Hepatitis A Virus
Authors: R. Monina Klevens, DDS, Melissa G. Collier, M.D., Noele P. Nelson, M.D.
Previous author: Raymond S. Koff, M.D.
General Description
Virology
Hepatitis A virus (HAV) is the prototypic hepatotropic member of the Hepatovirus genus within the family of picornaviruses (Picornaviridae). One human HAV serotype and four human genotypes are recognized. In the United States, most cases are associated with genotype IA. HAV is a non-enveloped, icosahedral particle, with a diameter of about 27- 29 nm. It is remarkably thermostable (may not be inactivated at 60°C for 10-12 hours and resists freezing) and acid-resistant (withstands pH levels as low as 3). Its RNA genome is a single-stranded, linear, positive-sense molecule with a length of about 7.5 kb. It serves as messenger RNA for translation into a single polyprotein from which the structural, capsid polypeptides and the non-structural HAV proteins necessary for viral replication are cleaved, predominantly by the action of a 3C protease (38).
Epidemiology
The virus is transmitted by the fecal-oral route with a mean incubation period of 28 to 30 days (range 15 to 50 days). HAV is shed in feces as early as 2 weeks after exposure, peaks with the onset of symptoms, and then declines rapidly during the first week of illness. The frequency of symptomatic infection increases with age. Viral shedding in stools may be prolonged but generally persists in adults and children for up to three months, but in infants can be present up to six months. Concentrations of virus in blood are lower than in stools but may be more infectious.
There are approximately 1.4 million hepatitis A illnesses every year around the world, but the rate of illness varies by geographic region (30). Countries are characterized as having high, intermediate, or low levels of HAV infection. Countries with poor sanitary conditions have high levels of infection in children aged less than 10 years. Epidemics are uncommon in these countries because immunity is high among adults. Developing countries with intermediate levels of infection, particularly those where economic conditions are in transition, improving sanitary conditions mean that children are less likely to be infected early in life, leading to more susceptible adults and the potential for large outbreaks. Developed countries with good sanitary conditions have low rates of infection in all age groups. However, depending on vaccination policies, outbreaks can occur in high risk populations. For example, food imported from endemic countries can cause outbreaks in developed countries (2, 13, 33, 40). The most recent such event in the US found that a product containing pomegranate arils imported from Turkey was associated with 165 hepatitis A cases from 10 states (12).
In the United States, hepatitis A causes about one-fourth of reported cases of acute viral hepatitis (CDC) (7). However, adjusting for asymptomatic infection and underreporting to surveillance, CDC estimates there are 2 new infections for each reported case (8). In 2011, the number of reported cases in the US reached an all-time low of only 1,398 cases; this is likely due to successful childhood vaccination. In 2011, only 5% of all reported cases were younger than 19 years of age. However, most US adults are unexposed to wild virus and remain unvaccinated; through 2006, only 30% of adults were immune to HAV (21).
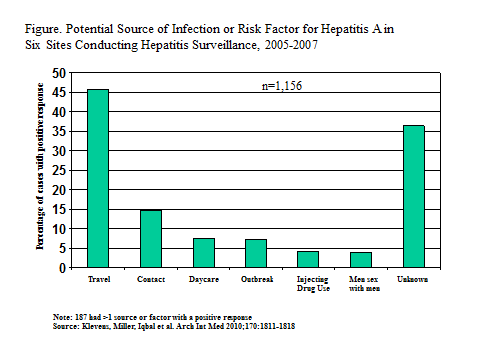
The most common risk factor in the US is international travel or contact with someone who traveled (22) (Figure 1). Other risk factors include employment or contact with someone at a day-care center, injection and other illicit drug use, high-risk behaviors among homosexual and bisexual men, and outbreaks from a common source, often contaminated, imported food.
Clinical Manifestation
While infections in young children may be asymptomatic, hepatitis A in adults is usually symptomatic and associated with jaundice, fever, nausea, vomiting, anorexia, and darkening of the urine. Most infections run their course with full recovery anticipated within 3 months of the onset of symptoms in 85% of infected persons. One or more relapses may be seen in as many as 20% of those infected, particularly in children, and a prolonged but reversible cholestatic phase is a well-known but infrequent complication of the acute disease. Acute liver failure is rare in infected children and young adults, but in adults older than 40 years of age, the risk of acute liver failure increases along with the frequency of hospitalization and death. The risk of severe disease is also increased in individuals with pre-existing chronic liver disease and immunosuppression such as from HIV infection. In the absence of effective antiviral therapy, acute liver failure due to hepatitis A is an indication for liver transplantation.
Laboratory Diagnosis
For persons presenting to the clinical setting with signs and symptoms of acute hepatitis, serologic detection of IgM anti-HAV allows the distinction of HAV infection from other types of hepatitis viruses (27). This antibody may be present 5 to 10 days before the onset of symptoms and during the acute phase of illness. The test may remain positive for 3 to 6 months thereafter. Anti-HAV of the IgG class also may be present early in the acute phase of infection and can persist for decades after recovery. The presence of IgG anti-HAV in the absence of the IgM anti-HAV can indicate that the individual had previously infection, has been vaccinated, or less frequently, has recently received immune globulin. Testing for antibodies should be limited to persons with symptoms to maximize the predictive value positive (7).
Pathogenesis
Virus likely passes through the stomach, replicates in the lower intestine, and travels to the liver. There, a direct cytopathic effect of HAV on hepatocytes seems unlikely. In contrast, hepatocyte injury in the form of necrosis and apoptosis appears to be mediated by cellular immune mechanisms, principally via CD8+ T-lymphocytes, natural killer cells, cytokines, including gamma-interferon, and possibly nitric oxide. From infected hepatocytes, virus is shed back into the intestine and excreted in feces (38).
SUSCEPTIBILITY AND ANTIVIRAL THERAPy
Therapy is symptom-specific and supportive only. Novel 3C proteinase inhibitors might serve as therapeutic antiviral agents but clinical studies in human subjects are not available. No information is currently available about susceptibility of HAV to antiviral therapy.
Immune Globulin
Prior to licensure of hepatitis A vaccines in the mid-1990s, passive immunization with human blood-derived immune globulin (IG) preparations was the only available form of immunoprophylaxis for hepatitis A. IG, prepared by serial cold ethanol fractionation from pools of human plasma, provides protection against hepatitis A through passive transfer of antibody. GamaSTAN® (Grifols Therapeutics Inc., Research Triangle Park, NC) is the only available hepatitis A immune globulin (IgG anti-HAV) product in the United States. The manufacturing process removes model enveloped and non-enveloped viruses, including HIV, HBV, HCV, as well as low levels of CJD/vCJD agent infectivity. Prior to licensure of hepatitis A vaccines in the mid-1990s, passive immunization with human blood-derived immune globulin (IG) preparations was the only available form of immunoprophylaxis for hepatitis A. IG, prepared by serial cold ethanol fractionation from pools of human plasma, provides protection against hepatitis A through passive transfer of antibody. GamaSTAN® (Grifols Therapeutics Inc., Research Triangle Park, NC) is the only available hepatitis A immune globulin (IgG anti-HAV) product in the United States. The manufacturing process removes model enveloped and non-enveloped viruses, including HIV, HBV, HCV, as well as low levels of CJD/vCJD agent infectivity.
Immunogenicity
Levels of circulating anti-HAV (i.e., 10-20 mIU/mL) are thought to be seroprotective and appear rapidly after pre-exposure intramuscular injection, reaching peak levels approximately 2 days after intramuscular injection of IG . When administered (0.02 mL/kg IM) before or within 2 weeks after an exposure to HAV, IG is 80%–90% effective in preventing hepatitis A (42). The concentrations of IG anti-HAV achieved after administration of IG intramuscularly are below the level of detection of most commercially available diagnostic tests (24). Although the prevalence of antibody to HAV in the population has been declining, no clinical or epidemiologic evidence of decreased protection from IG has been observed (15).
Indications
IG should be administered as soon as an exposure to HAV is suspected in children younger than 12 months, persons for whom the vaccine is contraindicated, and certain travelers (see Travel section). IG is unlikely to be effective if given more than 2 weeks after the exposure (4, 8).
Administration
IG should be administered intramuscularly only, preferably in the anterolateral aspects of the upper thigh and the deltoid muscle of the upper arm. The gluteal region should be avoided due to risk of sciatic nerve injury.
Dosage
The dose of IG recommended for household and institutional hepatitis A case contacts and for persons who plan to travel for< ≤ 3 months in areas where hepatitis A is common is 0.02 mL/kg (0.01 mL/lb). For travel >3 months an IG dose of 0.06 mL/kg should be given; repeat for travel duration >5 months (See Travel section for pre-travel recommendations for vaccine and IG) (8). GamaSTAN S/D is supplied in 2 mL and 10 mL single dose vials (Table 1).
Adverse Events, Contraindications and Precautions
Serious adverse events from IG are rare. IG is contraindicated for persons with IgA deficiency due to reports of anaphylaxis in these persons (14). Pregnancy and lactation are not contraindications to IG administration. IG is contraindicated for patients who have severe thrombocytopenia or any coagulation disorder.
Simultaneous Administration with Other Vaccines
IG can be administered with most inactivated vaccines. It does not interfere with the immune response to oral poliovirus vaccine or yellow fever vaccine, however, IG can interfere with the response to other live, attenuated virus vaccines (e.g., measles, mumps, and rubella (MMR) vaccine and varicella virus vaccine) when administered either as individual or combination vaccines (8, 14, 28).
VACCINES
Hepatitis A vaccine has multiple public health advantages for pre- and post-exposure prophylaxis compared with IG, including the induction of active immunity and longer protection, greater ease of administration, and often greater acceptability (25).
In 1995, the first FDA-approved HAV vaccine HAVRIX (GlaxoSmithKline Biologicals, Rixensart, Belgium) became available; a second HAV vaccine VAQTA (Merck & Co, Inc, West Point, PA) was introduced in 1996. In 2001, a combination hepatitis A and hepatitis B vaccine (TWINRIX, GlaxoSmithKline, Research Triangle Park, NC (35)) was approved for persons aged 18 years or older. HAVRIX and VAQTA are sterile suspensions containing inactivated whole virus vaccine derived from HAV grown in human MRC-5 diploid fibroblasts. TWINRIX contains inactivated HAV and noninfectious recombinant hepatitis B surface antigen. The purified HBsAg is obtained by culturing genetically engineered Saccharomyces cerevisiae yeast cells. Each antigen is separately adsorbed onto aluminum salts and pooled during TWINRIX formulation. HAVRIX, VAQTA and TWINRIX are formulated without preservatives.
Efficacy
Hepatitis A vaccines are highly effective in preventing clinically apparent disease based on clinical trials. The efficacy of HAVRIX in protecting against clinical hepatitis A was 94% among approximately 40,000 Thai children aged 1-16 years who received two doses 1 month apart while living in villages with high HAV disease rates (20). The efficacy of VAQTA in protecting against clinical hepatitis A was 100% among approximately 1,000 New York children aged 2-16 years who received one dose while living in a community with a high HAV disease rate (25). Because of the close genetic relatedness of human HAV isolates, all HAV infections are preventable by available vaccines (32). Although comparative trials assessing their protective efficacy are not available, the hepatitis A vaccines are likely to have similar effectiveness (20, 39).
Vaccine Recommendations
Following its introduction in 1996, hepatitis A vaccine was initially recommended for children and adolescents in communities with high rates of HAV disease or for outbreak control in communities with intermediate hepatitis A endemicity. Targeted hepatitis A vaccination was recommended by the Advisory Committee for Immunization Practices (ACIP) in 1996 for children at age 2 years in communities with high rates of disease and for older children and teens during outbreaks (4). In 1999, the Advisory Committee on Immunization Practices recommended vaccination in counties, communities, and 11 states (Washington, Oregon, Idaho, California, Nevada, Utah, Arizona, New Mexico, Oklahoma, South Dakota, Alaska) with hepatitis A rates twice the national average (i.e., >=20 cases per 100,000 population). Vaccination was to be considered in 6 additional states (Missouri, Montana, Wyoming, Colorado, Texas, Arkansas) with rates above the national average (i.e. ≥10 cases per 100,000, but <20 cases per 100,000 population, but lower than twice the national average) (Centers for Disease Control and, 1999) (5).
In 2006, ACIP expanded existing recommendations to include hepatitis A vaccine routinely for children aged 12-23 months and for any person wishing to obtain immunity. As stated in the recommendation, vaccination should be completed according to the licensed schedules and integrated into the routine childhood vaccination schedule. Children who are not vaccinated by age 2 years can be vaccinated at subsequent visits. States, counties, and communities with existing hepatitis A vaccination programs for children aged 2–18 years are encouraged to maintain these programs. In these areas, new efforts focused on routine vaccination of children aged 1 year should enhance, not replace, ongoing programs directed at a broader population of children. In areas without existing hepatitis A vaccination programs, catch-up vaccination of unvaccinated children aged 2–18 years can be considered (CDC, 2006) (8).
Indications for Use
See Table 2 for hepatitis A vaccine pre-exposure prophylaxis indications.
Vaccine Storage and Shipment
HAVRIX, VAQTA, and TWINRIX should be stored refrigerated at 2-8°C (36-46°F). Vaccines should not be frozen; discard if the vaccine has been frozen.
Immunogenicity
Antibody becomes detectable in serum shortly after administration in some vaccines (as early as 2 weeks) (8 , 26). Antibody concentrations after vaccination are 10- to 100- fold lower than those produced after natural infection. Among children age ≥ 1 year and adults, ≥ 90% will develop protective antibody within 4 weeks of a single dose of either vaccine; nearly 100% will develop protective antibodies after receiving two doses. Response to vaccine may be lower in patients aged >40 years, immunosuppressed patients, patients with advanced liver disease or recipients of liver transplants, and infants with passively acquired maternal antibody (8). The minimum protective antibody level of anti-HAV has not been defined. A concentration of vaccine-induced anti-HAV IG ≥20mIU/mL has served as a correlate of protection, although ≥10 mIU/mL has also been considered a protective level in some vaccination studies (23, 34).
Both monovalent hepatitis A vaccines are highly immunogenic. Administering the first and second doses with different monovalent hepatitis A vaccines has not been shown to impair immunogenicity (3). Pre-licensure clinical trials indicate that the immunogenicity of TWINRIX is equivalent to that of the single antigen hepatitis vaccine when administered in a three dose series (6).
Schedule and Dosage
Each of the hepatitis A vaccines is available in adult and pediatric dosage formulations (Table 3). The 2-dose hepatitis A vaccine series should be initiated at 12-23 months of age. The 2 doses should be separated by 6-18 months; 6 month minimum interval between the first and second dose. For monovalent hepatitis A vaccines, the dosage of the 2nd dose should be based on the person’s age at the time of the dose, not the age when the first dose was given. If the interval between the first and second doses of hepatitis A vaccine extends beyond 18 months, it is not necessary to repeat a dose (28).
Twinrix is only available for adults. Monovalent hepatitis A vaccine may be used to complete hepatitis A vaccination begun with Twinrix and vice versa (9).
Vaccine Administration
For children aged <2 years, the vaccine should be administered intramuscularly into the anterolateral area of the thigh. For adults, the vaccine should be administered intramuscularly into the deltoid muscle. A needle length appropriate for the vaccinee’s age and size (minimum of 1 inch) should be used (28).
Long Term Protection
Anti-HAV levels fall sharply after the last vaccine dose and then decline more gradually. Detectable antibodies are estimated to persist for 20–25 years or longer based on mathematical modelling and anti-HAV kinetic studies (17, 36).
Adverse Events
No serious adverse events in children or adults have been reported that could be attributed definitively to the hepatitis A vaccine. The most common adverse events were minor and of brief duration, such as fever, injection-site reactions (pain, tenderness, or erythema), rash, and headache. Systemic reactions that include fatigue, fever, diarrhea, and vomiting occur in fewer than 5% of vaccine recipients (8).
Contraindications and Precautions
Hepatitis A vaccine should not be administered to persons with a history of a severe allergic reaction to a previous dose of hepatitis A vaccine or to a vaccine component.
The safety of hepatitis A vaccination during pregnancy has not been determined. However, because hepatitis A vaccine is produced from inactivated HAV, the theoretic risk to the developing fetus is expected to be low. The risk to immunocompromised persons from inactivated HAV vaccine is also considered low and no special precautions need to be taken (8).
The tip caps of prefilled syringes of HAVRIX and TWINRIX, and the vial stopper, syringe plunger stopper, and tip caps of VAQTA may contain dry natural rubber which may cause allergic reactions in latex-sensitive persons. TWINRIX should not be administered to persons with a history of hypersensitivity to yeast.
Simultaneous Administration with Other Vaccines
Administration of hepatitis A vaccine does not impair the response to other vaccines commonly given concurrently.
Pre- and Post- Vaccination Testing
Pre-vaccination serologic testing may be considered to reduce costs by not vaccinating persons with prior immunity, but it should take into account the cost of testing, vaccine cost, and the likelihood that the person will return for vaccination if susceptible (26). Testing of children born in the United States is generally not cost effective. Vaccination of persons who are immune is not harmful. Screening for total anti-HAV before travel can be useful in some circumstances to determine susceptibility and eliminate unnecessary vaccination (26).
Post-vaccination serological testing is not indicated because of the high rate of vaccine response. Also, some commercially available testing methods cannot detect the low anti-HAV antibody concentrations generated by immunization.
Travel (pre-exposure)
All susceptible persons traveling to or working in countries that have high or intermediate hepatitis A endemicity should be vaccinated. Persons with contraindications listed above should receive IG before departure. Hepatitis A vaccine at the age-appropriate dose is preferred to IG for children and adults aged 1-40 years. For optimal protection, adults aged >40 years, immunocompromised persons, and persons with chronic liver disease or other chronic medical conditions planning to depart to an area in less than 2 weeks should receive the initial dose of vaccine along with IG (0.02 mL/kg) at a separate injection site. One dose of a monovalent hepatitis A vaccine protects most healthy persons aged 1-40 years, and should be administered as soon as travel is considered. No data on single dose hepatitis A vaccine efficacy are available for TWINRIX (1).
Travelers who are aged <12 months, are allergic to a vaccine component, or who otherwise elect not to receive vaccine should receive a single dose of IG (0.02 mL/kg), which provides effective protection against HAV infection for up to 3 months. Those who do not receive vaccination and plan to travel for more than 3 months should receive an IG dose of 0.06 mL/kg, which must be repeated if the duration of travel is more than 5 months. IG can be repeated every 6 months thereafter if the traveler remains in a high risk setting, though hepatitis A vaccination should be encouraged, if not contraindicated (1).
Postexposure Prophylaxis
Persons who recently have been exposed to HAV and who previously have not received hepatitis A vaccine should be administered one dose of monovalent hepatitis A vaccine or IG (0.02 mL/kg) as soon as possible, ideally within 2 weeks of exposure. The efficacy of IG or vaccine when administered >2 weeks after exposure, has not been established. The relative efficacy of vaccine is comparable to IG for postexposure prophylaxis among persons aged 1-40 years (1).
For healthy persons aged 1-40 years, a dose of monovalent hepatitis A vaccine is recommended. For persons aged >40 years, IG is preferred, but vaccine can be used if IG is unavailable. IG is recommended for infants aged <12 months, persons who are immunocompromised, persons who have chronic liver disease, and persons for whom vaccine is contraindicated. TWINRIX is not approved for use as postexposure prophylaxis (1).
Prevention and Control
Improvements in economic conditions and sanitary standards are likely to reduce the risk of HAV infection in endemic regions. Food contaminated with HAV must be heated to at least 85°C (185° F) before the virus is inactivated, and the virus is resistant to low pH and freezing. However, it can be killed by autoclave and common chemicals used for disinfection such as hypochlorite and quarternary ammonium products (38). Outbreaks of hepatitis A illness have been reported after consumption of frozen strawberries a year or more after harvest (18, 29). Better implementation of vaccine programs for targeted groups would be helpful in hepatitis A prevention. An estimated 20% of US travelers to potentially endemic countries self-reported vaccination (41). In the United States, complete hepatitis A vaccine coverage of international travelers could prevent 46% of reported cases (22). Major difficulties exist in reaching and educating travelers to endemic regions, persons who inject drugs, men who have sex with men, persons who have occupational risk for infection, and persons who have clotting-factor disorders about the need for hepatitis A vaccine (5). Similarly, vaccination of patients with chronic liver disease has been far from successful since only a minority (17%) of susceptible patients have been vaccinated and patients with advanced disease are less responsive (10, 41). Early vaccination after the diagnosis of chronic liver disease is critical to prevent complications in these patients.
COMMENTS AND CONTROVERSIES
a. The low incidence of hepatitis A disease in developing countries is associated with reduced circulating virus. In the US, children age of <19 years are more frequently immune from vaccination, but, as in other developed countries (31), there is a growing population of adults who are susceptible. These adults, if not vaccinated, are at risk through travel or from exposure to imported, contaminated food.
b. For the first time in 2013, the Strategic Group of Advisory Experts of the World Health Organization raised the possibility of immunization programs to consider a single dose of inactivated hepatitis A vaccine as it considered the level of effectiveness, the lower costs, and the ease of implementation compared to the current two dose schedule (43).
c. In the US, there is currently no vaccine recommendation and limited data are available for use of vaccine as post-exposure prophylaxis for adults aged >40 years. This is problematic because there are many susceptible adults in the population, immunoglobulin is not readily available for delivery, and IG provides only short term protection.
REFERENCES
1. Committee on Immunization Practices, Centers for Disease Control and Prevention Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007;56:1080-1084.
2. Bernard H, Frank C. Cluster of hepatitis A cases among travellers returning from Egypt, Germany, September through November 2008. Euro Surveill 2009;14(3). [PubMed]
3. Bryan JP, Henry CH, Hoffman AG, South-Paul JE, Smith JA, Cruess D, Spieker JM, de Medina M. Randomized, cross-over, controlled comparison of two inactivated hepatitis A vaccines. Vaccine 2000;19:743-750.[PubMed]
4. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization:recommendations of the Advisory Committee on Imunization Practices (ACIP). MMWR 1996;45:1-30.[PubMed]
5. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR RecommRep 1999;48:1-37.[PubMed]
6. Centers for Disease Control and Prevention. (2001) FDA approval for a combined hepatitis A and B vaccine. MMWR Morb Mortal Wkly Rep 2001;50:806-807.[PubMed]
7. Centers for Disease Control and Prevention. Positive test results for acute hepatitis A virus infection among persons with no recent history of acute hepatitis--United States, 2002-2004. MMWR MorbMortalWklyRep 2005;54:453-456.[PubMed]
8. Centers for Disease Control and Prevention. Prevention of Hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55:1-23.[PubMed]
9. Centers for Disease Control and Prevention. (2008) Hepatitis A. In: Epidemiology and Prevention of Vaccine-Preventable Diseases, 10th Edition (Atkinson W. H, J. , McIntyre, L., Wolfe S., , ed), pp 197-209. Washington DC: Public Health Foundation.
10. Centers for Disease Control and Prevention. The Adult Hepatitis Vaccine Project - California, 2007-2008. MMWR Morb Mortal Wkly Rep 2010;59:514-516. [PubMed]
11. Centers for Disease Control and Prevention. (2013) Surveillance for viral hepatitis, United States, 2011. In. Atlanta.,/
12. Collier MG, Khudyakov YE, Selvage D, Adams-Cameron M, Epson E, Cronquist A, Jervis RH, Lamba K, Kimura AC, Sowadsky R, Hassan R, Park SY, Garza E, Elliott AJ, Rotstein DS, Beal J, Kuntz T, Lance SE, Dreisch R, Wise ME, Nelson NP, Suryaprasad A, Drobeniuc J, Holmberg SD, Xu F; Hepatitis A Outbreak Investigation Team. Outbreak of hepatitis A in the USA associated with frozen pomegranate arils imported from Turkey: an epidemiological case study. Lacnet Infect Dis 2014; 14:976-981. [PubMed]
13. Couturier E, Roque-Afonso AM, Letort MJ, Dussaix E, Vaillant V, de Valk . Cluster of cases of hepatitis A with a travel history to Egypt, September-November 2008, France. Euro Surveill. 2009 ;14(3). [PubMed]
14. Ellis EF, Henney CS. Adverse reactions following administration of human gamma globulin. J Allergy 1969;43:45-54. [PubMed]
15. Farcet MR, Planitzer CB, Stein O, Modrof J, Kreil TR. Hepatitis A virus antibodies in immunoglobulin preparations. The Journal of allergy and clinical immunology 2010;125:198-202. [PubMed]
16. Havrix, Package Insert.
17. Hens N, Habteab Ghebretinsae A, Hardt K, Van Damme P, Van Herck K. Model based estimates of long-term persistence of inactivated hepatitis A vaccine-induced antibodies in adults. Vaccine 2014;32:1507-1513.[PubMed]
18. Hutin YJ, Pool V, Cramer EH, Nainan OV, Weth J, Williams IT, Goldstein ST, Gensheimer KF, Bell BP, Shapiro CN, Alter MJ, Margolis HS, for the National Hepatitis AIT. A multistate, foodborne outbreak of hepatitis A. N Engl J Med 1999;340:595-602. [PubMed]
19. Immune Globulin (Human) GamaSTAN S/D. Insert.
20. Innis BL, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik CA, Suntayakorn S, Suknuntapong T, Safary A, Tang DB. Protection against hepatitis A by an inactivated vaccine. JAMA 1994;271:1328-1334. [PubMed]
21. Klevens RM, Kruszon-Moran D, Wasley A, Gallagher K, McQuillan GM, Kuhnert W, Teshale EH, Drobeniuc J, Bell BP. Seroprevalence of hepatitis A virus antibodies in the U.S.: results from the National Health and Nutrition Examination Survey. Public Health Rep 2011;126:522-532. [PubMed]
22. Klevens RM, Miller JT, Iqbal K, Thomas A, Rizzo EM, Hanson H, Sweet K, Phan Q, Cronquist A, Khudyakov Y, Xia GL, Spradling P. The evolving epidemiology of hepatitis A in the United States: incidence and molecular epidemiology from population-based surveillance, 2005-2007. Arch Intern Med 2010;170:1811-1818. [PubMed]
23. Lemon SM, Binn LN. Serum neutralizing antibody response to hepatitis A virus. J Infect Dis 1983;148:1033-1039.
24. Lemon SM, Murphy PC, Provost PJ, Chalikonda I, Davide JP, Schofield TL, Nalin DR, Lewis JA. Immunoprecipitation and virus neutralization assays demonstrate qualitative differences between protective antibody responses to inactivated hepatitis A vaccine and passive immunization with immune globulin. J Infect Dis 1997;176:9-19. [PubMed]
25. Mayorga Perez O, Herzog C, Zellmeyer M, Loaisiga A, Frosner G, Egger M. Efficacy of virosome hepatitis A vaccine in young children in Nicaragua: randomized placebo-controlled trial. J Infect Dis 2003;188:671-677.
26. Murphy TV, Feinstone SM, Bell BP (2012) Hepatitis A Vaccine. In: Vaccines (Plotkin S, Orenstein WA, Offit PA, eds), pp 183-204. London: Saunders.
27. Nainan OV, Xia G, Vaughan G, Margolis HS. Diagnosis of hepatitis a virus infection: a molecular approach. Clin Microbiol Rev 2006;19:63-79.
28. National Center for Immunization and Respiratory Diseases, CDC General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60:1-64.
29. Niu MT, Polish LB, Robertson BH, Khanna BK, Woodruff BA, Shapiro CN, Miller MA, Smith JD, Gedrose JK, Alter MJ, et al. Multistate outbreak of hepatitis A associated with frozen strawberries. J Infect Dis 1992;166:518-524. [PubMed]
30. Ott JJ, Irving G, Wiersma ST. Long-term protective effects of hepatitis A vaccines. A systematic review. Vaccine.2012 Dec 17;31(1):3-11. [PubMed]
31. Payne L, Coulombier D. Hepatitis A in the European Union: responding to challenges related to new epidemiological patterns. Euro Surveill 2009;14(3). [PubMed]
32. Robertson BH, Jansen RW, Khanna B, Totsuka A, Nainan OV, Siegl G, Widell A, Margolis HS, Isomura S, Ito K, et al. Genetic relatedness of hepatitis A virus strains recovered from different geographical regions. J Gen Virol 1992;73 ( Pt 6):1365-1377. [PubMed]
33. Robesyn E, Micalessi MI, Quoilin S, Naranjo M, Thomas I. Cluster of hepatitis A cases among travellers returning from Egypt, Belgium, September through November 2008. Euro Surveill. 2009 Jan 22;14(3)[PubMed]
34. Stapleton JT. Host immune response to hepatitis A virus. J Infect Dis 1995;171 Suppl 1:S9-14.
35. Twinrix, Package Insert.
36. Van Damme P, Banatvala J, Fay O, Iwarson S, McMahon B, Van Herck K, Shouval D, Bonanni P, Connor B, Cooksley G, Leroux-Roels G, Von Sonnenburg F, International Consensus Group on Hepatitis AVI. Hepatitis A booster vaccination: is there a need? Lancet 2003;362:1065-1071. [PubMed]
37. VAQTA, Package Insert.
38. Wasley A, Stephen M. Feinstone, Bell* BP (2009) Principles and Practice of Infectious Diseases In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 7th ed., 7th ed. Edition (Mandell D, and Bennett, ed), p 2367: Churchill Livingstone, Elsevier.
39. Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, Miller W, Shouval D, Wiens B, Calandra G, et al. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med 1992;327:453-457. [PubMed]
40. Wheeler C, Vogt TM, Armstrong GL, Vaughan G, Weltman A, Nainan OV, Dato V, Xia G, Waller K, Amon J, Lee TM, Highbaugh-Battle A, Hembree C, Evenson S, Ruta MA, Williams IT, Fiore AE, Bell BP. An outbreak of hepatitis A associated with green onions. N Engl J Med 2005;353:890-897. [PubMed]
41. Williams WW, Lu P, Greby S, Bridges CB, Ahmed F. Non-influenza vaccination coverage among adults -- United States, 2011. MMWR 2013;62:66-72. [PubMed]
42. Winokur PL, Stapleton JT. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis 1992;14:580-586. [PubMed]
43. World Health Organization. WHO position paper on hepatitis A vaccines – June 2012. Weekly Epidemiologic Record 2012;87:261-276
Tables
Table 1. Immune Globulin
| Product | Trade Name (Manufacturer) | Dosing | Presentations | Route |
|---|---|---|---|---|
| GamaSTAN® S/D Immune Globulin (Human) | Grifols | 0.02 mL/kg for household, institutional contacts, and international travel prophylaxis < 3 months | 2 mL single dose vial |
IM |
0.06 mL/kg for international travel prophylaxis ≥ 3 months |
10 mL single dose vial |
Table 2. Indications for Pre-exposure Prophylaxis Hepatitis A Vaccine
Routine vaccination of children 12-23 months of age* |
| Any person aged 2 years and older seeking protection from hepatitis A virus (HAV) infection |
Previously unvaccinated persons who live in areas where vaccination programs target older children |
Persons traveling to or working in countries that have high or intermediate endemicity of infection§ |
Users of injection and non-injection illicit drugs |
Persons who work with HAV-infected primates or with HAV in a research laboratory |
Persons with clotting-factor disorders |
Persons with chronic liver disease, including from chronic hepatitis B or C virus infection |
Persons who anticipate close, personal contact (e.g., household or regular babysitting) with an international adoptee during the first 60 days after arrival in the United States from a country with high or intermediate endemicity‡ |
*Children who have received 1 dose of HepA vaccine before age 24 months should receive a second dose 6-18 months after the first dose
§See Travel section below
‡The first dose should be administered as soon as the adoption is planned, ideally 2 or more weeks before the arrival of the adoptee
Table 3. Hepatitis A Vaccines
| Vaccine |
Trade Name (Manufacturer) |
Age (Years) |
Dose |
Route |
Schedule |
|---|---|---|---|---|---|
Hepatitis A vaccine, inactivated |
Havrix (GlaxoSmithKline) |
1–18 ≥19 |
0.5 mL (720 ELU) 1.0 mL (1,440 ELU) |
IM IM |
0, 6–12 mo 0, 6–12 mo |
Hepatitis A vaccine, inactivated |
Vaqta (Merck & Co., Inc.) |
1–18 ≥19 |
0.5 mL (25 U) |
IM IM |
0, 6–18 mo 0, 6–18 mo |
Combined hepatitis A and B vaccine |
Twinrix (GlaxoSmithKline) |
≥18 (primary) ≥18 (accelerated) |
1.0 mL (720 ELU HAV + 20 μg HBsAg) 1.0 mL (720 ELU HAV + 20 μg HBsAg) |
IM IM |
0, 1, 6 mo 0, 7, 21–30 d (accelerate)* |
*FDA approved in 2007
Figure 1.
What's New
Guided Medline Search for Recent Reviews
Guided Medline Search for Historical Aspects:
None.
Table of Contents
- General Description
- Susceptibility and Antiviral Therapy
- Immune Globulin
- Vaccines
- Efficacy
- Vaccine Recommendations
- Indications for Use
- Vaccine Storage and Shipment
- Immunogenicity
- Simultaneous administration with other vaccines
- Immunogenicity
- Vaccine Administration
- Long Term Protection
- Adverse Events
- Contraindications and Precautions
- Simultaneous Administration with Other vaccines
- Pre- and Post- Vaccination Testing
- Travel (pre-exposure)
- Postexposure Prophylaxis
- Prevention and Control
- Comments and Controversies

