

Infective Endocarditis - Diagnosis
CLINICAL PRESENTATION
In the pre-antibiotic era, when infective endocarditis was uniformly fatal, a short duration of illness of less than 6 weeks prior to death was used to characterize acute endocarditis; in contrast, subacute and chronic infective endocarditis had a more indolent course until death in six weeks to two years. The distinction based on chronicity has continued to prove useful in the antibiotic era. Chronicity is now used in reference to the duration of illness prior to presentation. Acute infective endocarditis is usually (50-70%) caused by S. aureus, especially when accompanied by marked signs of general infection and suppurative embolic phenomena and has a rapidly fatal course if treatment is delayed. Acute infection may develop on a previously normal valve: In the non-intravenous drug users, the aortic valve is commonly involved; in the intravenous drug users, the tricuspid valve. Acute tricuspid valve lesions may be associated with multiple septic pulmonary emboli. Therefore, clinically acute infective endocarditis, especially in the presence of a pre-existing intravascular devise, e.g., intravenous catheter, can serve a diagnosis of presumptive S. aureus endocarditis, which will require use of empiric anti-staphylococcal antibiotic therapy that can be modified when results of initial blood cultures become available. Subacute infective endocarditis, commonly caused by streptococci and enterococci, in contrast, often develops on previously damaged endocardium, has a less dramatic clinical course with an onset within 14 days of the inciting bacteremia, and the clinical manifestations are characteristically fever, progressive wasting and debility, and non-suppurative peripheral vascular phenomena.
Clinical manifestations result from 1) the valvular infection itself; 2) embolization of fragments of the vegetation; 3) suppurative complications that result from hematogenous spread of infection; or 4) immunologic response to the infection in the form of immune complex vasculitis. Systemic manifestations of infective endocarditis include most commonly fever and other symptoms that may accompany fever, such as drenching night sweats, arthralgias, myalgias, pain in the low back and thighs, and weight loss. Fever is usually low grade, the temperature peaks rarely exceeding 39.4oC. However, fever may be high and spiking in patients with acute infective endocarditis or absent in a few patients, e.g., those who are very elderly or severely debilitated, have significant renal or heart failure, or are taking antipyretics or antibiotics.
Murmurs of cardiac valvular insufficiency due to destruction or distortion of the infected valve and its supporting structures are commonly present; less commonly murmurs of valvular stenosis occur due to large vegetations. Murmurs are likely to be absent in tricuspid infective endocarditis or may be absent when a patient with acute infective endocarditis is first seen. PVE may result in regurgitant systolic or diastolic murmurs as a result of dehiscence of the valve at the annulus, the suture lines being usual site of infection in early PVE, or muffling of the usual crisp prosthetic mechanical valve clicks.
In NVE, valve ring abscess due to local extension of infection occurs in the weakest portion of the annulus, which is near the membranous interventricular septum and AV node. Usually the noncoronary cusp of the aortic valve is involved. In post-operative PVE, valve ring abscess occurs commonly because the annulus, rather than the leaflet is the site of infection. Valve ring abscess can lead to persistent fever despite appropriate antimicrobial therapy, recurrent emboli, heart block as a result of destruction of conduction pathways in the area of the AV node and bundle of His in the upper interventricular septum, pericarditis or hemopericardium as a result of burrowing abscesses into the pericardium, or shunts between cardiac chambers or between the heart and aorta. Myocardial infarction may occur as a result of coronary artery embolization, and myocardial abscess can occur as a result of bacteremia. Diffuse myocarditis may occur as a consequence of immune-complex vasculitis.
Congestive heart failure (CHF) is a common complication of infective endocarditis and carries a grave prognosis; it develops in patients with infective endocarditis as a consequence of valvular or myocardial involvement during antimicrobial therapy (2/3 of patients will develop CHF within the first month of therapy), or may precede the onset of infective endocarditis as a consequence of the underlying cardiac lesion. CHF may develop indolently during the prolonged period prior to diagnosis in some patients with subacute infective endocarditis, or also may develop dramatically in patients with acute S. aureus infective endocarditis when it is often accompanied by the sudden onset of a new murmur of regurtitant blood flow secondary to destruction of the aortic valve or mitral valve or its supporting structures. CHF occurs more frequently with left-sided than right-sided infective endocarditis, and with aortic more frequently than mitral involvement, and CHF is more severe with sudden than insidious development.
Extracardiac manifestations include: 1) embolic events that result in infarction of numerous organs, such as the lung in right-sided infective endocarditis or the brain, heart, bowel, spleen, kidneys, or extremities in left-sided infective endocarditis; 2) suppurative complications that include abscesses, septic infarcts and mycotic aneurysms; and 3) immunologic reactions to the valvular infection that include glomerulonephritis, sterile meningitis, and polyarthritis, and a variety of vascular phenomena, such as mucocutaneous petechiae, splinter hemorrhages, Roth spots, and Osler’s nodes. The development of clinically apparent splenomegaly and many of the various non-suppurative peripheral vascular phenomena is related to the duration of illness prior to presentation. The frequency of these clinical manifestations (<50%) is currently less than in the past, as a result of shorter duration of illness prior to diagnosis.
Neurologic complications, which occur in about 20-40% of patients with left-sided infective endocarditis, are usually due to systemic embolization, often a devastating complication when involving the cerebral circulation. Up to 2/3 of emboli events involve the CNS, usually in the distribution of the middle cerebral artery. Systemic embolization 0ccurs in 22 to 50% of patients with infective endocarditis; it is more frequent with S. aureus, HACEK organisms, Abiotrophia species, and fungal infective endocarditis, increasing or stable vegetation size on therapy, vegetations larger than 1 cm in diameter on echocardiography, and vegetations of the anterior leaflet of the mitral valve. Frank cerebral abscess is rare, but occurs in patients with S. aureus endocarditis. Septic pulmonary emboli that appear as multiple round infiltrates commonly occur in patients with tricuspid valve S. aureus infective endocarditis and may cavitate or be complicated by empyema. The frequency of systemic embolization decreases dramatically during the first two weeks of successful antimicrobial therapy, as the vegetation heals. Mycotic aneurysms are an unusual, but important, complication of infective endocarditis. These aneurysms characteristically develop at arterial bifurcations, as a result of septic embolization or immune vasculitis involving the vasa vasorum, and involve more commonly cerebral arteries, followed by visceral arteries (e.g., splenic, superior mesenteric) and arteries of the extremities, the abdominal aorta and sinus of Valsalva.
Mycotic aneurysms may be asymptomatic, but can become clinically evident quite suddenly without warning as a result of precipitous rupture or gradually as a result of a slow leak, even months or years after completion of successful therapy. Unremitting headache, visual disturbance, cranial nerve palsy, meningeal signs, change in mental status, or a focal neurologic deficit is suggestive of an impending rupture of an intracranial mycotic aneurysm. Cerebrospinal fluid can show microscopic white and red blood and an elevated protein, or it can be grossly bloody. Signs of blood loss at any site in a patient with infective endocarditis should suggest rupture of a mycotic aneurysm.
Nosocomial Endocarditis
Nosocomial infective endocarditis has been reported to account for about 10% of cases of infective endocarditis. The clinical presentation of nosocomial infective endocarditis is similar to that of community-acquired infective endocarditis. The source of bacteremia can be identified in >90% of cases of nosocomial infective endocarditis. The most important bacteremia-inducing event during hospitalization that results in infective endocarditis is use of an intravascular device, present in up to 50% of cases. A major predisposing cardiac lesion for nosocomial infective endocarditis is a prosthetic cardiac valve (present in up to 50% of cases). Although community-acquired S. aureus bacteremia, without the presence of a primary focus of infection was thought to be a major indicator of underlying infective endocarditis, recent studies have documented that nosocomial S. aureus bacteremia is frequently complicated by infective endocarditis (10—30% of the time) with or without a removable focus of infection. Similarly, the risk of developing infective endocarditis during an episode of hospital-acquired enterococcal bacteremia had been considered very low (<1%), but nosocomial enterococcal infective endocarditis may be an emerging problem, especially if the patient has an underlying cardiac risk, such as a prosthetic valve, and the enterococcal species is E. faecalis. Sources for nosocomial enterococcal bacteremia include genitourinary or gastrointestinal procedures, intravascular catheter infection, or surgical wound infection. Although blood cultures are usually positive, the diagnosis of nosocomial infective endocarditis is frequently delayed due to failure to recognize the presence of early endocardial infection, which may be only evident on transesophageal echocardiography.
ECHOCARDIOGRAPHY
Echocardiography has become second in importance only to culture of blood in the
investigation of patients who are clinically suspected to have infective
endocarditis. Echocardiography can visualize valvular
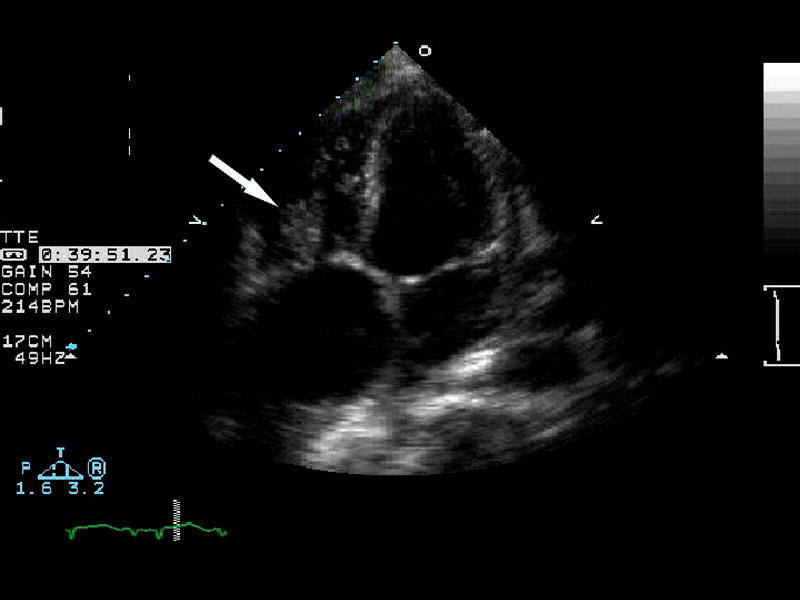
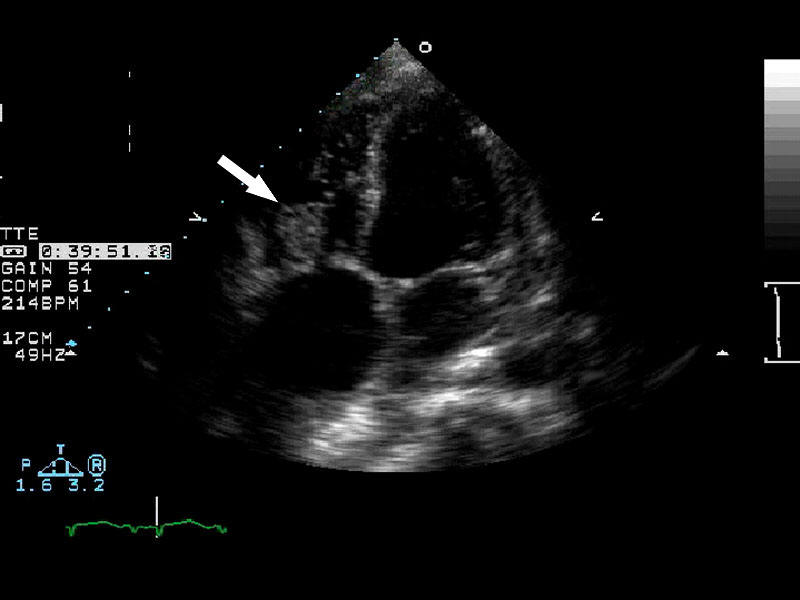
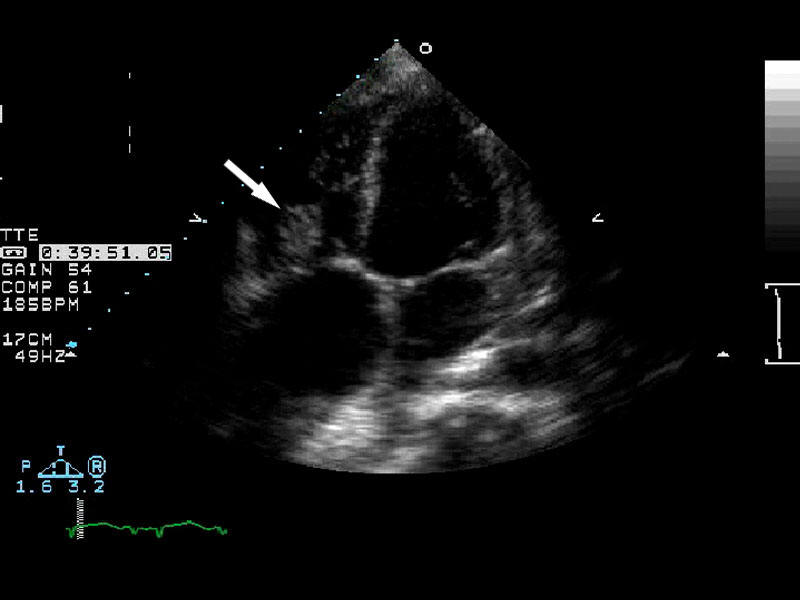
vegetations, satellite vegetations
![]() , flail valves, ruptured chordae, perivalvular
abscesses, fistulas, valvular perforations, and mycotic aneurysms.
Echocardiography is also relied upon to identify predisposing cardiac lesions,
and the causes and severity of congestive heart failure by assessment of
ventricular size, wall motion, and dynamic function. Two-dimensional
transthoracic echocardography (TTE) and transesophageal echocardiography (TEE),
the two currently performed types of echocardiography, are usually safe and
portable to the bedside. TTE is rapid, noninvasive, and relatively inexpensive.
TEE is invasive, requires sedation, and is more expensive. TTE can give more
general information as to cardiac structure and function. TEE is frequently
helpful in situations where TTE is not, e.g., in the presence of obesity,
emphysema, small vegetations, e.g., in early infective endocarditis, a
prosthetic cardiac valve the structural components of which may impair the image
of TTE, and infection in perivalvular tissue where PVE often starts. Hemodynamic
complications, such as central or perivalvular regurgitant flow in the presence
of a prosthetic valve can easily be detected and semiquantitated by additional
color flow Doppler.
, flail valves, ruptured chordae, perivalvular
abscesses, fistulas, valvular perforations, and mycotic aneurysms.
Echocardiography is also relied upon to identify predisposing cardiac lesions,
and the causes and severity of congestive heart failure by assessment of
ventricular size, wall motion, and dynamic function. Two-dimensional
transthoracic echocardography (TTE) and transesophageal echocardiography (TEE),
the two currently performed types of echocardiography, are usually safe and
portable to the bedside. TTE is rapid, noninvasive, and relatively inexpensive.
TEE is invasive, requires sedation, and is more expensive. TTE can give more
general information as to cardiac structure and function. TEE is frequently
helpful in situations where TTE is not, e.g., in the presence of obesity,
emphysema, small vegetations, e.g., in early infective endocarditis, a
prosthetic cardiac valve the structural components of which may impair the image
of TTE, and infection in perivalvular tissue where PVE often starts. Hemodynamic
complications, such as central or perivalvular regurgitant flow in the presence
of a prosthetic valve can easily be detected and semiquantitated by additional
color flow Doppler.
| Arrow demonstrating vegetation on the heart valve diagnostic of endocarditis. | ||
 |
 |
 |
The sensitivity of TEE is greater (90-100%) than that of TTE (< 80%), allowing for detection of vegetations as small as 1 mm. TEE also has a specificity (i.e., true negative rate), positive predictive accuracy (the probability of infective endocarditis when the echocardiogram is positive), and a negative predicative accuracy (the probability of no infective endocarditis when the echocardiogram is negative) of 95-100%. However, although low, the frequency of a falsely positive finding that can simulate a vegetation, due for example to nonspecific valvular thickening, rupture of a valve leaflet or mitral valve chordae, may be greater than the frequency of infective endocarditis in certain populations. Therefore TEE should only be done for diagnosis of endocarditis in patients in whom the diagnosis of infective endocarditis is at least “possible endocarditis” according to clinical or microbiologic criteria (see Duke criteria, Table 4). Use of TEE as the initial diagnostic test is recommended to detect small vegetations in patients with staphylococcal bacteremia and possible early endocarditis, suspected complicated infective endocarditis (e.g., perivalvular abscess or conduction block), and suspected PVE.
Echocardiography may be falsely negative if vegetations are very small or have already embolized. If initial echocardiography fails to reveal evidence of infective endocarditis in a patient strongly suspected of having infective endocarditis or its intracardiac complications and another diagnosis is still not apparent, the TEE should be repeated in about one week. Sequential echocardiography during the course of antimicrobial therapy is used to assess healing of vegetations and detect development or progression of complications and guide decisions as to the need and timing of surgery. The finding of early closure of the mitral valve, as a consequence of elevated left ventricular end-diastolic pressure, associated with acute aortic valve infective endocarditis, has been used to predict the need for surgery; and the detection of an enlarging vegetation during the course of therapy or an especially a large vegetation (e.g., >10 mm in its largest dimension) in some studies has been found to signify a poorer outcome, i.e., the greater likelihood of embolization, congestive heart failure, the need for surgery and death. However, echocardiographic results must be taken in context of the specific clinical situation, especially the hemodynamic status, prior emboli events, and the type of pathogen involved, e.g., antibiotic resistant gram-negative bacilli or fungi, for assessment of surgical intervention. Echocardiograph at the completion of antimicrobial therapy is needed to establish a new baseline as these patients remain at high risk for recurrent infective endocarditis and development of CHF that may require subsequent prosthetic valve placement if not done during period of antibiotic therapy.
Table 4. Modified Duke Criteria for the Diagnosis of Infective Endocarditis.
1.Definite diagnosis of infective endocarditis
A. Pathologic criteria a. Histological and/or microbiologic evidence of infection at surgery or autopsy B. Clinical criteria a. 2 major criteria; or b. 1 major/3 minor; or c. 5 minor 2.Possible diagnosis:
A. 1 major criteria and 1 minor criterion; or B. 3 minor 3.No endocarditis:
A. Firm alternate diagnosis B. Clinical resolution with ≤4 days of antimicrobial therapy C. No evidence of infective endocarditis at surgery or autopsy with ≤4 days of antimicrobial therapy; or D. Failure to meet criteria for possible infective endocarditis, as above Major Criteria 1. Blood culture A. 2 separate blood cultures positive for: a. viridans streptococci, Streptococcus bovis, HACEK*, Staphylococcus aureus. b. Community-acquired enterococci, in absence of primary focus. B. Microorganisms consistent with endocarditis isolated from: a. at least 2 blood cultures drawn >12 hours apart. b. 3 of 3, or a majority of 4 or more with 1st and last obtained >1hour apart. C. Single positive blood culture for Coxiella burnetii or antiphase 1 IgG antibody >1:800 2. Evidence of endocardial involvement
A. Echocardiography: Positive for oscillating intracardiac mass on valve or supporting
structure, in path of regurgitant jet, or on implanted material in the absence of an alternative
anatomic explanation; or valve ring abscess; or new partial dehiscence of valvular
prosthesis.
B. New valvular regurgitant murmur (increasing or changing or pre-existing murmur not sufficient). Minor Criteria 1. Predisposing heart condition or injection drug use. 2. Fever, >380C. 3. Major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage,
conjunctival hemorrhage, and Janeway lesions.
4. Immunologic phenomena: glomerulonephritis, Roth’s spot, Osler’s node, and rheumatoid factor.
5. Positive blood culture that does not meet major criterion (as noted above) or serologic evidence of active
infection with organism consistent with infective endocarditis.
*Haemophilus parainfluenzae, H. aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae
Other Investigative Procedures
Cardiac catheterization can provide important information and should not be avoided when indicated in selected patients with endocarditis for fear of dislodging emboli. Coronary angiography is used to assess the presence of significant coronary artery disease prior to elective placement of prosthetic cardiac valves in patients who are over 40 years of age and have additional atherogenic risk factors. Contrast-enhanced CT or MRI can best evaluate abscesses or infarcts of the intra-abdominal organs (spleen or liver). Contrast-enhanced CT or MRI/MRA of the head may provide evidence of mycotic aneurysms. Magnetic resonance angiography for the detection of intracranial aneurysms is promising, but 4-vessel cerebral angiography remains the standard for evaluation for aneurysms smaller than 5 mm.
ELECTROCARDIOGRAPHIC MANIFESTATIONS
A baseline electrocardiogram (EKG) should be obtained to assess the presence of conduction abnormalities that develop in about 10-20% of patients with infective endocarditis as a consequence of burrowing valve ring abscesses. Prolongation of the PR interval may be the initial indication of the sudden development of more severe conduction abnormalities, such as complete heart block. Other abnormalities that can be detected by EKG include myocardial infarction and pericarditis.
HEMATOLOGIC MANIFESTATIONS
Progressive anemia of chronic disease with normochromic, normocytic indices routinely develops in subacute infective endocarditis with relatively normal platelet, white blood cell and differential counts. In acute infective endocarditis due to S. aureus, anemia may be initially absent although the white blood cell count is usually elevated with a shift to the left, and the platelet count is often low. PVE with an unstable prosthesis may cause acute hemolysis. The erythrocyte sedimentation rate is routinely elevated in infective endocarditis except when there is CHF or hypofibrinogenemia secondary to disseminated intravascular coagulation.
RENAL MANIFESTATIONS
Proteinuria and microscopic hematuria are common findings, occurring in up to 50% of patients. Renal emboli or focal glomerulonephritis can cause microscopic hematuria, but gross hematuria usually indicates renal infarction. Renal failure that develops in a patient with infective endocarditis is usually due to diffuse immune-complex glomerulonephritis.
OTHER LABORATORY MANIFESTATIONS
Serologic evidence of circulating immune complexes (CIC) may by found in endocarditis, the frequency of which is related to the duration of illness. Occasional false-positive non-treponemal serologic tests for syphilis occur. The cerebrospinal fluid may show polymorphonuclear leukocytes and moderately elevated protein concentration in up to 15% of patients, with a normal glucose concentration and negative culture. Frank bacterial meningitis, although unusual, can occur in S. aureus infective endocarditis.
DIAGNOSIS
Evidence for persistent bacteremia and cardiac valvular involvement, the sine qua non of infective endocarditis, should be sought in patients with suspicious clinical findings (e.g., fever, risk factors, vascular or immune complex phenomena). Standardized criteria for the assessment of patients with suspected infective endocarditis were proposed in 1994 and subsequently modified in 2000 by the group at Duke University. These so-called “Duke Criteria” use findings at surgery or autopsy, predisposing factors, blood culture and other laboratory data, and echocardiographic data to rank the probability of the diagnosis of infective endocarditis as definitive, possible or rejected. These diagnostic infective endocarditis criteria have been validated in several different patient populations. Definitive diagnosis of infective endocarditis depends on proof of cardiac valvular infection by histology or culture of vegetations obtained at the time of surgery or autopsy or by histology or culture of vegetations obtained at the time of surgical removal of an arterial embolus. In lieu of surgery or autopsy, definitive diagnosis can be established by having 2 of 3 of the following major criteria: 1) echocardiographic demonstration of endocardial involvement with characteristic vegetation (oscillating intracardiac mass), valve ring abscess, or new prosthetic valve dehiscence; 2) a new murmur of valvular regurgitation (worsening or changing or pre-existing murmur is not sufficient); or 3) demonstration intravascular infection with multiple blood cultures obtained over an extended period of time that are positive for a microorganism consistent with endocarditis or a single positive blood culture or serology for Coxiella (see Table 4). A definite diagnosis can also be made if the patient has one of the above major criteria plus 3 minor criteria (see Table 4) or 5 minor criteria. The diagnosis of possible infective endocarditis is made if the patient has 1 major plus 1 minor or 3 minor criteria (see Table 4). The diagnosis of infective endocarditis is rejected if findings do not meet criteria for possible infective endocarditis as above, or there is either no pathologic evidence of infective endocarditis at autopsy or surgery with less than 4 days of antibiotic therapy, rapid resolution of clinical findings with short-term antibiotic therapy, or a firm alternate diagnosis. The Duke criteria are intended to be guides and not a substitute for clinical judgment; for example, empiric therapy must often be initiated based on the clinical picture (acute findings) and cardiac (e.g., presence of a prosthetic valve or history of prior infective endocarditis) or noncardiac risk factors (e.g., intravenous drug users) before results of blood cultures or other diagnostic studies are available to apply the Duke criteria.
Microbiologic Investigation:
Isolation of a pathogen from several blood cultures that are obtained over an extended period of time is important both to confirm the diagnosis of endocarditis and to enable determination of the optimal antibiotic regimen. Bacteremia in infective endocarditis is characterized by a constant number of organisms/ml of blood (usually 20-200 CFU/ml), the specific density being characteristic for that particular patient, unrelated to the height of the patient’s temperature or the site of blood sampling (e.g., arterial versus venous blood), except for a slight fall in numbers across the hepatic or splenic circulation. Intermittently positive blood cultures are unusual in the absence of prior antimicrobial therapy. Less than 5-15% of patients with infective endocarditis have sterile blood cultures if adequate an adequate number of blood cultures are obtained prior to the start of antimicrobial therapy. The proper method for obtaining blood for culture includes: 1) Disinfection of the skin with 80-95% alcohol and then 2% iodine or iodophor solution, allowing the disinfectant to remain on the skin for at least 1 minute; 2) Withdrawal of at least 10 ml of blood per blood culture in an adult through the least contaminated site, preferably an antecubital vein, rather than, for example, the femoral vein. Two to three blood cultures should be obtained in this manner at least 1 hour apart to demonstrate that the bacteremia is continuous. In septic patients suspected of having infective endocarditis who are in immediate danger of death, two to three blood cultures should be obtained by separate venipuncture within one hour prior to initiation of empiric antibiotic therapy. In patients suspected of having coagulase-negative staphylococcal infective endocarditis, e.g., patients with a prosthetic valve or other intracardiac foreign body, then it is advisable to draw 3 or more blood cultures initially to help distinguish coagulase-negative staphylococcal contamination of blood cultures from bacteremia.
The clinical microbiology laboratory should be advised of the suspected diagnosis of infective endocarditis, as some organisms require special media or more prolonged incubation of blood cultures (up to 3 weeks) for detection. In the absence of prior antibiotic therapy, the first three blood cultures are expected to be positive in over 95% of patients with positive cultures. Prior antibiotic therapy, fastidious bacteria (such as the Abiotrophia species, the HACEK group of organisms, Neisseria, Brucella, Legionella, Bartonella, Tropheryma, Chlamydia, Coxiella, and rickettsia), and fungi can result in negative cultures. Identification of these organisms will frequently require additional procedures, such as broad spectrum bacterial and fungal PCR and DNA sequencing on vegetations or emboli and histochemical stains and immunohistology of vegetations and emboli and serology, as well as Legionella urinary antigen testing, as outlined in Table 2, 3, 5.
Gram stain of the blood cultures may identify some pathogens, which may not be otherwise apparent. For example, Abiotrophia species that grow in blood cultures as chains of gram-positive cocci will fail to grow when subcultured on blood agar plates; these organisms will require subculture on pyridoxal HCl- or l-cysteine-enriched agar or on plates streaked with S. aureus.
In the face of a preceding course of antibiotic, if clinical conditions permit, further antibiotic therapy should be held and blood cultures repeated until positive. The longer the duration since that last dose of antibiotic or the shorter the preceding course of antibiotic, the more likely the blood cultures will be positive. Bacteriuria with either enterococci or S. aureus occurs in infective endocarditis due to the respective organism.
A variety of in vitro tests must be done on the pathogen isolated from blood to assess susceptibility to potential bactericidal drugs (Table 2). Enterococci should be tested for beta-lactamase production (which predicts resistance to anti-enterococcal beta-lactam antibiotics such as penicillin and ampicillin), for high-level-gentamicin and streptomycin resistance (which predicts lack of synergy with a combination of the respective aminoglycoside plus a cell-wall active drug, such as vancomycin, penicillin, or ampicillin), in addition to susceptibility to penicillin and vancomycin (see below). Synergism (see below) between cell wall-active antibiotics and aminoglycosides has been demonstrated for other microorganisms, including viridans streptococci, staphylococci, P. aeruginosa and aerobic enteric gram-negative bacilli in addition to enterococci and its presence can be assessed by a special in vitro test, the so-called time-kill assay.
Assay of peak serum bactericidal activity early in the course of therapy against the patient’s pathogen had been recommended in the past for non-standard regimens or unusual pathogens and, if inadequate, the dose of the antibiotic was increased (although not at the cost of toxicity) and the serum retested. However, the methodology of the serum bactericidal assay in general has not been well standardized, and scant clinical data exists to validate these recommendations. Measurements of vancomycin and aminoglycoside serum levels are helpful to assure adequate, but nontoxic antibiotic levels.
The pathogen should be retained in the laboratory for susceptibility testing to additional antibiotics if the need arises later, and relapse microorganisms or microorganisms obtained from tissue at surgery later in the course of antibiotic therapy should be retested for antimicrobial susceptibility.
Table 2: Considerations for Testing in Culture Negative IE
|
Special Culture Requirements |
Serologies |
Other Testing |
|
Histoplasma capsulatum (fungal cultures) |
H. capsulatum |
Tropheryma whippelii (PCR of tissue) |
|
Aspergillus (fungal cultures) |
Coxiella burnetii |
|
|
Blastomycosis dermatidis (fungal culture) |
Chlamydia psittaci |
|
|
Bartonella species (prolonged incubation) |
Legionella species |
|
|
Erysipelothrix sp. (fungal cultures) |
Brucella species |
|
|
|
Bartonella quintana or henselae |
|
Table 3. Epidemiological Clues in Etiological Diagnosis of Culture-Negative Endocarditis
|
Epidemiological Feature |
Common Microorganism(s) |
|
Injection drug use |
S aureus, including community-acquired oxacillin-resistant strains Coagulase-negative staphylococci ß-Hemolytic streptococci Fungi Aerobic Gram-negative bacilli, including Pseudomonas aeruginosa Polymicrobial |
|
Indwelling cardiovascular medical devices |
S aureus Coagulase-negative staphylococci Fungi Aerobic Gram-negative bacilli Corynebacterium sp |
|
Genitourinary disorders, infection, manipulation, including pregnancy, delivery, and abortion |
Enterococcus sp Group B streptococci (S agalactiae) Listeria monocytogenes Aerobic Gram-negative bacilli Neisseria gonorrhoeae |
|
Chronic skin disorders, including recurrent infections |
S aureus ß-Hemolytic streptococci |
|
Poor dental health, dental procedures |
Viridans group streptococci "Nutritionally variant streptococci" Abiotrophia defectiva Granulicatella sp Gemella sp HACEK organisms |
|
Alcoholism, cirrhosis |
Bartonella sp Aeromonas sp Listeria sp S pneumoniae ß-Hemolytic streptococci |
|
Burn patients |
S aureus Aerobic Gram-negative bacilli, including P aeruginosa Fungi |
|
Diabetes mellitus |
S aureus ß-Hemolytic streptococci S pneumoniae |
|
Early ( |
Coagulase-negative staphylococci S aureus Aerobic Gram-negative bacilli Fungi Corynebacterium sp Legionella sp |
|
Late (>1 y) prosthetic valve placement |
Coagulase-negative staphylococci S aureus Viridans group streptococci Enterococcus species Fungi Corynebacterium sp |
|
Dog–cat exposure |
Bartonella sp Pasteurella sp Capnocytophaga sp |
|
Contact with contaminated milk or infected farm animals |
Brucella sp Coxiella burnetii Erysipelothrix sp
|
|
Homeless, body lice |
Bartonella sp |
|
AIDS |
Salmonella sp S pneumoniae S aureus |
|
Pneumonia, meningitis |
S pneumoniae |
|
Solid organ transplant |
S aureus Aspergillus fumigatus Enterococcus sp Candida sp |
|
Gastrointestinal lesions |
S bovis Enterococcus sp Clostridium septicum |
Table 5. In Vitro Assays
|
Microorganism |
Test |
Result |
|
Viridans streptococcus |
Broth dilution test |
Penicillin MIC |
|
Enterococcus |
Broth dilution test Growth in: 500 ug/ml of Gentamicin 1000 ug/ml Streptomycin Nitrocephin degradation |
Penicillin MIC Vancomycin MIC High-level resistancea: Gentamicin Streptomycin Beta-lactamase production |
|
S. aureus and Coagulase- Negative Staphylococci |
Nitrocephin degradation Oxacillin/methicillin sensitivity Broth dilution test |
Beta-lactamase production MRSA/MRCNS Vancomycin MIC Rifampin MIC Gentamicin MIC TMP/SMX MIC |
|
Other pathogens |
Broth dilution tests |
Antibiotic MIC/MBCb |
|
All pathogens |
Serum antibiotic concentrations |
Peak and trough vancomycinc and aminoglycosided concentrations |
|
No pathogen isolated |
Histochemical stains of vegetations/emboli Immunohistology of vegetations/emboli Broad-spectrum bacterial and fungal PCR and DNA sequencing on vegetations/emboli Serology Legionella urinary antigen assay |
|
a The infecting strain of enterococcus recovered from patients with endocarditis should be tested for susceptibility to high levels of both gentamicin and streptomycin but not other aminoglycosides. Strains that are resistant to high levels of gentamicin are resistant to other aminoglycosides, except some of these strains may be susceptible to high levels of streptomycin.
Choice of an aminoglycoside for synergy should be based on in vitro high-level aminoglycoside susceptibility testing. If the strain is susceptible to high levels of both gentamicin and streptomycin, gentamicin is preferred because determination of gentamicin serum levels is more generally available. If the strain exhibits high-level resistance to one of these aminoglycosides, use only the aminoglycoside to which the strain is sensitive. If the strain is resistant to high levels of both gentamicin and streptomycin, no aminoglycoside is available to synergize with a cell wall-active antibiotic.
b MIC/MBC testing may be useful for nonstandard antimicrobial regimens or unusual pathogens
c Vancomycin “peak” serum levels should be obtained 1 h after completion of a 1-2h infusion and should be in the range of 30-45 ug/ml. Vancomycin trough levels obtained just before the next dose should be 10-15 ug/ml.
d Gentamicin “peak” serum levels obtained 1 h after start of a 20-30 min IV infusion or IM injection of 1 mg/kg should be about 3-4 ug/ml and trough level should be <1 ug/ml. Streptomycin peak serum level 1h after IM administration of 7.5 mg/kg is about 15-20 ug/ml and trough should be about 5 ug/ml.
MIC, minimal inhibitory concentration; MBC, minimal bactericidal concentration; MRSA, methicillin-resistant S. aureus; MRCNS, methicillin-resistant coagulase-negative staphylococci; TMP/SMX, trimethoprim/sulfamethoxazole;