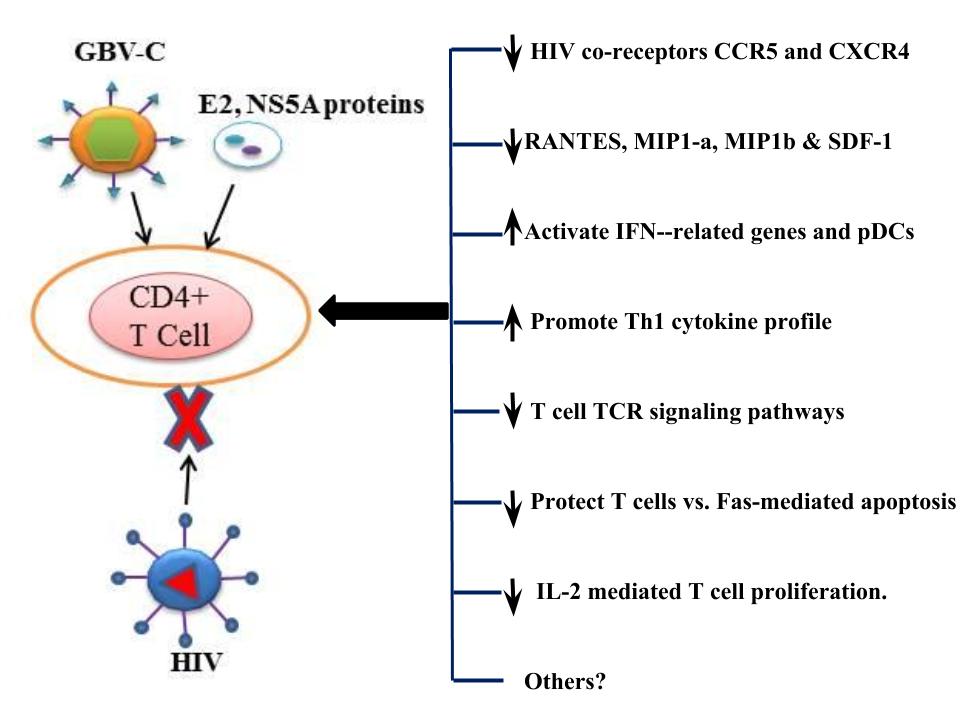
Figure 1. Effects of HPgV infection that may be beneficial in HIV coinfection.GBV-C replication increases chemokine expression, reduces the expression of CCR5 and CXCR4 and alters Th1/Th2 cytokines (30). It also induces IFN-g expression and activates pDCs and reduce the frequency of Fas-expressing in T cells (reviewed in (5)). GBV-C viremia is associated with decreased CCR5 expression (30) and polarization of Th cytokines towards a Th1 profile in clinical and laboratory (5). These effects may be mediated by the GBV-C E2 protein via interactions with CD81 on the surfaces of CD4 cells (30). In addition to the effects of GBV-C on chemokine and cytokine expression, other undefined effects of GBV-C may also influence HIV disease progression. The E2 protein interferes with HIV cellular binding and/or fusion (27), block T cell receptor (TCR) signaling by competing for Lymphocyte-specific protein tyrosine kinase (Lck) activation (4). The NS5A protein downregulates CD4 and CXCR4 expression and induces SDF-1 that is soluble ligand for CXCR4 (15). The NS5A protein also induces Th1 cytokines and downregulates expression of Th2 cytokines (27). Both GBV-C E2 and NS5A proteins inhibit HIV replication. Other mechanisms to alter GBV-C interaction with HIV are underway.
What's New
None
Guided Medline Search For:
Reviews
Review Article:Funk GA, et al. Viral dynamics in transplant patients: implications for disease. Lancet Infectious Diseases 2007;7:460-472.

