
Diabetic Foot Infections - Assessment
Initial Assessment
Not all ulcers are infected. Since all wounds are colonized by microorganisms, infection must be diagnosed clinically rather than microbiologically. Various authoritative committees (Infectious Disease Society of America [IDSA], International Working Group on the Diabetic Foot [IWGDF], and American Diabetes Association) have defined infection in the diabetic foot as the presence of purulent secretions or at least two symptoms or signs of infection (erythema, warmth, tenderness, pain, or induration). Importantly, local and systemic inflammatory responses to infection may be diminished in those with peripheral neuropathy or arterial insufficiency. In a patient with limb ischemia, infection may reach a limb-threatening state before the patient or clinician recognizes the problem. Eradicating infection in a wound will certainly facilitate healing, but it will usually take additional time for the wound to completely heal. As long as the wound remains, it is at continued risk of re-infection. Thus, curing infection is a separate, albeit related, issue to wound healing.
Because of the complex nature of diabetic foot infection and the potential for
rapid worsening (sometimes within hours), the clinician must assess the patient
promptly, methodically and repeatedly (Figure 1 and 2, IDSA guidelines).
Evaluate for systemic evidence of infection (e.g., fever, chills, leukocytosis),
examine the affected limb (i.e., for foot deformities, altered biomechanics,
neuropathy, and arterial insufficiency) and finally the wound (size
![]() (I), depth,
tissues involved
(I), depth,
tissues involved
![]() (II), necrosis/gangrene
(II), necrosis/gangrene
![]() (III), foreign objects). There are several
classification schemes for diabetic foot ulcers
(III), foreign objects). There are several
classification schemes for diabetic foot ulcers
![]() (IV) and the lack of consensus on
wound definitions and infection classification makes comparison of published
studies difficult and is confusing to clinicians. Most, however agree that the
critical factors in evaluating a diabetic foot wound are its depth and the
limb’s vascular status. The recently published guidelines from the IWGDF and IDSA are similar (Table 3). The IDSA scheme has been validated and
predicts clinical outcome. Asessing the component features should
influence decisions regarding site of therapy (inpatient vs. outpatient), the
spectrum, route of administration and sduration of antibiotic therapy, the
urgency of any necessary surgical intervention, and likely the outcome.
Identifying causative pathogens using proper wound culturing techniques guides
antibiotic therapy, especially for chronic infections and persons recently
treated with antibiotics. A Gram-stained smear of a wound specimen can provide
real-time information on the likely causative organisms. When selecting an
initial antibiotic regimen, it is most helpful for deciding whether or not to
add coverage for gram-negative rods in a patient with mild infection.
(IV) and the lack of consensus on
wound definitions and infection classification makes comparison of published
studies difficult and is confusing to clinicians. Most, however agree that the
critical factors in evaluating a diabetic foot wound are its depth and the
limb’s vascular status. The recently published guidelines from the IWGDF and IDSA are similar (Table 3). The IDSA scheme has been validated and
predicts clinical outcome. Asessing the component features should
influence decisions regarding site of therapy (inpatient vs. outpatient), the
spectrum, route of administration and sduration of antibiotic therapy, the
urgency of any necessary surgical intervention, and likely the outcome.
Identifying causative pathogens using proper wound culturing techniques guides
antibiotic therapy, especially for chronic infections and persons recently
treated with antibiotics. A Gram-stained smear of a wound specimen can provide
real-time information on the likely causative organisms. When selecting an
initial antibiotic regimen, it is most helpful for deciding whether or not to
add coverage for gram-negative rods in a patient with mild infection.
|
I. Infected diabetic foot ulcer |
II. Infected diabetic bullae |
|
|
|
III.
|
a. Necrotic toe |
b. Diabetic foot infection with gangrene |
c. Necrotic toe and dorsal abscess from tight shoes |
d. 4th toe necrotic cellulitis in a diabetic |
|
|
|
|
|
IV.
|
Lateral foot infected ulcer in a diabetic patient |
Probing of a diabetic foot ulcer after debridement |
Calcaneal and midfoot diabetic foot ulcer |
Neuropathic uninfected ulcer |
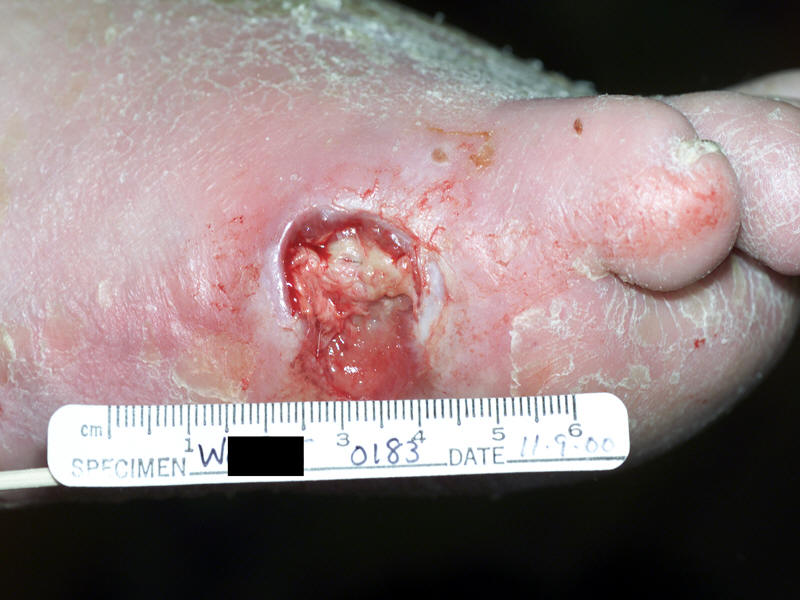 |
|
|
|
Table 3. Clinical Classification of Diabetic Foot Infections
Infection* severity
Clinical manifestations of infection
Uninfected
Wound lacking purulence or any manifestations of inflammation
Mild
Infection localized to the skin and subcutaneous tissue (cellulitis/erythema extends ≤2 cm around an ulcer) without evidence of systemic illness
Moderate
More extensive local infection (i.e., local spread ≥2cm beyond an ulcer, lymphangitic streaking, abscess, gangrene, or involvement of deep soft tissue, muscle, fascia, tendon, joint or bone) without evidence systemic illness or severe metabolic derangements
Severe
Infection with systemic toxicity or severe metabolic derangements
* Infection defined as the presence of purulent secretions (pus) or ≥2 signs or symptoms of inflammation
(erythema, warmth, tenderness, induration, pain)
Figure 1. Approach to treating a diabetic patient with a foot wound
Figure 2. Approach to treating a diabetic patient with a foot infection. 1
Consider hospitalization if any of the following criteria are present: systemic toxicity (e.g., fever and leukocytosis); metabolic instability (e.g., severe hypoglycemia or acidosis); rapidly progressive or deep tissue infection, substantial necrosis or gangrene, or presence of critical ischemia; requirement of urgent diagnostic or therapeutic interventions; and inability to care for self or inadequate home support.
Determining the Severity of Infection
Systemic Evidence of Infection:
Systemic symptoms and signs of infection include fevers, chills, diaphoresis, anorexia, hemodynamic instability (tachycardia, hypotension), metabolic derangements (e.g., acidosis, dysglycemia, volume depletion, renal failure), leukocytosis and inflammatory markers. Surprisingly to many clinicians, these are uncommon in patients with a diabetic foot infection. When systemic signs or symptoms are present they generally signify severe infection with extensive tissue involvement or more virulent pathogens. But, elevated temperature, white blood cell count, or sedimentation rate are absent in up to 50% of severe diabetic foot infection.
Extent of Tissue Involvement:
A key factor in determining the outcome of a diabetic foot infection is to assess the wound depth and which tissues are involved. This requires first debriding
(V) any necrotic material or callus, then gently probing
(VI) to uncover any abscesses, sinus tracts, foreign bodies
(VII) or bone or joint involvement. Occasionally, defining the extent of infection requires an imaging study (usually MRI) or surgical exploration. If there is any concern for necrotizing deep space infection
(VIII), an experienced surgeon should evaluate the patient. Deeper and more extensive infections may respond more slowly to appropriate antibiotic therapy. Palpating bone
(IX) in a diabetic foot ulcer using a steel probe (a positive “probe-to-bone” test) is a simple and useful bedside test. The positive predictive value approaches 90% when the pre-test probability of osteomyelitis is high, but is closer to 55% when the prevalence (usually ~20%) is lower. Visibly exposed bone probably provides similar information as probing bone.
V. Debridement of an infected foot ulcer
VII. XR demonstrating calcaneal foreign body
IX. Visible bone as a consequence of a diabetic foot infection
VI.
Probing of a diabetic foot ulcer after debridement
Ulcerating callus which probed through the foot
Probing of a diabetic foot ulcer
VIII.
Diabetic foot infection with associated necrotizing fasciitis
Assessment of Peripheral Arterial Perfusion
The presence of peripheral arterial disease is an independent risk factor for developing a diabetic foot infection and is present in up to 40% of cases
(see above pictures III a, b, and c) . The presence of significant arterial insufficiency in an infected limb adversely affects host immunological responses and wound healing and impairs delivery of systemic antibiotics to the infected tissues. The absence of pedal pulses suggests peripheral arterial disease, but this method of assessment of arterial perfusion is often not reliable, especially in persons with diabetes. Determining the ratio of ankle to brachial artery systolic blood pressure ankle/brachial index is a simple, reliable, non-invasive, bedside procedure to assess for peripheral arterial disease and should be performed in most patients with diabetic foot infection, especially if pedal pulses are absent or diminished. An ankle/brachial index <0.90 is abnormal, and <0.40 signifies severe ischemia (Table 4), but arterial calcification of vessels may falsely elevate the ankle/brachial index in patients with diabetes. Assessing the transcutaneous partial pressure of oxygen (TcpO2) in the skin of the foot is another non-invasive method of assessing peripheral arterial perfusion. TcpO2 values of less than 30mmHg signify critical limb ischemia and predict poor wound healing. If significant peripheral arterial disease is suspected based on history, physical examination, or non-invasive testing, vascular surgery consultation is appropriate. Limb revascularization may be necessary to cure infection and promote healing. Having access to an active lower extremity revascularization program can decrease amputation rates and increase the incidence of foot sparing surgeries that have a more favorable long-term outcome.
Table 4. Interpretation of Ankle-Brachial Index Results
Ankle-brachial index (ABI)*
Interpretation
>1.30
Poorly compressible vessels, arterial calcification
0.90-1.30
Normal
0.60-0.89
Mild obstruction
0.40-0.59
Moderate obstruction
<0.40
Severe obstruction
* Obtained by measuring the systolic blood pressure in the ankle divided by that in the brachial artery
Laboratory Diagnosis
Properly obtained wound cultures (Table 5) are useful for guiding antibiotic
therapy in diabetic foot infections, particularly in patients with chronic
infections or who have recently been treated with antibiotics. Culture specimens
should be obtained after the wound has been cleansed and debrided. A sample
obtained by curettage, the aseptic scraping of tissue at an ulcer base using a
scalpel blade or dermal curette, more accurately identifies pathogens than a
wound swab
![]() (X). Swabs are often contaminated with normal skin flora or colonizers and are less likely to grow anaerobic, and some fastidious aerobic
organisms. Specimens must be promptly transported to the laboratory, in an
appropriate sterile transport system, where they should be processed for aerobic
and anaerobic cultures and a Gram-stained smear. Other acceptable methods of
culturing wounds include aspiration of cellulitic tissue or purulent secretions,
and tissue biopsy obtained either at the bedside or at surgery. A bone biopsy,
obtained surgically or percutaneously
(X). Swabs are often contaminated with normal skin flora or colonizers and are less likely to grow anaerobic, and some fastidious aerobic
organisms. Specimens must be promptly transported to the laboratory, in an
appropriate sterile transport system, where they should be processed for aerobic
and anaerobic cultures and a Gram-stained smear. Other acceptable methods of
culturing wounds include aspiration of cellulitic tissue or purulent secretions,
and tissue biopsy obtained either at the bedside or at surgery. A bone biopsy,
obtained surgically or percutaneously
![]() (XI), processed for culture (and histological
assessment, if possible) is the criterion standard for diagnosing osteomyelitis.
The results of wound or sinus tract cultures do not accurately reflect those of
bone culture. Blood cultures are not frequently positive in these
infections but should be obtain in patients with systemic symptoms and signs of
infection. In the minority of cases with bacteremia, S. aureus is the
most frequently isolated pathogen.
(XI), processed for culture (and histological
assessment, if possible) is the criterion standard for diagnosing osteomyelitis.
The results of wound or sinus tract cultures do not accurately reflect those of
bone culture. Blood cultures are not frequently positive in these
infections but should be obtain in patients with systemic symptoms and signs of
infection. In the minority of cases with bacteremia, S. aureus is the
most frequently isolated pathogen.
X. Proper methods for culturing wounds
XI. Bone biopsy demonstrated on XR
Table 5. Recommendations for Collection of Specimens for Culture from Diabetic Foot Wounds
Do
Cleanse and debride wound before obtaining specimen(s) for culture
Obtain tissue specimen for culture by scraping with a sterile scalpel or dermal curette (curettage) or biopsy from the base of a debrided ulcer
Aspirate any purulent secretions using sterile needle/syringe
Promptly send specimens for culture in sterile container or appropriate transport media for aerobic and anaerobic culture
Do Not
Culture clinically uninfected lesions, unless for epidemiological studies
Obtain specimen for culture without first cleansing or debriding the wound
Obtain specimen for culture by swabbing the wound or wound drainage
Imaging Studies
Imaging studies may be useful in a patient with diabetic foot infection to
assess for any foreign material, soft tissue abscesses
![]() (XII), or bony abnormalities.
Plain radiographs are usually the appropriate first study but have limited
diagnostic utility in assessing for osteomyelitis
(XII), or bony abnormalities.
Plain radiographs are usually the appropriate first study but have limited
diagnostic utility in assessing for osteomyelitis
![]() (XIII). They lack sensitivity early
in infection because abnormalities on plain film may not be evident until 50% of
the bone is resorbed which typically requires 2-3 weeks. They also lack
specificity because neuroarthropathy (Charcot foot) may have a similar
radiographic appearance. If suspicion for osteomyelitis remains despite an
initial negative radiograph, repeating plain films in a few weeks can either
exclude the diagnosis (if still negative) or suggest that it has developed (if
there is cortical erosion, periosteal elevation or other suggestive changes in
one underlying the affected soft tissue).
(XIII). They lack sensitivity early
in infection because abnormalities on plain film may not be evident until 50% of
the bone is resorbed which typically requires 2-3 weeks. They also lack
specificity because neuroarthropathy (Charcot foot) may have a similar
radiographic appearance. If suspicion for osteomyelitis remains despite an
initial negative radiograph, repeating plain films in a few weeks can either
exclude the diagnosis (if still negative) or suggest that it has developed (if
there is cortical erosion, periosteal elevation or other suggestive changes in
one underlying the affected soft tissue).
| XII. Plantar abscess in a diabetic | XIII a. Calcaneal osteomyelitis & fracture | XIII b. Bone biopsy demonstrated on XR |
|
|
|
|
| XIII c. Osteomyelitis demonstrated clinically and radiographically | XIII d. Xray demonstrating great toe regeneration after antibiotic treatment | XIII e. Sequential radiographic demonstration of 1st metatarsal destruction |
|
|
|
|
Radionucleotide bone scans (using bisphosphonate-linked technetium or other
radionuclides) are more sensitive than plain radiographs for diagnosing
osteomyelitis, but uptake occurs with any type of inflammation
![]() (XIV), resulting in
poor specificity (~50%). Labeled (e.g., with Indium111) white cell or
immunoglobulin scans have better specificity (~75%) than bone scans
(XIV), resulting in
poor specificity (~50%). Labeled (e.g., with Indium111) white cell or
immunoglobulin scans have better specificity (~75%) than bone scans
![]() (XV). Among
imaging modalities, magnetic resonance imaging
(XV). Among
imaging modalities, magnetic resonance imaging
![]() (XVI)
has the best overall sensitivity
(>90%) and specificity (>80%) for detecting osteomyelitis and higher resolution
for soft tissue abnormalities. It is now considered the imaging procedure of
choice, but it is still relatively expensive and often not readily
available.
(XVI)
has the best overall sensitivity
(>90%) and specificity (>80%) for detecting osteomyelitis and higher resolution
for soft tissue abnormalities. It is now considered the imaging procedure of
choice, but it is still relatively expensive and often not readily
available.
XIV. Bone scan demonstrating osteomyelitis
XVI. MRI (STIR&T1) demonstrating osteomyelitis, gas in the soft tissue and a sinus tract
XV a. Heel ulcer followup xray and scans showing resolution of osteomyelitis
XV b. Bone scan (right upper panel) and tagged WBC scan (right lower panel) demonstrating osteomyelitis