Staphylococcus aureus in Transplant Recipients
Authors: Paschalis Vergidis and Robin Patel
PATHOGEN
Staphylococcus aureus is a major cause of healthcare and community-acquired infections encountered in both immunocompetent and immunosuppressed individuals. Staphylococci are Gram-positive cocci, typically arranged in irregular clusters (Figure 1). S. aureus is coagulase-positive, an important feature used to differentiate it from other staphylococcal species. The organism usually forms gray to deep golden yellow colonies (Figure 2).
S. aureus may have one or more of a variety of types of antimicrobial resistance. Production of β-lactamase confers resistance to penicillin, and was reported shortly after the introduction of penicillin in the 1940’s. Resistance was initially encountered in nosocomial strains, but eventually spread to strains in the community. Nowadays, penicillin resistance is found in 80-95% of isolates (32). Since the 1970’s, resistance to penicillinase-stable penicillins (e.g., nafcillin, oxacillin, methicillin) has emerged. Resistance is conferred by a modified penicillin binding protein (PBP2a) with reduced affinity to methicillin. Methicillin is not used in clinical practice today; however these strains, historically termed methicillin-resistant S. aureus (MRSA), are resistant to all available β-lactam antibiotics. PBP2a is encoded by mecA which is part of a chromosomal element called the staphylococcal cassette chromosome (SCCmec) (1). The majority of healthcare-associated MRSA isolates are SCCmec types I, II, or III, and are multidrug resistant. In contrast, community-associated MRSA isolates are mainly SCCmec type IV.
Resistance to clindamycin may be constitutive or inducible. To evaluate for inducible clindamycin resistance, clindamycin and erythromycin disks may be placed in proximity to one another on an agar plate. Flattening of the clindamycin zone adjacent to the erythromycin disk indicates inducible clindamycin resistance (Figure 3). S. aureus isolates which test susceptible to clindamycin but exhibit inducible clindamycin resistance (detected as described) may be associated with clinical failure of clindamycin (i.e., even in the absence of erythromycin) (36).
The majority of S. aureus strains are susceptible to vancomycin. Vancomycin heteroresistance has been recognized among strains containing subpopulations with intermediate susceptibility to vancomycin (with the population as a whole falling within the susceptible range). The Clinical and Laboratory Standards Institute (CLSI) and the US Food and Drug Administration (FDA) have established a minimal inhibitory concentration (MIC) to vancomycin of 2 µg/ml or below as indicating susceptibility (60). This breakpoint was introduced since 2006 to address the issue of heteroresistance. Isolates with MICs between 4 and 8 µg/ml are vancomycin intermediate [vancomycin intermediate S. aureus (VISA), or glycopeptide intermediate S. aureus (GISA)]. Vancomycin resistant isolates [(vancomycin resistant S. aureus (VRSA)] have been identified since 2002, but are rare. In the clinically isolated strains, resistance was conferred by a plasmid-mediated transfer of vanA from resistant enterococci (51). VRSA strains demonstrate an MIC to vancomycin of 16 µg/ml or above.
Regarding the newer anti-staphylococcal agents, linezolid resistance among S. aureus strains is rare, but reported. Chromosomal mutations leading to daptomycin non-susceptibility have been described, but this type of resistance is also rare (9). Increases in the MIC to vancomycin have been linked to increases in the MIC to daptomycin not associated with genetic mutations. It has been suggested that vancomycin induces formation of thick cell walls impairing the activity of daptomycin, but the mechanism of non-susceptibility for these strains has not been clearly elucidated.
EPIDEMIOLOGY
Approximately 30% of the population carries S. aureus in the anterior nares or on the skin. The prevalence of MRSA carriage in the acute care setting has been estimated between 1 and 12%, but varies geographically. Colonization with MRSA is a risk factor for invasive disease; however infection can occur in the absence of prior colonization. Other risk factors for healthcare-associated MRSA infections include prolonged hospitalization and exposure to antibiotics, both of which are common among transplant recipients. The association between methicillin-susceptible S. aureus (MSSA) carriage and infection is weaker (6). Interestingly, MSSA may compete with MRSA for colonization of the anterior nares.
Despite current infection control measures, MRSA is causing an increasing number of infections in healthcare institutions. Most healthcare-associated infections derive from invasive procedures or devices, such as indwelling catheters. In a study conducted from 1990 through 1998 among deceased donor liver transplant recipients, MRSA infections occurred in 23% of patients (53). An exponential increase in the number of such infections was noted over the years of the study. The median time of onset of MRSA infection was 24 days post-transplant. The sources of infection were intravascular catheters, wounds, the abdomen, and lungs.
Hemodialysis and peritoneal dialysis are risk factors for MRSA infection in renal transplant candidates or recipients. Among liver transplant candidates with MRSA colonization who received a hepatic allograft there was an increased risk of MRSA infection post-transplant (18, 44). However, no difference in survival was seen between those colonized with MRSA prior to transplantation and those who were not. Patients with MRSA nasal carriage tend to develop infection due to MRSA sooner after transplantation compared to non-carriers. Other risk factors for MRSA infection include cytomegalovirus seronegativity, primary cytomegalovirus infection (53), alcoholic cirrhosis (7) and severity of liver disease. Carriers of MRSA tend to have higher Model for End-Stage Liver Disease (MELD) scores than non-MRSA carriers (18). Nasal MRSA carriage can be acquired in the post-transplant period (24, 46). Hence, when surveillance is performed, surveillance cultures should be obtained post-operatively in patients who are not found to be colonized prior to the transplant. Among patients without nasal carriage, hepatic explant iron overload has been identified as a risk factor for S. aureus bacteremia in liver transplant recipients (56), presumably because iron is a nutrient source for staphylococci.
CLINICAL MANIFESTATIONS
Skin and soft tissue infections S. aureus is a common cause of skin and soft tissue infection. Cellulitis is most often caused by Streptococcus pyogenes or S. aureus; however in transplant recipients, cellulitis may occasionally be caused by Gram-negative organisms and even fungi. Necrotizing fasciitis produces severe pain at the initial stage of infection. Skin findings are less prominent. Fasciitis may be caused by hematogenous spread of bacteria. Community-acquired MRSA has been implicated in severe cases of fasciitis. Again, Gram-negative organisms may be involved in the transplant patient.
Surgical Site Infections
Surgical site infections manifest with progressive edema, erythema, and pain around the surgical incision. S. aureus has been identified as the causative agent in 15% of surgical site infections in liver transplant recipients (2) and 5% of surgical site infections in kidney transplant recipients (41); most surgical site infections in transplant recipients are, however, caused by Gram-negative aerobic organisms. In a study of surgical site infections in heart transplant recipients, the inciting organisms were mainly coagulase-negative staphylococci, followed by MRSA (40).
Pulmonary Infections
Community acquired pneumonia due to S. aureus (especially MRSA) is increasingly reported, especially with antecedent influenza infection. Among hospitalized patients, S. aureus is a common cause of nosocomial and ventilator-associated pneumonia. Clinically, staphylococcal pneumonia may be indistinguishable from other causes of bacterial pneumonia, but tends to progress rapidly and cause lung necrosis and cavitation. Complications include lung abscess formation and pleural empyema.
There are certain features pertinent to lung transplant recipients. Because of the communication with the external environment, lung allografts are particularly vulnerable to respiratory tract infections, not only in the immediate post-operative period, but throughout the post-transplant course. Lung allografts are denervated and are subjected to decreased mucociliary clearance and cough reflex. The use of antimicrobial prophylaxis has decreased the rate of early post-operative pneumonia. In the era of antiviral prophylaxis, cytomegalovirus pneumonitis also occurs infrequently. Among the Gram-positive pathogens, S. aureus infection predominates. In patients with cystic fibrosis or bronchiectasis as the underlying lung disorder, pretransplant colonizing bacteria can be the causative agents of pneumonia post-transplant. Hence antibacterial prophylaxis is guided based on these organisms (11). Among children with cystic fibrosis, S. aureus pulmonary infections in the pretransplant period were associated with decreased post-transplant survival (28). Recent evidence suggests an association between Gram-positive respiratory tract infections (either bronchitis or pneumonia) and surgical airway complications, such as airway stenosis, dehiscence, bronchomalacia, and pseudomembrane formation (22). Bronchiolitis obliterans, a late complication mainly associated with acute cellular rejection, has also been linked to Gram-positive respiratory tract infections (61).
In recipients of solid organ transplants other than the lungs, S. aureus has also been identified as a common cause of pneumonia (8). Empiric coverage for this organism should be considered in lower respiratory tract infections.
Bloodstream Infections
S. aureus is the leading cause of healthcare associated bloodstream infections. Commonly these infections are associated with an intravascular catheter. Fever or other manifestations of systemic infection may not be prominent in the immunocompromised host. Even a single blood culture positive for S. aureus should prompt the initiation of antimicrobial therapy. Blood stream infections may be complicated by metastasis of infection. Persistent bacteremia should raise the suspicion of an endovascular infection (e.g., endocarditis) or an undrained focus of infection.
Among heart and liver transplant recipients there is a trend towards a predominance of Gram-negative blood stream infections in the post-transplant period (42, 54). Gram-positive blood stream infections affect morbidity and mortality, particularly in cases of hepatic retransplantation. Among Gram-positive organisms causing bacteremia, S. aureus carries the highest 30-day mortality (5).
Endocarditis, Pericarditis
S. aureus endocarditis typically have an acute course with fever and tachycardia. Dyspnea may ensue as a result of congestive heart failure. S. aureus endocarditis is associated with large (i.e., 1 cm or larger) vegetations and a high risk of septic emboli. Central nervous system involvement may be the result of septic emboli or mycotic aneurysms. Endocarditis is a rare, yet well-described, complication of heart transplantation. In a published series of 10 cases, S. aureus and Aspergillus fumigatus were the most commonly involved pathogens (48). The mitral and tricuspid valves were predominantly involved. Central venous catheters and frequent myocardial biopsies predispose to S. aureus infection.
Purulent MRSA pericarditis complicated by tamponade has been described in a liver transplant recipient (19).
Heart Transplantation In the Setting of an Infected Ventricular-Assist Device
Patients with severe refractory congestive heart failure may receive a ventricular-assist device as a bridge to heart transplantation. Infection can occur in the blood-contacting prosthetic surfaces, the device-related pockets and cavities, or the transcutaneous drivelines and power cables. Among other pathogens, S. aureus has been reported as the etiologic agent of these infections (47, 63). The organism may be isolated from blood or purulent material surrounding the device or the transcutaneous line. Device-related infections are associated with increased mortality, but are not a contraindication to transplantation (25). Use of appropriate antimicrobial agents in combination with adequate debridement of infected tissue and drainage of purulent material generally prevents infection of the cardiac allograft.
Infection of the Hepatic Allograft
There are differences between deceased donor and living donor liver transplantation. In living donor transplantation, the allografts are frequently small-for-size. This may lead to postoperative liver dysfunction with coagulopathy and cholestasis, predisposing to septic complications. Biliary leakage from the cut surface of the graft is another complication of living donor transplantation. MRSA infections have been studied in this patient population (23). The incidence of MRSA infection was found to be 10%. Preoperative MRSA colonization, preoperative use of antimicrobial agents, operative time of 16 hours or longer, and postoperative apheresis, suggesting allograft dysfunction, were independently associated with postoperative MRSA infection in living donor transplants.
Osteomyelitis
Acute osteomyelitis is usually the result of hematogenous seeding. S. aureus is the predominant pathogen. Symptoms include fever and local pain. Cases have been described in transplant patients (15, 31). Sternal wound osteomyelitis may complicate open heart surgery. Mediastinitis, a feared complication following heart transplantation, is predominantly caused by S. aureus (58). Besides antibiotic treatment, surgical debridement with or without flap closure is necessary (14).
Donor-derived Infections, Contamination of the Allograft
The risk of infection is generally higher in donor-derived infection than allograft contamination, as a result of the larger inoculum of microorganisms transmitted. Donor-derived infection is generally more severe than is infection associated with allograft contamination; however donor-derived infection is less common than is infection associated with allograft contamination. In a study conducted in Spain, donor bacteremia was detected in 14% of donors, with the most common organisms detected being coagulase-negative staphylococci followed by S. aureus (35). Fatal staphylococcal pneumonia in a liver transplant recipient derived from an infected but asymptomatic donor has been described (34).
Bacterial contamination of the allograft can occur during recovery, preservation and handling, or at transplantation (43). Isolation of S. aureus from the donor or preservation fluids has been associated with graft loss and even death post transplant.
LABORATORY DIAGNOSIS
The diagnosis of staphylococcal infection is established by isolation of the organism from affected sites (e.g., blood, sputum or tracheal secretions, urine, purulent material). Visualization of Gram-positive cocci on Gram stain of the direct specimen provides an early clue to S. aureus infection. Rapid diagnostic techniques can expedite characterization of Gram-positive cocci in clusters in blood cultures as either S. aureus or coagulase-negative staphylococci. A fluorescent in situ hybridization (FISH) assay employing peptide nucleic acid (PNA) probes to target specific ribosomal RNA in live bacteria (PNA FISH) allows for identification of S. aureus in fewer than 3 hours after blood cultures turn positive (21). Molecular assays, such as real-time polymerase chain reaction (PCR), can also be used for identification of S. aureus (and MRSA) in blood culture bottles yielding Gram-positive cocci resembling staphylococci (64).
Surveillance cultures may be performed at body sites that may be colonized, such as the anterior nares, throat, axillae, rectum or open wound areas. Traditional culture techniques yield results within 24 to 72 hours. Rapid culture results can be obtained with the use of chromogenic agar which changes color in the presence of S. aureus. The turnaround time is typically 24 to 48 hours (13). Chromogenic agar can also be used to detect MRSA (Figure 4). Molecular techniques targeting DNA sequences within SCCmec allow for MRSA detection in 2 to 6 hours (12). Cost and operator skills required for molecular assays limit their widespread use.
PATHOGENESIS
S. aureus colonizes the skin and mucosal surfaces. Microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) play a key role in adherence of S. aureus to cell and extracellular matrix proteins (38). S. aureus may be introduced into deeper tissue through abrasions, open wounds, administration of parenteral medications or via intravenous catheters. Production of bacterial enzymes allows for further replication and spread of infection. Several bacterial toxins have been described which facilitate invasion and tissue destruction. The Panton-Valentine leukocidin, a staphylococcal pore forming toxin often present in community-acquired MRSA, damages human neutrophils. The earliest tissue response is acute inflammation through recruitment of polymorphonuclear lymphocytes. Tissue necrosis leads to abscess formation.
S. aureus isolates form biofilms, a layer of microorganisms adherent to an organic or inorganic surface and enclosed in a self-produced polymeric matrix. Biofilm formation plays a significant role in the pathogenesis of device-associated, wound and other deep-seated infections. Bacteria in biofilms demonstrate resistance to conventional antimicrobial agents predominantly as a result of their altered growth (16).
Therapy
Antimicrobial agents used in the management of staphylococcal infections are listed in Table 1. Recommended durations of treatment for individual types of infections are presented in Table 2.
Penicillin-Susceptible S. aureus
Penicillin (penicillin G for parenteral administration, or penicillin VK or amoxicillin for oral administration) are used for penicillin-susceptible S. aureus. Since isolates testing susceptible to penicillin may still produce β-lactamase, if the MIC to penicillin is in the susceptible range (≤ 0.12 mg/L), an induced β-lactamase test should be performed. Even then, occasional resistant isolates may remain undetected (26). Thus, for serious infections, the penicillinase-stable penicillins are preferred.
Penicillin-Resistant, Methicillin-Susceptible S. aureus (MSSA)
The drugs of choice are the penicillinase-stable penicillins, nafcillin and oxacillin. Methicillin has fallen out of favor due to adverse events, primarily intersitial nephritis. Combinations of β-lactams with β-lactamase inhibitors (e.g., ampicillin/sulbactam, piperacillin/tazobactam both given intravenously and amoxicillin/clavulanate given orally) have activity against MSSA. Due to their broad antimicrobial spectrum, these drugs are mainly reserved for mixed infections.
Cephalosporins may be used in patients allergic or intolerant to penicillins. However, in the setting of severe allergy, such as angioedema or anaphylaxis, all β-lactams should be avoided. Commonly used first generation cephalosporins are cephalexin and cefadroxil for oral use, and cefazolin for intravenous use. Cefuroxime, ceftriaxone, cefotaxime and cefepime are active against MSSA, but ceftazidime has poor activity. The carbapenems (e.g., imipenem/cilastatin, meropenem, ertapenem) are also active against MSSA.
In serious infections with known severe allergy to the β-lactams, vancomycin is the recommended drug. However, for MSSA infections treatment failure is higher with the use of vancomycin than with the β-lactams (59). Tolerance to vancomycin (high ratio of minimal bactericidal to bacteriostatic concentrations) may play a role.
Methicillin-Resistant S. aureus (MRSA)
The drug of choice for serious infections, such as bacteremia, endocarditis, pneumonia or osteomyelitis, is vancomycin. It inhibits bacterial cell wall synthesis by blocking glycopeptide polymerization. Teicoplanin can also be used but is not marketed in the United States.
Linezolid inhibits bacterial protein synthesis, hence it is bacteriostatic against staphylococci. It is approved by the United States FDA for the treatment of complicated skin and skin-structure infections and nosocomial pneumonia caused by MRSA. An analysis of two randomized double-blind trials suggests that linezolid may be associated with better survival and clinical cure rates compared to vancomycin for nosocomial pneumonia (65). There are no randomized controlled trials regarding its use in primary bacteremias or infectious endocarditis. Individual treatment successes and failures have been reported in cases of endocarditis; however its use is not routinely recommended.
Daptomycin acts by disrupting the functional integrity of the plasma membrane and is rapidly bactericidal. For bacteremias and endocarditis, daptomycin met primary endpoints for non-inferiority compared to penicillins and vancomycin for MSSA and MRSA infections, respectively (20). As the medication is inactivated by pulmonary surfactant it should not be used in lower respiratory infections (52).
An alternative agent for mild to moderate infections is clindamycin. Clinicians should verify whether testing for inducible clindamycin resistance is performed in the microbiology laboratory. For strains with inducible clindamycin resistance, the drug should not be used. Of note, there is an increasing prevalence of clindamycin-resistant community-acquired MRSA (10).
Other oral treatment options for less severe infections include high-dose trimethoprim-sulfamethoxazole, and doxycycline or minocycline. The new generation fluoroquinolones, levofloxacin, moxifloxacin and gemifloxacin, are associated with rapid development of resistance and should be avoided. The macrolides (e.g., erythromycin, azithromycin, clarithromycin) may be active against S. aureus, but are not recommended for empiric treatment due to increasing rates of resistance.
Vancomycin-Intermediate and Resistant S. aureus (VISA, VRSA)
Treatment options are linezolid and daptomycin. Of note, VISA strains may also demonstrate non-susceptibility to daptomycin (9).
Alternative Therapy
Tigecycline, a derivative of minocycline, acts by inhibiting protein synthesis. It has been approved for the treatment of complicated skin and soft tissue infections, including those caused by MSSA and MRSA, and for complicated intra-abdominal infections, including those caused by MSSA but not MRSA. Tigecycline was shown to be non-inferior to imipenem/cilastatin for the treatment of hospital-acquired pneumonia. However, for patients with ventilator-associated pneumonias the medication did not meet non-inferiority criteria (29). Moreover, clinicians should be cautious when using tigecycline for the treatment of patients with suspected or proven bacteremia given the drug’s low serum levels and bacteriostatic activity. Treatment with tigecycline should generally be reserved for patients who cannot receive other anti-staphylococcal agents.
Quinupristin-dalfopristin is active against S. aureus, including MRSA, and has been approved for the treatment of Gram-positive bacteremia. Severe associated myalgias limit the use of the drug.
The lipoglycopeptides are semisynthetic derivatives of vancomycin that demonstrate more rapid bactericidal activity than vancomycin. Telavancin has been approved by the United States FDA for the treatment of complicated skin and soft tissue infections due to susceptible Gram-positive bacteria. Dalbavancin and oritavancin are under investigation. Dalbavancin has the unique feature of a long half-life, possibly allowing once-weekly intravenous dosing. In clinical studies it has been found effective in the treatment of catheter-related BSIs due to Gram-positive organisms, including MRSA (39).
Ceftobiprole is a novel investigational intravenous cephalosporin with activity against Gram-positive pathogens, including MRSA. The agent binds tightly to PBP2a. In a randomized, double-blind study ceftobiprole monotherapy was as effective as the combination of vancomycin and ceftazidime for complicated skin and skin-structure infections due to either Gram-positive or Gram-negative bacteria (33).
Fusidic acid acts by inhibiting protein synthesis. It is used mainly for the treatment of MRSA, but is not marketed in the United States. Due to the high risk of emergence of resistance, the drug is typically used in combination with rifampin (62). The medication can be administered orally or intravenously. A topical formulation for skin infections is also available. Finally, fosfomycin inhibits cell wall synthesis and is mainly used parenterally for serious MRSA infections.
Combination Treatment
The combination of nafcillin with gentamicin for native valve infective endocarditis due to MSSA is associated with a decreased duration of bacteremia compared to nafcillin monotherapy. However, combination treatment does not have an effect on mortality and does not reduce the frequency of cardiac complications (27). Thus combination treatment is recommended for only 3 to 5 days and for strains susceptible to the aminoglycoside. Similarly, there are no robust data for superior clinical efficacy with the combination of vancomycin and gentamicin.
Many S. aureus isolates are susceptible to rifampin. Monotherapy should be avoided due to the risk of emergence of resistance. Combination treatment with rifampin may be used in device-associated infections due to activity against staphylococcal biofilms. For S. aureus prosthetic valve endocarditis combination of an antistaphylococcal penicillin or vancomycin with rifampin is recommended (4). Concomitant administration of gentamicin for the first 2 weeks of treatment is also recommended. Resistance to rifampin can rapidly emerge due to a single-step mutation, especially in the presence of high grade bacteremia. Some experts recommend addition of rifampin only after clearance of bacteremia. Finally, combination treatment is recommended for S. aureus prosthetic joint infection managed with debridement and retention.
Treatment in Transplant Recipients
Treatment of staphylococcal infections does not differ in transplant recipients compared to other patients. Extensive drug-drug interactions can be seen with the use of rifampin (3). More specifically, rifampin results in decreased cyclosporine levels. Cyclosporine levels need to be increased by ~2.5-fold to achieve appropriate levels. Rifampin also decreases tacrolimus levels. Monitoring of tacrolimus levels is necessary. No empiric dose changes are recommended. Rifampin decreases the levels of mycophenolate mofetil. Finally, rifampin decreases the levels of azoles (e.g., used for antifungal prophylaxis). Concomitant use of quinolones and azoles has the potential to result in QT prolongation. ECG monitoring is therefore advised when the drugs are administered concomitantly.
ADJUNCTIVE THERAPY
For skin and soft tissue infections, surgical drainage of abscess collections is indicated in addition to antimicrobial therapy. For necrotizing fasciitis, aggressive surgical debridement of devitalized tissue in the involved fascia is indicated. For surgical site infections, opening of the wound with removal of purulent material and necrotic tissue may be necessary.
Pleural empyemas should be adequately drained. In the presence of free flowing fluid, chest tube thoracostomy is usually adequate. Drainage of empyemas may be facilitated by intrapleural instillation of fibrinolytic agents. For multiloculated collections, video-assisted thoracoscopic surgery with débridement is usually required. In advanced pleural fibrosis, lung reexpansion is achieved with surgical decortication.
For catheter-related bloodstream infections due to S. aureus, the intravascular catheter should be removed (30). This applies to both temporary and chronic indwelling catheters. Placement of a new catheter should be performed only after documentation of clearance of bacteremia. On rare occasions where removal of the catheter is not possible, the patient should receive both systemic and antimicrobial lock therapy.
In native or prosthetic valve endocarditis due to S. aureus, valve replacement should be considered in cases of congestive heart failure, persistent bacteremia despite appropriate antimicrobial treatment or embolic events within the first two weeks of treatment (4). Surgical intervention, including valve replacement, should also be considered if there is echocardiographic evidence of valve dehiscence, perforation, rupture or fistula formation, and in the presence of a large paravalvular abscess or an increase in vegetation size despite treatment. The routine use of antiplatelet agents, such as aspirin, is not recommended. Purulent pericarditis necessitates adequate drainage of the pericardial collection.
For osteomyelitis in the presence of prosthetic material and for prosthetic joint infection, removal of the hardware should be combined with antimicrobial treatment. Candidates for debridement and retention of the hardware are patients with a stable functioning prosthesis who fulfill the following criteria: 1) symptoms for less than 3 weeks, 2) infection within 3 months of implantation or hematogenous infection, and 3) no abscess or sinus tract (17).
ENDPOINTS FOR MONITORING THERAPY
Appropriate response to antimicrobial treatment is monitored by clinical improvement. For endocarditis, blood cultures should be obtained to demonstrate clearance of bacteremia. Blood cultures should also be obtained if recurrence of fever or if symptoms suggestive of breakthrough bacteremia develop. Persistence of bacteremia for more than 72 hours after initiation of effective treatment should prompt evaluation for a deep focus of infection. Finally, in cases of endocarditis, followup cultures should be obtained after completion of treatment. Inflammatory markers (e.g., erythrocyte sedimentation rate, C-reactive protein) may be followed in osteomyelitis to assess response to treatment.
Vancomycin dosing is adjusted based on the weight and renal function of the patient. Therapeutic drug monitoring is recommended if renal function is unstable or if the expected duration of treatment is longer than five days. Trough levels correlate better with efficacy than peak levels. A trough serum concentration should be obtained after the steady state has been achieved. For patients with normal renal function steady state is usually achieved after the third drug dose. Trough serum concentrations should be obtained just before the anticipated dose. For patients with stable renal function, therapeutic drug monitoring should be repeated at least weekly. In general, trough serum concentrations should be maintained above 10 mg/L; however for serious infections, such as bacteremia, endocarditis, pneumonia, osteomyelitis, and meningitis, trough serum concentrations of 15-20 mg/L have been recommended (45). This recommendation is based on consensus opinion and is not supported by evidence from randomized controlled trials (level III evidence).
VACCINES
There are currently no approved vaccines for protection against S. aureus infections. A bivalent conjugated vaccine using type 5 and 8 capsular polysaccharides was tested in patients receiving hemodialysis in a double-blind trial. The estimated efficacy in preventing bacteremia was 57% between weeks 3 and 40 (49). However, in a phase III placebo-controlled trial no reduction in S. aureus infections was found. Other vaccines for prevention of staphylococcal infections are under investigation.
PROPHYLAXIS AND INFECTION CONTROL
Standard precautions should be implemented for all patients based on the principle that all body fluids may contain transmissible agents (50). These include hand hygiene and use of gloves, gowns, and/or mask depending on the anticipated exposure to body fluids or secretions. Patients who have known colonization or infection due to MRSA should be placed in “contact precautions”. These patients are placed in a private room or cohorted with other patients infected or colonized with MRSA. Healthcare workers should use gloves and gowns when entering the room and remove them before exiting the room. Proper hand hygiene should always be used. Environmental cleaning with routine hospital disinfectants is imperative.
Decolonization has not been consistently found to decrease the risk of MRSA infection. More specifically, nasal decolonization with mupirocin as a single infection control measure was not found to be efficacious in prevention of post-transplant infections in liver transplant recipients (37). However, a bundle of infection control interventions including nasal and rectal surveillance cultures, strict isolation precautions, and cohorting of patients, in combination with decolonization with mupirocin, has been effective in decreasing MRSA infections in liver transplant recipients (55). The use of chlorhexidine gluconate baths and /or oral antimicrobial agents may also be considered as part of a decolonization regimen. Colonization with MRSA at body sites other than the nares may necessitate aggressive infection control interventions for prevention of infection. Indeed, patients with both rectal and nasal S. aureus colonization were significantly more likely to develop infection than were those with nasal carriage alone (57).
Measures for general surgical peri-operative prophylaxis have been recommended, but randomized trials have not been conducted in transplant recipients. In order to decrease the risk of infection, bacterial prophylaxis should be administered within 60 minutes prior to surgical incision. Cefazolin, which has activity against streptococci and staphylococci, is recommended (for procedures not involving the bowel). Vancomycin should be used instead in patients known to be colonized with MRSA. Some centers have implemented combined use of cefazolin and vancomycin for major cardiothoracic procedures in patients colonized with MRSA.
REFERENCES [PubMed]
1. Appelbaum PC. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin Infect Dis 2007;45:S165-70. [PubMed]
2. Asensio A, Ramos A, Cuervas-Mons V, Cordero E, Sanchez-Turrion V, Blanes M, Cervera C, Gavalda J, Aguado JM and Torre-Cisneros J. Effect of antibiotic prophylaxis on the risk of surgical site infection in orthotopic liver transplant. Liver Transpl 2008;14:799-805. [PubMed]
3. Baciewicz AM, Chrisman CR, Finch CK and Self TH. Update on rifampin and rifabutin drug interactions. Am J Med Sci 2008;335:126-36. [PubMed]
4. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr., Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M and Taubert KA. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394-434. [PubMed]
5. Bedini A, Codeluppi M, Cocchi S, Guaraldi G, Di Benedetto F, Venturelli C, Masetti M, Prati F, Mussini C, Borghi V, Girardis M, Gerunda GE, Rumpianesi F and Esposito R. Gram-positive bloodstream infections in liver transplant recipients: incidence, risk factors, and impact on survival. Transplant Proc 2007;39:1947-9. [PubMed]
6. Bert F, Galdbart JO, Zarrouk V, Le Mee J, Durand F, Mentre F, Belghiti J, Lambert-Zechovsky N and Fantin B. Association between nasal carriage of Staphylococcus aureus and infection in liver transplant recipients. Clin Infect Dis 2000;31:1295-9. [PubMed]
7. Bert F, Bellier C, Lassel L, Lefranc V, Durand F, Belghiti J, Mentre F, Fantin B. Risk factors for Staphylococcus aureus infection in liver transplant recipients. Liver Transpl 2005;11:1093-9. [PubMed]
8. Bonatti H, Pruett TL, Brandacher G, Hagspiel KD, Housseini AM, Sifri CD and Sawyer RG. Pneumonia in solid organ recipients: spectrum of pathogens in 217 episodes. Transplant Proc 2009;41:371-4. [PubMed]
9. Boucher HW, Sakoulas G. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis 2007;45:601-8. [PubMed]
10. Braun L, Craft D, Williams R, Tuamokumo F and Ottolini M. Increasing clindamycin resistance among methicillin-resistant Staphylococcus aureus in 57 northeast United States military treatment facilities. Pediatr Infect Dis J 2005;24:622-6. [PubMed]
11. Campos S, Caramori M, Teixeira R, Afonso J, Jr., Carraro R, Strabelli T, Samano M, Pego-Fernandes P, and Jatene F. Bacterial and fungal pneumonias after lung transplantation. Transplant Proc 2008;40:822-4. [PubMed]
12. Carroll KC. Rapid diagnostics for methicillin-resistant Staphylococcus aureus: current status. Mol Diagn Ther 2008;12:15-24. [PubMed]
13. Carson J, Lui B, Rosmus L, Rennick H and Fuller J. Interpretation of MRSASelect screening agar at 24 hours of incubation. J Clin Microbiol 2009;47:566-8. [PubMed]
14. Chou NK, Wang JL, Chi NH, Wu IH, Huang SC, Chen YS, Yu HY, Tsao CI, Ko WJ, Su HY, Chang SC, Chu SH and Wang SS. Surgical treatment of mediastinitis after cardiac transplantation. Transplant Proc 2008;40:2629-30. [PubMed]
15. Datta S, Hussain IR and Madden B. Spinal osteomyelitis and diskitis: a rare complication following orthotopic heart transplantation. J Heart Lung Transplant 2001;20:1213-6. [PubMed]
16. Del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther 2007;82:204-9. [PubMed]
17. Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 2009;361:787-94. [PubMed]
18. Desai D, Desai N, Nightingale P, Elliott T and Neuberger J. Carriage of methicillin-resistant Staphylococcus aureus is associated with an increased risk of infection after liver transplantation. Liver Transpl 2003;9:754-9. [PubMed]
19. Durao D, Fernandes AP, Marum S, Marcelino P and Mourao L. Cardiac tamponade secondary to methicillin-resistant Staphylococcus aureus pericarditis. Rev Port Cardiol 2008;27:953-8. [PubMed]
20. Fowler VG, Jr., Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fatkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A and Cosgrove SE. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006;355:653-65. [PubMed]
21. Gonzalez V, Padilla E, Gimenez M, Vilaplana C, Perez A, Fernandez G, Quesada MD, Pallares MA and Ausina V. Rapid diagnosis of Staphylococcus aureus bacteremia using S. aureus PNA FISH. Eur J Clin Microbiol Infect Dis 2004;23:396-8. [PubMed]
22. Gupta MR, Valentine VG, Walker JE, Jr., Lombard GA, Laplace SG, Seoane L, Taylor DE and Dhillon GS. Clinical spectrum of gram-positive infections in lung transplantation. Transpl Infect Dis 2009 [PubMed]
23. Hashimoto M, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Moriya K, Koike K and Makuuchi M. Methicillin-resistant Staphylococcus aureus infection after living-donor liver transplantation in adults. Transpl Infect Dis 2008;10:110-6.[PubMed]
24. Hashimoto M, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, Moriya K, Koike K and Makuuchi M. Acquisition of methicillin-resistant Staphylococcus aureus after living donor liver transplantation: a retrospective cohort study. BMC Infect Dis 2008;8:155. [PubMed]
25. Herrmann M, Weyand M, Greshake B, von Eiff C, Proctor RA, Scheld HH and Peters G. Left ventricular assist device infection is associated with increased mortality but is not a contraindication to transplantation. Circulation 1997;95:814-7.
26. Kaase M, Lenga S, Friedrich S, Szabados F, Sakinc T, Kleine B and Gatermann SG. Comparison of phenotypic methods for penicillinase detection in Staphylococcus aureus. Clin Microbiol Infect 2008;14:614-6. [PubMed]
27. Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: A prospective study. Ann Intern Med 1982;97:496-503. [PubMed]
28. Liou TG, Adler FR, Cox DR and Cahill BC. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med 2007;357:2143-52. [PubMed]
29. Maroko R, Cooper A, Dukart G, Dartois N, Gandjini H. Results of Phase 3 study comparing a tigecycline (TGC) regimen with an imipenem/cilastatin (IMI) regimen in treatment of patients (Pts) with hospital-acquired pneumonia (HAP). 47th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 17–20 September; Chicago, IL (2007). [PubMed]
30. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad, II, Rijnders BJ, Sherertz RJ and Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009;49:1-45. [PubMed]
31. Nicholls A, Edward N and Catto GR. Staphylococcal septicaemia, endocarditis, and osteomyelitis in dialysis and renal transplant patients. Postgrad Med J 1980;56:642-8. [PubMed]
32. Nimmo GR, Bell JM, Mitchell D, Gosbell IB, Pearman JW and Turnidge JD. Antimicrobial resistance in Staphylococcus aureus in Australian teaching hospitals, 1989-1999. Microb Drug Resist 2003;9:155-60. [PubMed]
33. Noel GJ, Bush K, Bagchi P, Ianus J and Strauss RS. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin Infect Dis 2008;46:647-55. [PubMed]
34. Obed A, Schnitzbauer AA, Bein T, Lehn N, Linde HJ and Schlitt HJ. Fatal pneumonia caused by Panton-Valentine leucocidine-positive methicillin-resistant Staphylococcus aureus (PVL-MRSA) transmitted from a healthy donor in living-donor liver transplantation. Transplantation 2006;81:121-4. [PubMed]
35. Paredes D, Gambra MP, Cervera C, Linares L, Almela M, Rodriguez C, Ruiz A, Vilardell J and Moreno A. Characterization of the organ donor with bacteremia. Transplant Proc 2007;39:2083-5. [PubMed]
36. Patel M, Waites KB, Moser SA, Cloud GA and Hoesley CJ. Prevalence of inducible clindamycin resistance among community- and hospital-associated Staphylococcus aureus isolates. J Clin Microbiol 2006;44:2481-4. [PubMed]
37. Paterson DL, Rihs JD, Squier C, Gayowski T, Sagnimeni A, Singh N. Lack of efficacy of mupirocin in the prevention of infections with Staphylococcus aureus in liver transplant recipients and candidates. Transplantation 2003;75:194-8. [PubMed]
38. Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol 1994;48:585-617. [PubMed]
39. Raad I, Darouiche R, Vazquez J, Lentnek A, Hachem R, Hanna H, Goldstein B, Henkel T, Seltzer E. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005;40:374-80. [PubMed]
40. Ramos A, Asensio A, Munez E, Torre-Cisneros J, Blanes M, Carratala J, Segovia J, Munoz P, Cisneros JM, Bou G, Aguado JM, Cervera C, Gurgui MM. Incisional surgical infection in heart transplantation. Transpl Infect Dis 2008;10:298-302. [PubMed]
41. Ramos A, Asensio A, Munez E, Torre-Cisneros J, Montejo M, Aguado JM, Cofan F, Carratala J, Len O, Cisneros JM. Incisional surgical site infection in kidney transplantation. Urology 2008;72:119-23. [PubMed]
42. Rodriguez C, Munoz P, Rodriguez-Creixems M, Yanez JF, Palomo J, Bouza E. Bloodstream infections among heart transplant recipients. Transplantation 2006;81:384-91. [PubMed]
43. Ruiz I, Gavalda J, Monforte V, Len O, Roman A, Bravo C, Ferrer A, Tenorio L, Roman F, Maestre J, Molina I, Morell F, Pahissa A. Donor-to-host transmission of bacterial and fungal infections in lung transplantation. Am J Transplant 2006;6:178-82. [PubMed]
44. Russell DL, Flood A, Zaroda TE, Acosta C, Riley MM, Busuttil RW, Pegues DA. Outcomes of colonization with MRSA and VRE among liver transplant candidates and recipients. Am J Transplant 2008;8:1737-43. [PubMed]
45. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr., Craig W, Billeter M, Dalovisio JR and Levine DP. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009;66:82-98. [PubMed]
46. Santoro-Lopes G, de Gouvea EF, Monteiro RC, Branco RC, Rocco JR, Halpern M, Ferreira AL, de Araujo EG, Basto ST, Silveira VG, Ribeiro-Filho J. Colonization with methicillin-resistant Staphylococcus aureus after liver transplantation. Liver Transpl 2005;11:203-9. [PubMed]
47. Schmid C, Schneider M, Etz C and Scheld HH. Heart transplantation in a patient with a left ventricular assist device and methicillin-resistant Staphylococcus aureus infection. Ann Thorac Surg 2004;78:1820-1. [PubMed]
48. Sherman-Weber S, Axelrod P, Suh B, Rubin S, Beltramo D, Manacchio J, Furukawa S, Weber T, Eisen H, Samuel R. Infective endocarditis following orthotopic heart transplantation: 10 cases and a review of the literature. Transpl Infect Dis 2004;6:165-70. [PubMed]
49. Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, Muenz L, Fuller S, Johnson J, Fireman B, Alcorn H and Naso R. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002;346:491-6. [PubMed]
50. Siegel JD, Rhinehart E, Jackson M and Chiarello L. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control 2007;35:S65-164. [PubMed]
51. Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ and Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin Infect Dis 2008;46:668-74. [PubMed]
52. Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149-52. [PubMed]
53. Singh N, Paterson DL, Chang FY, Gayowski T, Squier C, Wagener MM and Marino IR. Methicillin-resistant Staphylococcus aureus: the other emerging resistant gram-positive coccus among liver transplant recipients. Clin Infect Dis 2000;30:322-7. [PubMed]
54. Singh N, Wagener MM, Obman A, Cacciarelli TV, de Vera ME and Gayowski T. Bacteremias in liver transplant recipients: Shift toward gram-negative bacteria as predominant pathogens. Liver Transpl 2004;10:844-9. [PubMed]
55. Singh N, Squier C, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Impact of an aggressive infection control strategy on endemic Staphylococcus aureus infection in liver transplant recipients. Infect Control Hosp Epidemiol 2006;27:122-6. [PubMed]
56. Singh N, Wannstedt C, Keyes L, Mayher D, Tickerhoof L, Akoad M, Wagener MM, Frye R, Cacciarelli TV. Hepatic iron content and the risk of Staphylococcus aureus bacteremia in liver transplant recipients. Prog Transplant 2007;17:332-6. [PubMed]
57. Squier C, Rihs JD, Risa KJ, Sagnimeni A, Wagener MM, Stout J, Muder RR, Singh N. Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Infect Control Hosp Epidemiol 2002;23:495-501. [PubMed]
58. Stolf NA, Fiorelli AI, Bacal F, Camargo LF, Bocchi EA, Freitas A, Nicoletti A and Meira D. Mediastinitis after cardiac transplantation. Arq Bras Cardiol 2000;74:419-30. [PubMed]
59. Stryjewski ME, Szczech LA, Benjamin DK, Jr., Inrig JK, Kanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller LB, Corey GR, Fowler VG, Jr. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 2007;44:190-6. [PubMed]
60. Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007;44:1208-15. [PubMed]
61. Valentine VG, Gupta MR, Walker JE, Jr., Seoane L, Bonvillain RW, Lombard GA, Weill D, Dhillon GS. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant 2009;28:163-9. [PubMed]
62. Verbist L. The antimicrobial activity of fusidic acid. J Antimicrob Chemother 1990;25 Suppl B:1-5. [PubMed]
63. Wang SS, Chou NK, Hsu RB, Ko WJ, Yu HY, Chen YS, Huang SC, Chi NH, Liau CS, Lee YT. Heart transplantation in the patient under ventricular assist complicated with device-related infection. Transplant Proc 2004;36:2377-9. [PubMed]
64. Wellinghausen N, Siegel D, Gebert S, Winter J. Rapid detection of Staphylococcus aureus bacteremia and methicillin resistance by real-time PCR in whole blood samples. Eur J Clin Microbiol Infect Dis 2009;28:1001-5. [PubMed]
65. Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV and Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003;124:1789-97. [PubMed]
Figure 1. Gram Stain of Staphylococcus aureus.
The Gram stain shows Gram-positive cocci in irregular “grape-like” clusters, characteristic of staphylococci.
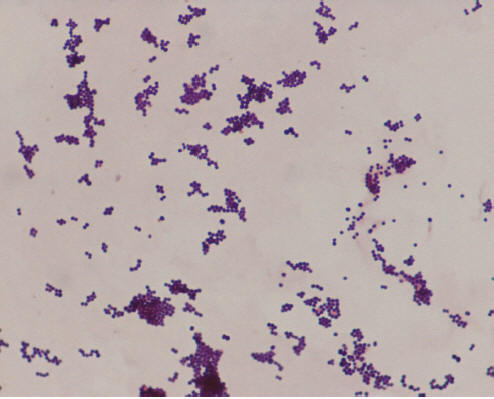
Figure 2. Sheep Blood Agar Plate Growing Staphylococcus aureus.
Grayish-yellow, smooth colonies are shown on 5% sheep blood agar after 24 hours of incubation.
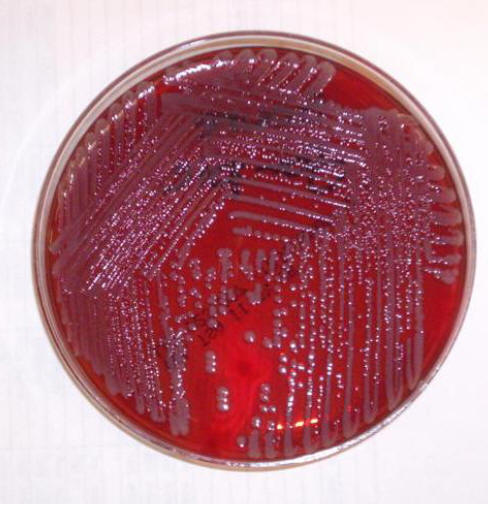
Figure 3. Detection of Inducible Clindamycin in Staphylococcus aureus.
The S. aureus isolate shown is resistant to erythromycin (disc on left). The blunting of the zone of inhibition around the clindamycin disc (on the right) adjacent to the erythromycin disc is consistent with inducible clindamycin resistance. 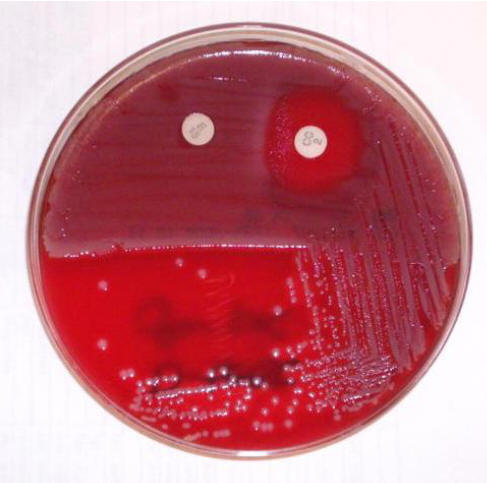
Figure 4. Detection of Methicillin-Resistant Staphylococcus aureus on BBL™ CHROMagar™ MRSA (BD, Sparks, MD).
This selective and differential medium which includes cefoxitin is used to detect methicillin-resistant S. aureus. Chromogens mimic metabolic substrates. Once cleaved, the chromogen becomes colored and accumulates within the cell giving the mauve appearance of the colonies. 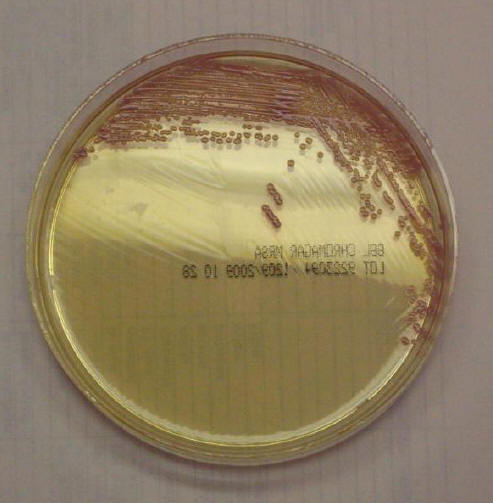
Table 1. Antimicrobials Used in the Treatment of Staphylococcal Infections
| DRUG | DOSAGE |
|---|---|
| Intravenous treatment for MSSA infections | |
Nafcillin IV |
1-2 g q 4-6h No renal adjustment required |
Oxacillin IV |
500 mg to 2 g q 4-6hNo renal adjustment required |
Cefazolin IV |
CrCl ≥30: 1-2 g q 8h CrCl 10-29: 1-2 g q 12hClCr <10: 1 g q 24hIHD: 1 g q 24h; on dialysis days, give after HDAlternatively 1-2 g after HD on dialysis days onlyCRRT: 1-2 g q 12h |
| Intravenous treatment for MRSA infections | |
Vancomycin IV* |
Consider 25 mg/kg load for serious infections (e.g., meningitis) and for health care-associated pneumonia, endocarditis, and critically ill patients
CrCl ≥65: 15-20 mg/kg q 12h; q 8h may be considered for younger patientsCrCl 35-64: 15-20 mg/kg q 24hFor severe infections consider q12h initial dose for CrCl >50 and adjust based on levelsCrCl 21-34: 15-20 mg/kg q 48hFor severe infections consider q 24h initial dose and adjust based on levelsCrCl ≤20: Re-dose based on serum levelsIHD: Give 25 mg/kg and monitor serum levelsCRRT: 15-20 mg/kg q 24-48h. Monitor serum levels and adjust dose accordingly |
Linezolid IV |
600 mg q12h
No renal adjustment required |
Daptomycin IV |
CrCl ≥30: 4 mg/kg q 24h for complicated skin and soft tissue infections6 mg/kg q 24h for bacteremia, endocarditis, bone or joint infectionCrCl <29, IHD, CRRT: 4 mg/kg q 48h for complicated skin and soft tissue infection6 mg/kg q 48h for bacteremia, endocarditis, bone or joint infectionsOn dialysis days give after HD |
Clindamycin IV |
300-900 mg iv q 6-8hNo renal adjustment required |
| Oral treatment options for MSSA infections | |
Dicloxacillin PO |
125-500 mg qidNo renal adjustment required |
Cephalexin PO |
CrCl ≥30: 250 mg to 1 g qidCrCl 10-29: 250 mg to 1 g bid or tidCrCl <10: 250-500 mg bid or dailyIHD: 250-500 mg q 24h; on dialysis days, schedule after HD |
Cefadroxil PO |
CrCl ≥50: 500 mg to 1 g bidCrCl 25-49: 500 mg bidCrCl 10-24: 500 mg q 24 hCrCl <10 or IHD: 500 mg q 24h or every other day |
| Oral treatment options for MRSA infections (if susceptible) | |
Trimethoprim-sulfamethoxazole PO[One double strength (DS) tablet contains 160 mg of trimethoprim] |
8-10 mg/kg q 24h based on trimethoprim component in 2 divided doses. (Usually 1-2 DS tab bid).CrCl 10-30: 1DS tab q 24hCrCl <10, IHD: Avoid or use 1 DS tab q48h |
Clindamycin PO |
150-450 mg qidNo renal adjustment required |
Doxycycline PO |
200 mg then 100 mg bidNo renal adjustment required |
Linezolid PO |
600 mg q12hNo renal adjustment required |
| Alternative therapy for MRSA infections | |
Tigecycline IV |
100 mg load, then 50 mg q 12hNo renal adjustment required |
Quinupristin-dalfopristin IV |
7.5 mg/kg q 12h for complicated skin and soft tissue infections7.5 mg/kg q 8h for bacteremiaNo renal adjustment required |
Telavancin IV |
CrCl ≥50: 10 mg/kg q24hCrCl 30-50: 7.5 mg/kg q24hCrCl 10-29: 10 mg/kg q48hCrCl <10 or IHD: No data available |
| Combination treatment (use in combination with other antistaphylococcal agent) | |
Gentamicin IV [Synergy for staphylococcal bacteremia] |
CrCl ≥80: 1 mg/kg q 8hCrCl 50-80: 1 mg/kg q 12hCrCl 30-49: 1 mg/kg q 12-24hCrCl 15-29: 1 mg/kg q 24-36hCrCl <15: based on serum levelDose for serum peak level: 3-4 mcg/mL , serum trough level: 0.6-1.2 mcg/mL |
Rifampin PO [For prosthetic valve endocarditis, prosthetic joint infection with retention of the hardware] |
CrCl ≥10 450 mg po bidCrCl<10 and IHD: Give 50-100% of usual dose |
CrCl: Creatinine clearance in mL/min
IHD: Intermittent hemodialysis
CRRT: Continuous renal replacement therapy
bid: twice daily
tid: three times daily
qid: four times daily
Table 2. Recommended Duration of Treatment
| Type of staphylococcal infection | Recommended duration of treatment |
|---|---|
| Skin and soft tissue infections | 7-14 days |
| Pneumonia | Treat for at least 14 days. Necrotizing pneumonia usually requires longer treatment. |
| Primary bacteremia | 14 days (after clearance of bacteremia)*If complicated, treat for at least 4 weeks |
| Catheter-associated bacteremia | 14 days (after catheter removal)*If complicated, treat for at least 4 weeks |
| Infective endocarditis | Native valve: Antistaphylococcal agent for 4-6 weeks (after clearance of bacteremia), with gentamicin for the first 3-5 days of treatmentProsthetic valve: Antistaphylococcal agent and rifampin for 6 weeks (after clearance of bacteremia), with gentamicin for the first 2 weeks |
| Osteomyelitis | 4-6 weeks |
| Prosthetic joint infection | 4-6 weeks if prosthesis is exchanged.3 months for hip infection, 6 months for knee infection, if prosthesis is retained. This may be followed by chronic suppressive treatment. |
Bard JD, et al. Rationale for Eliminating Staphylococcus Breakpoints for β-lactam Agents Other Than Penicillin, Oxacillin or Cefoxitin, and Ceftaroline. Clin Infect Dis 2014;58:1287-1296.
Hall RG, et al. Empiric guideline-recommended weight-based vancomycin dosing and nephrotoxicity rates in patients with methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol 2013;14:12.
Chua K, et al. Not Community-Associated Methicillin-Resistant Staphylococcus aureus (CA-MRSA)! A Clinician's Guide to Community MRSA - Its Evolving Antimicrobial Resistance and Implications for Therapy. Clin Infect Dis 2011;52:99-114.
van Hal SJ, et al. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureusbacteraemia in a daptomycin-naive patient--a review of the literature. Eur J Clin Microbiol Infect Dis 2011;30:603-610.
Jung JY, et al. Effect of vancomycin plus rifampicin in the treatment of nosocomial methicillin-resistant Staphylococcus aureus pneumonia. Crit Care Med 2010;38:175-180.
Lauderdale TLY, et al. Carriage rates of methicillin-resistant Staphylococcus aureus (MRSA) depend on anatomic location, the number of sits cultured, culture methods, and the distribution of clonotypes. Eur J Clin Microbiol Infect Dis 2010;29:1553-1559.
Vesga O, et al. Generic Vancomycin Products Fail In Vivo Despite Being Pharmaceutical Equivalents of the Innovator. Antimicrob Agents Chemother 2010;54:3271-3279.
Goldberg E, Paul M, et al. Co-trimoxazole Versus Vancomycin for the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteraemia: a Retrospective Cohort Study. J Antimicrob Chemother 2010;65:1779-83.
Zeller V, et al. Continuous Clindamycin Infusion, an Innovative Approach to Treating Bone and Joint Infections. Antimicrobial Agents and Chemotherapy. 2010;54:88-92.
Bode, LGM, et al. Preventing Surgical-Site Infections in Nasal Carriers of Staphylococcus aureus. N Engl J Med 2010;362 (1):9-17.
Jang HC, Kim SH, et al. Salvage Treatment for Persistent Methicillin-Resistant Staphylococcus aureus Bacteremia: Efficacy of Linezolid With or Without Carbapenem. Clin Infect Dis. 2009;49:395-401.
Svetitsky S, Leibovici L, Paul M. The comparative efficacy and safety of vancomycin vs. teicoplanin: Systematic review and meta-analysis. Antimicrob Agents Chemother. 2009 Jul 13. [Epub ahead of print]
Forouzesh A, Moise PA, Sakoulas G. Vancomycin Ototoxicity: A Re-Evaluation in an Era of Increasing Doses. Antimicrob Agents Chemother. 2009;53:483-6.
Horne KC, et al. Prospective comparison of the clinical impacts of heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-susceptible MRSA. Antimicrob Agents Chemother. 2009; 53:3447-52.
Rutland BE, Weese JS, et al. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg Infect Dis. 2009;15:1328-30.
Kosowska-Shick K, Clark C, et al. Activity of Telavancin Against Staphylococci and Enterococci Determined by MIC and Resistance Selection Studies. Antimicrob Agents Chemother. 2009;53:4217-24.
Climo MW, et al. The Effect of Daily Bathing with Chlorhexidine on the Acquisition of Methicillin-Resistant Staphylococcus aureus, Vancomycin-resistant Enterococci, and Healthcare-associated Bloodstream Infections: Results of a Quasi-experimental Multicenter Trial. Crit Care Med. 2009;37:1858-65.
Elliott DJ, Zaoutis TE, et al. Empiric Antimicrobial Therapy for Pediatric Skin and Soft-Tissue Infections in the Era of Methicillin-Resistant Staphylococcus aureus. Pediatrics. 2009;123:e959-66.
Euba G, et al. Long-Term Follow-Up Trial of Oral Rifampin-Cotrimoxazole Combination versus Intravenous Cloxacillin in Treatment of Chronic Staphylococcal Osteomyelitis. Antimicrob Agents Chemother. 2009;53:2672-6.
Patel N, Lubanski P, et al. Correlation Between Vancomycin MIC Values and Those of Other Agents Against Gram-Positive Bacteria Among Patients with Bloodstream Infections Caused by Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:5141-4.
Zaoutis, T et al. Prolonged Intravenous Therapy Versus Early Transition to Oral Antimicrobial Therapy for AcuteOsteomyelitis in Children. Pediatrics 2009;123:636-642.
Mitrano JA, Spooner LM, et al. Excretion of Antimicrobials used to Treat Methicillin-Resistant Staphylococcus aureus Infections during Lactation: Safety in Breastfeeding Infants. Pharmacotherapy. 2009 Sep;29:1103-9.
Muñoz P, Hortal J, et al. Nasal carriage of S. aureus increases the risk of surgical site infection after major heart surgery. J Hosp Infection 2008;68:25-31.
Deresinski S. Active surveillance for detection of MRSA colonization. Clin Infect Dis 2008;47:v-vi.
Mariani-Kurkdjian P. et.al. Monitoring serum vancomycin concentrations in the treatment of Staphylococcus infections in children. Arch Pediatr. 2008;15:1625-9.
Soriano A, Marco F, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008;46:193-200.
Rubinstein E, et al. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46:S378-85.
Kim SH, Kim KY, et al. Outcome of Vancomycin Treatment in Patients with Methicillin-Susceptible Staphylococcus aureus Bacteremia. Antimicrob Agents Chemother 2008;52:192-197.
Lodise TP, Lomaestro B, et al. Larger Vancomycin Doses (>=4 grams/day) are Associated with an Increased Incidence of Nephrotoxicity. Antimicrob Agents Chemother 2008;52:1330-6.
Malhotra-Kumar S, Haccuria K, et al. Current Trends in Rapid Diagnostics for Methicillin-Resistant Staphylococcus aureusand Glycopeptide-Resistant Enterococcus Species. J Clin Microbiol 2008;46:1577-1587.
Proctor RA. Role of Folate Antagonists in the Treatment of Methicillin-resistant Staphylococcus aureus Infection. Clin Infect Dis. 2008;46:584-593.
Lee D, et al. Successful Treatment of Methicilin-Resistant Staphylococcus aureus Meningitis with Daptomycin. Clin Infect Dis 2008;47:588-590.
Rajendran PM, Young D, et al. Randomized, Double-Blind, Placebo-Controlled Trial of Cephalexin for Treatment of Uncomplicated Skin Abscesses in a Population at Risk for Community-Acquired Methicillin-Resistant Staphylococcus aureus Infection. Antimicrob Agents Chemother 2007;51:4044-4048.
Awad SS, et al. Increasing incidence of methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: reconsideration of empiric antimicrobial therapy. Am J Surg 2007;194:606-610.
Lodise TP, McKinnon PS, Levine DP, Rybak MJ. Impact of Empirical-Therapy Selection on OUtcomes of Intravenous Drug Users with Infective Endocarditis Caused by Methicillin-Susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2007;51:3731-3733.
van Loo I, Huijsdens X, et al. Emergence of Methicillin-Resistant Staphylococcus aureus of Animal Origin in Humans.Emerg Infect Dis. 2007;13:1834-9.
Bhalla A, et al. Staphylococcus aureus intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis. 2007;7:105.
Kallweit U, et al. Successful treatment of methicillin-resistant Staphylococcus aureus meningitis using linezolid without removal of intrathecal infusion pump. Case report. J Neurosurg 2007;107:651-653.
Barberan J, Aguilar L, Carroquino G, Gimenez M-J, Sanchez B, Martinez D, Prieto J. Conservative Treatment ofStaphylococcal Prosthetic Joint Infections in Elderly Patients. Am J Med 2006;119:993.e7-993.e10.
Fowler VG Jr, Boucher HW, Corey GR, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006;355:653-65.
McKinnon PS, et al. Impact of Linezolid on Economic Outcomes and Determinants of Cost in a Clinical Trial Evaluating Patients with MRSA Complicated Skin and Soft-Tissue Infections. Ann Pharmacother. 2006;40:1017-23.
Guided Medline Search for
Turnidge J., Rao N., Chang FY., et al. Staphylococcus aureus
Baron EJ. Staphylococci
Baron EJ. Flow Chart from Colony on BAP
Baron EJ. Flow Chart Aerobic Gram Positive Organisms
Lentino JR, Narita M, Yu VL. New Antimicrobial Agents as Therapy for Resistant Gram-Positive Cocci.
Efflux Pumps as a Mechanism of Antimicrobial Resistance.
Patient Handout: Methicillin-resistant Staphylococcus aureus (MRSA)
Leid, JG. Bacterial Biofilms Resist Key Host Defenses. Microbe/Volume 4, Number 2, 2009.
Rybak M, et al. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health-Syst Pharm 2009;66:82-98.
Raad, I., Hanna, H. and Maki, D. Intravascular Catheter-related Infections: Advances in Diagnosis, Prevention and Management. The LANCET Infectious Diseases 2007; Vol.7, Issue 10, 645-657.
Schaudin C, Stoodley P, Kainovic' A, O'Keeffe T, Costerton B, Robinson D, Baum M, Ehrlich G, Webster P. Bacterial Biofilms, Other Structures Seen as Mainstream Concepts. Microbe 2007;2:231-237.
Jorgenson L, et al. Vancomycin disposition and penetration into ventricular fluid of the central nervous system following intravenous therapy in patients with cerebrospinal devices. Pediatr Neurosurg 2007;43:449-455.
Wertheim HFL, Melles DC, et al. The role of nasal carriage of Staphylococcus aureus infections. Lancet Infectious Diseases 2005;5:751-762.
CDC. MRSA infecting the St. Louis Rams football team. N Eng J Med February 3, 2005
Rybak MJ, LaPlante KL.Community-Associated Methicillin-Resistant Staphylococcus aureus: A Review. Pharmacotherapy 2005;25(1):74-85.
Menace in the Locker Room, Sports Illustrated , February 28, 2005.
Chang FY. Staphylococcus aureus Bacteremia Recurrence and the Impact of Antibiotic Treatment in a Prospective Multicenter Study. Medicine 2003.
Chang FY. A Prospective Multicenter Study of Staphylococcus aureus Bacteremia Incidence of Endocarditis, Risk Factors for Mortality, and Clinical Impact of Methicillin Resistance. Medicine 2003.
Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. October 2001.
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
Richard L. Oehler: MRSA: Historical Perspective
Abigail Orenstein: The Discovery and Naming of Staphylococcus aureus
Lina G, Vandenesch F, Etienne J. A brief history of Staphylococcus aureus Panton Valentine leucocidin
John JF, Lindsay JA. Clones and Drones: Do Variants of Panton-Valentine Leukocidin Extend the Reach of Community-Associated Methicillin-Resistant Staphylococcus aureus?J Infect Dis 2008;197:175-178.
