Pneumonia Infection In Organ Transplant Recipients
Authors: Nina Singh, M.D., Kevin M. Chan, M.D., Garth Garrison, M.D.
More than 28,000 solid organ transplants were performed in the United States in 2009 (84). The majority were kidney (60%) followed by liver (23%), heart (8%) and lung (5%) transplants. All solid organ transplants (SOT) receive immunosuppressive agents with the highest levels applied within the first three months after surgery when the risk for allograft rejection is greatest. The complexity of the surgical procedure and need for post-operative intensive care and/or mechanical ventilation are directly related to the development of pulmonary infection after transplantation (10, 13). These risk factors along with the pre-operative comorbidities related to the predisposing disease, make pneumonia a leading cause of infection in solid organ transplant recipients. Knowledge on infection risk, the temporal relationship between immunosuppression and infectious etiology, as well as the prompt diagnostic pursuit of organism identification together with empiric therapy; are required to reduce the consequences of this complication.
EPIDEMIOLOGY
Rates of Infection
Although the overall rate of clinically significant infection has decreased with the addition cyclosporine (22, 44), pneumonia remains a significant cause of morbidity and mortality in the post-transplant population. The highest risk for pulmonary infection occurs in the first month post-transplant (2, 71). Lung transplant recipients have the highest incidence of pneumonia, estimated as high as 72% per year in one cohort (2). The incidence of pneumonia following heart (17-28%) (8, 21, 74) and liver (8-23%) (56, 93) transplantation are lower, with renal transplant recipients having the lowest incidence of pulmonary infection (2.9-30%) (41, 71, 117). The importance of pneumonia in this population cannot be underestimated since mortality can be greater than 50% (13, 107, 109).
Risks for Pulmonary Infection
The risk for pneumonia is increased in solid organ transplant recipients due to an elevated "net state of immunosuppression" (37). The immunosuppressive agents used in transplantation are comprised of a triple regimen of cyclosporine or tacrolimus, azathiaprine or mycophenolate mofetil, and corticosteroids. Higher doses of each are administered early post-operatively when the risk of acute rejection is greatest and therefore, infection is of major concern; especially during the first 1-3 months after transplantation. Both cyclosporine and tacrolimus inhibit the activation and proliferation of T cells by inhibiting IL-2 production (50). Azathiaprine is a purine analog that prevents the proliferation of activated B and T lymphocytes; therefore, both cell-mediated and humoral mediated immunity are affected. Mycophenolate mofetil is often substituted for azathiaprine as it inhibits de novo purine synthesis and lymphocyte proliferation (50). Corticosteroids are non-specific in their effect and lead to a reduction of neutrophil chemotaxis, antigen presentation, T cell activation and proliferation, and macrophage function (50). Induction therapy with anti-lymphocyte antibodies (cytolytic agents) or interlukin-2 receptor antagonists are often administered in the perioperative period to prevent acute allograft rejection. Anti-lymphocyte antibodies are polyclonal or monoclonal agents directed against circulating lymphocytes and result in the depletion of these cells by complement mediated lysis as well as entrapment of the antibody coated cells by the reticuloendothelial tissues (50). Interleukin-2 receptor antagonists are monoclonal humanized or chimeric (human/mouse) monoclonal antibodies that bind to the interleukin-2 receptor α chain (CD 25 antigen,IL-2Rα) on the surface of activated T lymphocytes (49, 52, 64). High dose corticosteroids or cytolytic agents are also administered when acute allograft rejection is encountered thereby elevating the risk for infection. Patients at risk for cytomegalovirus (CMV) infection are predisposed to reactivation when cytolytic agents are used (16). Sirolimus, also known as rapamycin, inhibits the proliferative T-cell response to interleukin-2 (113). Sirolimus is used as a substitute agent in patients intolerant or not responsive to the calcinurin inhibitors or purine analogs.
Lung transplant recipients are at the greatest risk for the development of pneumonia due to exposure to the external environment as well as additional unique factors. The donor lung(s), native lung (in single lung transplant), and the upper airway can be colonized with pathogenic bacteria (16). In the initial post-operative period, ischemia/reperfusion injury leads to impairment in the mucosal barrier. Bronchial anastamotic narrowing and denervation of the lungs with subsequent impairment of the cough reflex impairs clearance of pathogens. Chronic allograft rejection manifesting histologically as obliterative bronchiolitis (OB) or its clinical correlate, bronchiolitis obliterans syndrome (BOS), affects up to 50% of patients 5 years post-transplant(19). OB is associated with an increased risk for infection due to the use of augmented immunosuppression to treat the process as well as structural alterations of the airway leading to colonization by Pseudomonas and aspergillus species (16).
ETIOLOGIC AGENTS
Time Course for Infection
Among the different solid organ transplant populations, the spectrum of isolated pathogens follows a similar pattern. During the initial month, infections tend to be secondary to bacterial pathogens acquired in the hospital and intensive care unit. From the first to sixth months post-transplant, patients are exposed to sustained levels of augmented immunosuppression leading to the appearance of opportunistic pathogens. After six months, immunosuppression is typically decreased and infections with community acquired pathogens become more common (67). The typical timeline for the appearance of pathogens is shown in Figure 1. With the widespread use of trimethoprim-sulfamethoxazole, the incidence of Pneumocystis jerovecii has declined (30) but remains of concern when prophylaxis is discontinued (13, 62, 73). The use of fungal and CMV prophylaxis may delay the expected appearance of these organisms.
Bacterial
Bacterial pneumonia remains the most common cause of lower respiratory tract infections in solid organ transplant recipients (4, 10, 13, 16, 107, 109). In the initial perioperative period, nosocomial pathogens including Pseudomonas aeruginosa, Escheria coli, Klebsiella species, Acinetobacter species, and Staphylococcus aureus (including MRSA) are common causes of infection. Prolonged mechanical ventilation following transplant increases the risk for nosocomial pneumonia (10, 13). After the initial post-transplant period, community acquired pathogens including Haemophilus influenza, Streptococcus pneumonia, and Legionella species may be seen. The incidence of Nocardia pneumonia has declined substantially with the use of trimethoprim-sulfamethoxazole prophylaxis, however, it continues to be reported (125).
Bacterial pneumonia or bronchitis encompass 32 – 63% of all infections in lung transplant recipients (16). The incidence of bacterial pneumonia peaks in the first 4-8 perioperative weeks and declines by the fourth month. The use of perioperative antibiotics has reduced the incidence of early bacterial pneumonia in this patient group from 35-48% to less than 10 % (16). Likewise, bacterial pneumonia occurs in 15% of cardiac, 9% of liver and 4-6% of kidney transplant recipients (4, 17, 74, 109). In recent cohort studies of predominantly liver and kidney transplant recipients, overall mortality was between 21 and 35%, however, mortality differences between nosocomial and community acquired infection were extreme at 58% compared to 8%, respectively (10, 13). Mechanical ventilation and nosocomial infection increase the risk for death (10, 13, 107, 109).
In lung transplant recipients, preoperative cultures from the recipient and donor improve the ability to focus post-transplant prophylactic antibiotics. If pseudomonas organisms are found preoperatively, dual anti-pseudomonal coverage is tailored to organism sensitivity. Aminoglycosides are avoided, especially during the early perioperative period due to the high risk of nephrotoxicity. The development of a new radiographic infiltrates after the fifth post-operative day leads to a higher possibility of infection or rejection whereas prior to day five, radiographic change is likely due to reperfusion injury. Patients who develop OB frequently have recurrent purulent bronchitis with pseudomonas sp.(23). These patients require aggressive therapy with appropriate intravenous and/or oral antibiotics to prevent progression of infection and OB. Aerosolized aminoglycoside therapy may be helpful to prevent recurrent infection. Cystic fibrosis (CF) patients require special attention in terms of bacterial pneumonia. Of 27 cystic fibrosis patients described by Flume et al., 89% were colonized with pseudomonas aeruginosa, 19% with Burkholderia complex and 63% with Aspergillus sp. pre-transplantation (39). Despite the preoperative presence of these organisms, the incidence of post-transplant infection and survival was no different between this group and non-CF patients although subgroups of Burkholderia complex, specifically B. cenocepacia and B. gladioli, have a higher risk of death after transplantation(39, 82).
Bacterial infection with Legionella species has been rarely reported but should be suspected in patients with severe pneumonia (109). Antibiotic therapy should include a fluoroquinolone due to the interaction between macrolides and calcineurin inhibitors (cyclosporine and tacrolimus).
Mycobacterial Infections
Mycobacterium Tuberculosis: The incidence of M. tuberculosis in solid organ transplant recipients is 30 to 100-times higher than in the general population (110). Overall, the incidence has been reported to be 0.5-2% in the United States and Europe but as high as 15% in endemic areas (67). Infection is most likely to be the result of reactivated latent infection although primary infection and transmission in the donor allograft may also occur.
The lungs are the most common organ affected with tuberculosis infection. Fever is common at presentation but is not universally present. Radiographic presentations include focal infiltrate, miliary pattern, cavitary disease, diffuse infiltrates, and pleural effusions (67). Patients respond to usual therapy for M. tuberculosis, however, rifabutin is often utilized since metabolism by the cytochrome P450 system results in less interaction with the calcineurin inhibitors than rifampin(77).
Non-tuberculous mycobacteria: Non-tuberculous mycobacterial infection occurs late in the post-transplant period. The incidence is higher than with Mycobacterium tuberculosis and occurs more frequently in lung transplant recipients than other solid organ transplant patients (67). These infections have been reported to occur in 2% to 9.0% of lung transplant recipients and can involve both the allograft and native lung (65, 78, 101). Donor allografts have been a major source of transmission with presentation of infection developing between 6 weeks and 13 months after transplant(12, 20, 28, 99, 127). Acquisition of the organism can also occur through reactivation in the native lung and from primary infection(28, 65). Myocbaterium kansasii and Mycobacterium avium complex are the most common pathogens (85, 90). Colonization with Mycobacterium abscessus in cystic fibrosis recipients can lead to progressive infection and a poor prognosis despite therapy(116, 129).
Viral Infections
Cytomegalovirus (CMV): Cytomegalovirus (CMV) infection is the most clinically important opportunistic viral infection in solid organ transplant patients. It is also associated with the development of obliterative bronchiolitis and increased rates of bacterial infections in the lung transplant recipient (9, 29, 112). Duncan described a twofold greater prevalence of chronic rejection (49%) and bacterial pneumonia (1.02 episodes/patient) in CMV serology positive lung recipients when compared to CMV serology negative patients (22% and 0.50 episodes/patient, respectively) (31).
Cytomegalovirus infection or “reactivation” is defined as asymptomatic shedding of the virus in blood or secretions, while CMV pneumonitis requires symptoms of dyspnea, fever and malaise with the identification of characteristic CMV cells in lung tissue. The infection occurs 1-3 months post-transplant without antibiotic prophylaxis, otherwise it will present after prophylaxis is discontinued, typically 3-6 months post-transplantation. Symptoms include the “CMV syndrome” with fever, malaise, transaminitis and leukopenia. CMV pneumonitis can be found in up to 24% of asymptomatic at risk lung transplant recipients during surveillance bronchoscopy (106). Radiographic changes associated with CMV disease are non-specific and include diffuse haziness (60%), focal haziness (33%), focal lobar consolidation (7%) and no change (29%) (106).
The risk of developing CMV infection and/or disease is dependent on the serologic status of the donor and recipient. Lung allograft recipients have the greatest risk of developing CMV disease. Recipients who are negative for CMV IgG and receive a CMV positive organ, are at greatest risk for primary CMV disease which has been diagnosed in 80% of lung allograft recipients prior to ganciclovir prophylaxis (33). The risk for CMV disease in D-/R+ and D+/R+ patients is lower at 25% and 33%, respectively(33). CMV disease has been described in 25% of donor positive, recipient negative and in 10-15% of recipient positive kidney-pancreas patients (115). Cytomegalovirus naive recipients who receive a CMV negative allograft (D-/R-) have a 10-15% risk of developing CMV infection even when CMV seronegative blood products are used (115). Leukocyte filters and/or irradiated blood products are recommended to reduce the transmission of CMV to these patients when CMV seronegative blood products are not available.
Cytolytic therapy causes additional risk for CMV infection. When used for induction or corticosteroid resistant acute rejection, the risk for CMV illness increases in the 30 days following administration (16).
The detection of CMV in the lung is made by cell culture viral isolation, which typically requires 14-28 days to evaluate (24). The rapid shell vial assay technique allows the detection of CMV by immediate-early antigen staining with labeled monoclonal antibodies after only 12-48 hours of culture (51).
The detection of CMV DNA by polymerase chain reaction (PCR) techniques indicates replication in peripheral blood leukocytes and is a helpful adjunct for screening and the diagnosis of CMV viremia (5, 24, 34). A correlation between viral load and the development of CMV pneumonitis has been shown using quantitative PCR techniques for the detection of CMV DNA in peripheral leukocytes (80, 100). Michaelides and colleagues found that the detection of CMV DNA in blood leukocytes predicted CMV pneumonitis with a sensitivity of 92% and a specificity of 76% (80).
Ganciclovir is the antibiotic of choice for CMV infection and effectively has reduced the morbidity and mortality of CMV disease (16). It is administered intravenously or orally as valganciclovir, the monovalyl ester of ganciclovir. It is also used as a preventive agent in at-risk patients (53). Ganciclovir administration effectively delays CMV infection and/or disease until it is discontinued (6, 7, 106). This avoids the period of greatest risk since immunosuppressive medications have been reduced by the time prophylaxis is stopped however, delayed CMV infection occurs in up to 30% of at risk patients (115). Nine percent of at risk liver transplant recipients have been described to develop CMV disease despite the use of CMV prophylaxis at a median onset of 4.5 months after transplantation (115). Relapse is not uncommon after treatment and repeated therapy may be required up to 20% of the time (102).
Foscarnet and cidofovir are effective alternative agents for CMV therapy although their side effects make them less desirable agents (32, 96, 122). The use of CMV hyperimmune globulin in conjunction with ganciclovir for prophylaxis and treatment of CMV infection/disease has been shown to be beneficial in some studies .
Other Herpesviruses
Other members of the Herpesvirus family including herpes simplex virus (HSV)-1 and varicella-zoster (VZV) have been implicated as rare causes of lower respiratory tract infections (55). Pneumonia due to HSV and VZV is typically due to reactivation of a latent infection and is now infrequent since the institution of valganciclovir and acyclovir prophylaxis. In addition, human herpesvirus 7 (HHV-7) infection has been described and has been associated with early chronic rejection (BOS) in lung transplant patients (47).
Community Acquired Respiratory Viruses (CARV)
Non-CMV viral infections can occur in any solid organ transplant patient but have the greatest incidence and consequence in lung transplant recipients (8% to 34%) (45). The majority of viral infections are community acquired and present several months after transplantation (45, 57, 66). Early viral infection can be obtained by viral reactivation or nosocomial spread. Adenovirus has been documented to occur by donor transmission (83). Contemporary studies evaluating CARV using highly sensitive PCR detection techniques have found respiratory syncytial virus (RSV), parainfluenza (PIV), and human metapneumovirus (hMPV) as the three most commonly detected community acquired viral infections in lung transplant recipients (45, 57, 66). Influenza A and B (including Swine H1N1), adenovirus, coronavirus and rhinovirus have also been reported (3, 38, 45, 57, 66).
Respiratory syncytial virus (RSV), adenovirus, hMPV and parainfluenza viruses typically present with cough, wheeze, coryza and dyspnea with seasonal variation noted between January and April only with RSV (57, 87). Significant mortality of up to 20% is associated with RSV infection in SOT recipients after the development of lower respiratory tract infection. An association between CARV pneumonia and BOS, as well as increased mortality in lung transplant recipients, has been noted (45, 57, 66).
There are four major serotypes of parainfluenza viruses responsible for human infection (PIV-1-PIV-4). PIV-1 and PIV-2 are responsible for seasonal outbreaks in winter months while PIV-3 persists in low levels throughout the year with seasonal outbreaks in spring and summer. Bacterial coinfection is common. In immunocompromised patients with LRTI, mortality rates range from 15-73% and there is a strong association with BOS development in lung transplant recipients (45).
Human metapneumovirus (hMPV) was first isolated from children in 2001 but now has been shown to cause clinically important infection in adults as well (25). In one series, it was the most common respiratory viral infection isolated in lung transplant recipients with an incidence similar to RSV (57). It has also been associated with signs of acute lung allograft rejection (72).
Chest radiographic changes will occur in 58-71% of patients with CARV pneumonia and will include diffuse interstitial infiltrates, lobar infiltrates and mixed interstitial-alveolar infiltrates (69, 121). High-resolution computed tomography may reveal peribronchial ground glass opacities and scattered peripheral reticular changes or patchy consolidation in the transplanted lungs (69). The presence of radiographic change may be indicative of the development of respiratory failure, especially when RSV is suspected (87).
Bronchoalveolar lavage is the most sensitive method for the detection of these viruses. When using BAL culture as the gold standard, BAL has a sensitivity of 86-90% compared to 60% with nasal swab/upper respiratory tract cultures (45, 121). Unfortunately, the median time to viral detection by culture can be greater than 5 days (average 13 days), thereby delaying specific therapy. Rapid antigen testing increases the sensitivity and shortens the time to identification of viral infection. This test is available for influenza, parainfluenza I-III, RSV and adenovirus (45). Polymerase chain reaction (PCR) amplification of specific viral RNA or DNA has greater sensitivity than either antigen detection or culture (85%, 40-54%, 31-67%, respectively) and is now routinely used for CARV detection (45, 120). Fifty percent of reported patients with RSV and parainfluenza virus fail to recover lung function if they present with an initial decline in spirometry, (87, 121). Obliterative bronchiolitis is later diagnosed in a high proportion of these patients (87, 121). This relationship between viral infection and OB is not surprising since it is a recognized complication of adenovirus infections in children and it induces acute allograft rejection in lung and kidney transplant recipients (87, 111).
Adenovirus usually presents with severe constitutional symptoms, loss in lung function and the pathologic finding of a necrotizing pneumonia with intraalveolar hemorrhage and diffuse alveolar damage (55, 108). The virus may be transmitted from person to person in a manner similar to other community acquired respiratory viruses although clinical infection is often due to reactivation of latent infection. (60). This may lead to progressive respiratory failure and a high fatality rate (108).
Ribavirin is a synthetic nucleoside antiviral agent that is effective against RSV infection. Aerosolized and/or systemic ribavirin in conjunction with intravenous immunoglobulin, with or without palivizumab (humanized monoclonal IgG targeting RSV) has been reported to be effective against RSV, parainfluenza and human metapneumovirus infection (45, 54, 76, 92). No effective treatment has been found for adenovirus infection although some authors suggest that intravenous ribavirin or immunoglobulin may be helpful (87).
Seasonal influenza can present with respiratory symptoms, myalgias, fever and headache. Infection in SOT recipients mirrors the seasonal pattern in the general population with outbreaks in winter months. Development of lower respiratory tract infection is uncommon although bacterial superinfection, particularly with Staphylococcal species, is a concern. Fever is the hallmark of influenza and may be associated with rhinorrhea, myalgias, and headache. Therapy with amantidine or rimantadine can be used for the treatment or prophylaxis against influenza A. Zanamivir and oseltamivir are effective against both influenza A and B.
A worldwide pandemic due to novel influenza A (H1N1) was declared in June 2009. This viral infection is distinct from seasonal influenza due to the development of high acuity disease in young, healthy individuals (70). Early reports in lung transplant recipients describe severe disease with nonspecific viral symptoms although fever was less prevalent than expected (3, 38, 95). Respiratory failure requiring mechanical ventilation is common (63). Therapy with the neuraminidase inhibitors oseltamivir or zanamivir is recommended. In cases of possible oseltamivir resistance, therapy with both a M2 inhibitor (amantadine or rimantadine) and oseltamivir or zanamivir alone is indicated (70).
Coronaviruses and rhinoviruses are frequent causes of URI in immunocompetent patients. Although data is limited on the frequency of infection, both have been described as causative agents for lower respiratory tract infection in solid organ transplant patients. In particular, the SARS Coronavirus (SARS-CoV) may cause severe disseminated disease in solid organ transplant patients (35).
Fungal Infections
Two contemporary microbiologic evaluations of pneumonia in predominantly liver and kidney transplant recipients found the incidence of opportunistic fungal pneumonia (not including pneumocystis jirovecii) to range between 8 and 17% (10, 13). Additional studies have found fungal pneumonia to encompass 7% of pulmonary infections in liver transplant patients and 1 to 4% of kidney transplant recipients (4, 107). Although the incidence of fungal pneumonia is lower than bacterial pneumonia in SOT recipients, infections by fungal organisms carry a mortality rate of up to 80-100% when invasive disease occurs (43, 58). The majority of fungal infections in these patients are due to either candida (35-91%) or aspergillus species (9-52%) (58).
The prevalence of fungal colonization in lung transplant recipients’ ranges between 20-40% with 25% of these patients having tracheo-bronchial or invasive disease (61). Difficulties in dealing with these infections are due to the problems of early diagnosis, differentiation between colonization and invasive disease, the lack of effective therapy, the toxicity of therapeutic agents and the limited data on effective antifungal prophylactic regimens. Risk factors for the development of invasive fungal infection in transplant recipients include airway complications requiring the placement of a stent or obliterative bronchiolitis in lung allografts, renal failure, post operative complications, recurrent bacterial infection, recurrent augmented immunosuppression and older age, (43, 89, 91).
Candida sp. are the most frequent cause of nosocomial fungal colonization/infection in solid organ transplant patients causing 98% of fungal infections in a series of patients in a transplant ICU (94). Infection was associated with a longer ICU stay (30 vs. 5 days) and greater mortality (35% vs. 3.5%) than patients without candida infection. Candida appears as a frequent airway colonizer of lung transplant recipients but less than 10% of patients colonized with candida species develop invasive disease (39).
Aspergillus sp. is a more serious saprophytic organism with higher rates of mortality than candida sp. In a case control study evaluating the risk factors for invasive aspergillosis in SOT recipients, the overall incidence was found to be 1.4% in over 11,000 patients, ranging from 3% in lung, 2.4% in heart, 2% in liver and 0.2% in kidney transplant recipients (43). The majority of cases occurred within the first 90 days with risk factors for invasive disease in this early group including a complicated postoperative course, repeated bacterial infections or cytomegalovirus disease, and renal failure. Patients with disease development more than 90 days after transplantation were older, had a greater immunosuppressed state and again had renal failure (43). Aspergillus is isolated from the respiratory tract in 20 to 40% of lung transplant recipients with complicated infections affecting 6%-13% of all patients (61, 67, 79, 81). The risk for developing a complicated aspergillus infection has been found to be 3 times greater in single lung over double lung transplant recipients due to harboring of organisms in the native lung (123). Patients with invasive disease can present with purulent sputum, fever, malaise, respiratory distress, and rarely with hemoptysis (123).
Other opportunistic mycelial fungal infections are of growing consequence and include scedosporium, fusarium, cladosporium, rhizopus, and mucor species (58). A multicenter, prospective study of heart and liver transplant recipients found that 30% of opportunistic mycelial fungi were due to non-aspergillus organisms (58). Overall mortality was high at 55%, however, compared to a mortality rate of 54% for invasive aspergillosis patients, mortality was up to 80-100% in patients with zygomycosis (rhizopus and mucor species) or non-aspergillus hyalohyphomycosis (scedosporium apiospermum and fusarium species).
Tissue invasion by fungal organisms is required for a definitive diagnosis of invasive pneumonia, but a presumptive diagnosis can be made if a fungus is cultured from the airways (usually obtained by fiberoptic bronchoscopy) and the clinical picture is consistent with disease. When fungi are isolated from the airways, especially aspergillus in a lung transplant recipient, differentiation between colonization and invasive disease is difficult. In addition, positive BAL findings may be present in only 25% of cases with invasive aspergillosis (124). The detection of serum galactomannan antigen (a cell wall component of aspergillus) by enzyme immunoassay (EIA) has a sensitivity of 30% for the diagnosis of invasive aspergillosis (59). Greater sensitivity and specificity (67-100% and 91-98%, respectively) using this test in BAL fluid may allow improved differentiation between aspergillus colonization and invasive disease in SOT patients (59, 124).
Chest radiographs can remain normal, can reveal ill-defined nodules, cavitary lesions, focal consolidation or patchy densities (27, 58, 79, 89, 123). A halo of decreased density in the aspergillus nodules, wedge-shaped pleural based lesions, a feeding pulmonary artery branch to the lesion (feeding vessel sign), air filled bronchi with an intraluminal lesion (open bronchus sign), or an “air crescent” sign, can be characteristically seen on computerized tomography of the chest (27, 67).
Endemic dimorphic fungi have been reported including Coccidioides immitis, histoplasma capsulatum and blastomyces dermatitidis (42). Patients with blastomycosis frequently present with respiratory failure (78%) but fortunately the incidence of blastomycosis in solid organ transplant recipients is only 0.14% which is lower than that of histoplasmosis (0.4-2.1%) or coccidioidomycosis (0.59-8.7%) (42). Cryptococcus accounts for 3% of invasive fungal infections in SOT recipients but is associated with a mortality of up to 40% (40).
Three classes of antifungal agents are available for treatment of systemic invasive fungal infection; intravenous polyenes, intravenous and oral extended spectrum triazoles, and intravenous echinocandins (114). Systemic amphotericin B is effective against candida, aspergillus, Zycomycetes and other opportunistic mycoses, however, due to its renal toxicity, especially when used in conjunction with cyclosporine or tacrolimus, it is generally avoided. Liposomal preparations of amphotericin B have proved to be effective with less nephrotoxicity (75). Aerosolized amphotericin B has been used to prevent aspergillus colonization and for therapy of patients with fungal airway lesions(81, 114).
Fluconazole is effective against candida albicans, candida tropicalis, coccidioides immitis and Cryptococcus neoformans. It is ineffective against Torulopsis glabrata and candida krusei (91). Itraconazole is a triazole that is active against aspergillus but suffers from poor oral bioavailability. Its use has been displaced by the newer oral wide spectrum triazoles voriconazole and posaconazole, both which have predictable bioavailability and have been found to be effective in treating acute invasive aspergillosis (26, 114, 119). All triazoles cause a rise in serum calcineurin inhibitors and to a greater degree, sirolimus. A 50% reduction in medication dose is recommended although voriconazole appears to have the greatest effect, so close monitoring of calcineurin and/or sirolimus levels is required(40). Posaconazole has additional activity against the Zygomycetes.
The echinocandins (anidulafungin, caspofungin, micafungin) are glucan synthesis inhibitors that interfere with fungal cell wall synthesis (114, 126). They are active against azole, as well as amphotericin, sensitive and resistant candida sp. (114, 126). Only caspofungin has regulatory approval for treatment of aspergillus sp. however, monotherapy for aspergillosis is not recommended (40, 114, 126). These agents provide alternatives to amphotericin B with less toxicity and perhaps improved efficacy for the management of invasive fungal infections.
Pneumocystis jirovecii
Prior to the use of trimethoprim-sulfamethoxazole (TMP-SMX) for the prevention of pneumocystis jirovecii (formerly pneumocystis carinii) pneumonia (PCP), the incidence of infection ranged between 15% and 88% in heart-lung recipients and 0.6 to 11.5% in renal transplant patients (68, 105). Infection was typically discovered between three and 6 months post transplant as well as after augmented immunosuppression. Shreeniwas et al. found that asymptomatic PCP was the cause of 16% (6/27) of opportunistic infections in a group of lung transplant recipients (106). Three patients were on aerosolized pentamidine for prophylaxis while the second three had stopped TMP-SMX therapy due to side effects. Lobar haziness was noted on the chest radiographs in the second group. Patients can present with subacute onset of dyspnea, nonproductive cough, and low grade fever. Progression to respiratory failure may be more rapid than in HIV populations. Chest x-ray typically shows fine reticular interstitial infiltrates, starting peripherally and extending more centrally as the disease progresses. Recent reports of PCP pneumonia after rituximab use for treatment of acute humoral rejection and the suggestion of human to human transmission leading to an outbreak of PCP pneumonia in renal transplant recipients, have added to possible risk factors for the development of this opportunistic infection (105, 128).
Antibiotic prophylaxis with trimethoprim-sulfamethoxazole, inhaled pentamidine, or dapsone for 4-12 months in renal transplant patients and 12 months in lung transplant recipients is recommended to prevent disease (36, 128).
DIAGNOSTIC TECHNIQUES
Fiberoptic Bronchoscopy
Bronchoscopy with BAL, protected specimen brushings (PSB), and/or transbronchial biopsies are the gold standard for obtaining a microbiological diagnosis in transplant patients (11). The procedure should be considered in all transplant patients unless there is a strong suspicion of a community acquired bacterial infection (focal infiltrate in a non-lung transplant recipient). Transbronchial biopsies are essential in excluding lung allograft rejection and can be helpful in the identifying invasive fungal infection and CMV pneumonitis. When conducted on properly selected patients, lavage is a safe procedure with a reported mortality rate of 0.01 – 0.02% and a major complication rate of 0.08-0.3% (1, 118). Pneumothorax and hemorrhage are risks associated with the addition of transbronchial biopsies, occurring in 1-5% and 9% of cases respectively (130, 131). Mortality from the performance of transbronchial biopsies remains low at 0.1% (131). Bronchoalveolar lavage alone can be safely performed on patients with severe respiratory failure requiring mechanical ventilation. Transbronchial biopsy has generally been contraindicated in this population but can be done with a higher but acceptable morbidity(86, 88). O’Brien and colleagues described TBBx in 51 mechanically ventilated patients of which 34 were lung transplant recipients(86). A histologic diagnosis was made in 35% of the procedures and patient management was changed as a result of TBBx 41% of the time. An overall complication rate of 18% was observed with 6.5% of pneumothoraces developing in lung transplant recipients compared to 18.9% in non-lung transplant patients. Other complications included hemorrhage (6%), hypoxemia (8.4%) and hypotension (7.2%). No deaths occurred as a result of the procedure(86).
In solid organ transplant patients, the sensitivity of bronchoalveolar lavage for obtaining microbiological diagnosis has been reported to be between 58% and 69% (15, 97). The sensitivity is highest for the detection of PCP (90%) (103) and lower for mycobacterial and fungal infections (50%) (103). A threshold of 105 CFU/mL is commonly used to define bacterial pneumonia by BAL(46) with 103 CFU/mL defining pneumonia when plated using a PSB.
Image Guided and Surgical Lung Biopsy
Percutaneous needle biopsy can be considered for a persistent focal or nodular infiltrate should bronchoscopy and transbronchial biopsies be negative. There is an increased rate of pneumothorax following percutaneous biopsy compared to bronchoscopic biopsy although the major complication rate and mortality rate is similar (98). Surgical lung biopsy should be reserved for patients with nodular, diffuse, or cavitary disease, nondiagnostic bronchoscopy and clinical deterioration despite empiric antimicrobial therapy. Complication rates of OLB are quoted to range from 8-20% with 3% of the complications being major (18, 104). These complications include bronchopleural fistulas, prolonged air leaks, empyema, and hemothorax. Procedure related mortality is about 1% (104).
APPROACH TO DIAGNOSIS
Clinical intuition, physical examination, chest radiographs and laboratory findings do not always suggest the correct diagnosis in immunosuppressed patients, especially in the first 6 months after transplantation (48, 104). Overall agreement between clinical impression and final diagnosis in lung transplant recipients has been found to be only 54% (48). In addition, common processes can present atypically, rare processes can occur and two or more infectious processes can present simultaneously in immunosuppressed individuals (104).
Solid organ transplant patients with a new infiltrate should be urgently evaluated and empirically treated for possible infection, especially when presenting less than six months post-transplant. In lung transplant recipients, differentiating lung allograft rejection from infectious pneumonia is of particular concern. The decision to hospitalize should be based on the patient’s clinical condition, ability to coordinate testing, and the type of antimicrobials prescribed for therapy (14). Noninvasive testing may be helpful to obtain a microbiological diagnosis although fiberoptic bronchoscopy remains the gold standard. Attempts are made to perform bronchoscopy with BAL (and transbronchial biopsy in all lung transplant recipients), but in cases of acute respiratory failure, this may not be possible. Acute patient instability is an indication for empiric therapy that is guided by the appearance of the radiographic abnormality, the patient’s course after transplant, CMV serology, and the time elapsed since organ transplantation.
The presence of focal lobar consolidation is more likely to represent nosocomial bacterial pneumonia in the first 6 months after surgery. Acute allograft rejection is also of concern in lung transplant recipients during this time period. Empiric antimicrobial therapy should focus on nosocomial bacterial organisms, avoiding nephrotoxic medications if possible. Previous airway cultures and microbial sensitivities should be considered when antibiotic choices are made, especially if antimicrobial resistant organisms are suspected. If the patient is serologically CMV donor or recipient positive, and CMV prophylaxis has been discontinued, intravenous ganciclovir or oral valganciclovir can be added. High dose corticosteroids to cover the possibility of severe acute rejection can be initiated in lung transplant recipients after antibiotics are started.
In solid organ transplant recipients presenting with findings consistent with bacterial pneumonia more than six months after transplantation, therapy for community acquired organisms should be administered, especially in non-complicated kidney, heart and liver transplant patients. If there is no improvement in 48 to 72 hours after treatment with empiric antibiotic therapy, fiberoptic bronchoscopy with BAL should be done (14). The early performance of bronchoscopy with BAL combined with a reduction in immunosuppression and empiric antibiotic administration has been advocated, although a conservative approach to therapy is also effective (14, 107).
Patients with diffuse or nodular disease should have fiberoptic bronchoscopy with or without transbronchial biopsy performed. Diffuse pulmonary opacities, especially “ground glass opacities” seen on high resolution CT of the chest should lead to the consideration of an opportunistic viral or fungal infection such as CMV or PCP, especially if antibiotic prophylaxis against these organisms has been discontinued. Empiric treatment should commence until a diagnosis has been made.
In patients with nodular or cavitary changes suggestive of fungal or atypical organisms, bronchoscopy with BAL and/or transbronchial biopsy is suggested for diagnosis. If findings are non-diagnostic, radiological guided percutaneous biopsy of a lesion or surgical lung biopsy can be considered.
Suggested non-invasive tests, radiographic chest CT findings, and bronchoalveolar lavage studies to assist in obtaining a diagnosis are listed in Tables 1, 2, and 3 respectively.
CONCLUSION
An increasing number of solid organ transplant recipients are likely to be seen by non-transplant physicians. Pulmonary infections are the most common cause of morbidity in this population and prompt recognition and treatment is necessary to prevent poor outcomes. An understanding of the temporal relationship between immunosuppression and the risk of developing infection can assist the clinician with appropriate treatment. Bacterial pneumonia is common within the first 3 months after transplantation while CMV infection or disease becomes prevalent after the discontinuation of prophylaxis in at-risk patients. Fungal infections, especially aspergillosis, can be fatal if not treated early and the risk of infection is present throughout the transplant period. Community acquired viral infections present with upper-respiratory symptoms and wheezing that may lead to obliterative bronchiolitis in lung transplant recipients. The immediate suspicion of infection in these immunosuppressed individuals should lead to urgent bronchoscopy and empiric antimicrobial therapy, especially in the early post-transplant period.
REFERENCES
1. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56 Suppl 1:i1-21. [PubMed]
2. Aguilar-Guisado M, Givalda J, Ussetti P, Ramos A, Morales P, Blanes M, Bou G, de la Torre-Cisneros J, Roman A, Borro JM, Lama R, Cisneros JM. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant 2007;7(8):1989-96. [PubMed]
3. Ahluwalia J, Ahya VN, Lee J, Kotloff RM, Christie JD, Hadjiliadis D. H1N1 INFLUENZA IN LUNG TRANSPLANT RECIPIENTS: A REPORT OF THREE CASES. Chest 2009;136(4):47S-e-. [PubMed]
4. Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, West MS, Sillix DH, Chandrasekar PH, Haririan A. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant 2006;20(4):401-9. [PubMed]
5. Allice T, Enrietto M, Pittaluga F, Varetto S, Franchello A, Marchiaro G, Ghisetti V. Quantitation of cytomegalovirus DNA by real-time polymerase chain reaction in peripheral blood specimens of patients with solid organ transplants: comparison with end-point PCR and pp65 antigen test. J Med Virol 2006;78(7):915-22. [PubMed]
6. Asberg A, Humar A, Jardine AG, Rollag H, Pescovitz MD, Mouas H, Bignamini A, Toz H, Dittmer I, Montejo M, Hartmann A. Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant 2009;9(5):1205-13. [PubMed]
7. Asmi AE, Chan KM, Nguyen PT, Allenspach LL, Klosterman KG, Thomson LC, Higgins RSD. Efficacy of IV ganciclovir followed by oral therapy for the prevention of CMV infection in lung transplant recipients. Am J Respir Crit Care Med 1999;159:A738. [PubMed]
8. Atasever A, Bacakoglu F, Uysal FE, Nalbantgil S, Karyagdi T, Guzelant A, Sayiner A. Pulmonary complications in heart transplant recipients. Transplant Proc 2006;38(5):1530-4. [PubMed]
9. Bando K, Paradis IL, Similo S, Konishi H, Komatsu K, Zullo TG, Youwsem SA, Close JM, Zeevi A, Duquesnoy RJ, Manzetti J, Keenan RJ, Armitage JM, Hardesty RL, Briffith BP. Obliterative bronchiolitis after lung and heart-lung transplantation: an analysis of risk factors and management. Journal of Throacic and Cardiovascular Surgery 1995;110:4-14. [PubMed]
10. Bonatti H, Pruett TL, Brandacher G, Hagspiel KD, Housseini AM, Sifri CD, Sawyer RG. Pneumonia in solid organ recipients: spectrum of pathogens in 217 episodes. Transplant Proc 2009;41(1):371-4. [PubMed]
11. Bowden RA, Ljungman P, Paya CV. Transplant infections. Philadelphia: Lippincott-Raven; 1998. [PubMed]
12. Carlsen SE, Bergin CJ. Reactivation of Tuberculosis in a donor lung after transplantation. AJR 1990;154:495-407. [PubMed]
13. Cervera C, Agusti C, Angeles Marcos M, Pumarola T, Cofan F, Navasa M, Perez-Villa F, Torres A, Moreno A. Microbiologic features and outcome of pneumonia in transplanted patients. Diagn Microbiol Infect Dis 2006;55(1):47-54. [PubMed]
14. Chakinala MM, Trulock EP. Pneumonia in the solid organ transplant patient. Clin Chest Med 2005;26(1):113-21. [PubMed]
15. Chan CC, Abi-Saleh WJ, Arroliga AC, Stillwell PC, Kirby TJ, Gordon SM, Petras RE, Mehta AC. Diagnostic yield and therapeutic impact of flexible bronchoscopy in lung transplant recipients. J Heart Lung Transplant 1996;15(2):196-205. [PubMed]
16. Chan KM, Allen SA. Infectious pulmonary complications in lung transplant recipients. Semin Respir Infect 2002;17(4):291-302. [PubMed]
17. Chang GC, Wu CL, Pan SH, Yang TY, Chin CS, Yang YC, Chiang CD. The diagnosis of pneumonia in renal transplant recipients using invasive and noninvasive procedures. Chest 2004;125(2):541-7. [PubMed]
18. Chaparro C, Maurer JR, Chamberlain DW, Todd TR. Role of open lung biopsy for diagnosis in lung transplant recipients: ten-year experience. Ann Thorac Surg 1995;59(4):928-32. [PubMed]
19. Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Taylor DO, Kucheryavaya AY, Hertz MI. The registry of the international society for heart and lung transplantation: twenty-sixth official adult lung and heart-lung transplantation Report-2009. J Heart Lung Transplant 2009;28(10):1031-49. [PubMed]
20. Chusid MJ, Splaingard ML, Tweddell JS, Rice TR, Adams M, Havens PL, Kehl KS. Pulmonary tuberculosis after lung-liver transplantation for cystic fibrosis. Pediatric Infectious Disease Journal 1996;15(5):462-464. [PubMed]
21. Cisneros JM, Munoz P, Torre-Cisneros J, Gurgui M, Rodriguez-Hernandez MJ, Aguado JM, Echaniz A. Pneumonia after heart transplantation: a multi-institutional study. Spanish Transplantation Infection Study Group. Clin Infect Dis 1998;27(2):324-31. [PubMed]
22. d'Ivernois C, Dupon M, Dartigues JF, Potaux L, Aparicio M, Lacut JY. Decreased incidence of infection after renal transplantation with the use of cyclosporine. Eur J Clin Microbiol Infect Dis 1991;10(11):911-6. [PubMed]
23. Dauber JH, Paradis IL, Dummer JS. Infectious complications in pulmonary allograft recipients. Clinics in Chest Medicine 1990;11:291-308. [PubMed]
24. de Maar EF, Verschuuren EA, Harmsen MC, The TH, van Son WJ. Pulmonary involvement during cytomegalovirus infection in immunosuppressed patients. Transpl Infect Dis 2003;5(3):112-20. [PubMed]
25. Deffrasnes C, Hamelin ME, Boivin G. Human metapneumovirus. Semin Respir Crit Care Med 2007;28(2):213-21. [PubMed]
26. Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, Haas A, Ruhnke M, Lode H. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis 2002;34(5):563-71. [PubMed]
27. Diederich S, Scadeng M, Dennis C, Stewart S, Flower DR. Aspergillus infection of the respiratory tract after lung transplantation: chest radiographic and CT findings. Eur Radiol 1998;8:306-312. [PubMed]
28. Dromer C, Nashef SA, Velly JF, Martigne C, Couraud L. Tuberculosis in transplanted lungs. J Heart Lung Transplant 1993;12(6 Pt 1):924-7. [PubMed]
29. Duncan AJ, Dummer JS, Paradis IL, Dauber JH, Yousem SA, Zenati MA, Kormos RL, Griffith BP. Cytomegalovirus infection and survival in lung transplant recipients. J Heart Lung Transplant 1991;10(5 Pt 1):638-44; discussion 645-6. [PubMed]
30. Duncan MD, Wilkes DS. Transplant-related immunosuppression: a review of immunosuppression and pulmonary infections. Proc Am Thorac Soc 2005;2(5):449-55. [PubMed]
31. Duncan SR, Paradis IL, Yousem SA, Similo SL, Grgurich WF, Williams PA, Dauber JH, Griffith BP. Sequelae of cytomegalovirus pulmonary infections in lung allograft recipients. Am Rev Respir Dis 1992;146(6):1419-25. [PubMed]
32. Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant 2008;22(2):162-70. [PubMed]
33. Ettinger NA, Bailey TC, Trulock EP, Storch GA, Anderson D, Raab S, Spitznagel EL, Dresler C, Cooper JD. Cytomegalovirus infection and pneumonitis. Impact after isolated lung transplantation. Washington University Lung Transplant Group. Am Rev Respir Dis 1993;147(4):1017-23. [PubMed]
34. Fica A, Cervera C, Perez N, Marcos MA, Ramirez J, Linares L, Soto G, Navasa M, Cofan F, Ricart MJ, Perez-Villa F, Pumarola T, Moreno A. Immunohistochemically proven cytomegalovirus end-organ disease in solid organ transplant patients: clinical features and usefulness of conventional diagnostic tests. Transpl Infect Dis 2007;9(3):203-10. [PubMed]
35. Fischer SA. Emerging viruses in transplantation: there is more to infection after transplant than CMV and EBV. Transplantation 2008;86(10):1327-39. [PubMed]
36. Fisher JH. Infectious complications of lung transplantation. Seminars in Respiratory and Critical Care Medicine 1996;17(2):167-171. [PubMed]
37. Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med 1998;338(24):1741-51. [PubMed]
38. Flagg A, Danziger-Isakov L, Foster C, Nasman C, Smedira N, Carl J, Kwon C, Davis S, Boyle G. Novel 2009 H1N1 influenza virus infection requiring extracorporeal membrane oxygenation in a pediatric heart transplant recipient. J Heart Lung Transplant 2009. [PubMed]
39. Flume PA, Egan TM, Paradowski LJ, Detterbeck FC, Thompson JT, Yankaskas JR. Infectious complications of lung transplantation. Impact of cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1994;149:1601-1607. [PubMed]
40. Gabardi S, Kubiak D, W. , Chandraker A, K., Tullius S, G. . Invasive fungal infections and antifungal therapies in solid organ transplant recipients. Transplant International 2007;20(12):993-1015. [PubMed]
41. Gasink LB, Blumberg EA. Bacterial and mycobacterial pneumonia in transplant recipients. Clin Chest Med 2005;26(4):647-59, vii. [PubMed]
42. Gauthier GM, Safdar N, Klein BS, Andes DR. Blastomycosis in solid organ transplant recipients. Transpl Infect Dis 2007;9(4):310-7. [PubMed]
43. Gavalda J, Len O, San Juan R, Aguado JM, Fortun J, Lumbreras C, Moreno A, Munoz P, Blanes M, Ramos A, Rufi G, Gurgui M, Torre-Cisneros J, Montejo M, Cuenca-Estrella M, Rodriguez-Tudela JL, Pahissa A. Risk factors for invasive aspergillosis in solid-organ transplant recipients: a case-control study. Clin Infect Dis 2005;41(1):52-9. [PubMed]
44. Gorensek MJ, Stewart RW, Keys TF, McHenry MC, Longworth DL, Rehm SJ, Babiak T. Decreased infections in cardiac transplant recipients on cyclosporine with reduced corticosteroid use. Cleve Clin J Med 1989;56(7):690-5. [PubMed]
45. Gottlieb J, Schulz TF, Welte T, Fuehner T, Dierich M, Simon AR, Engelmann I. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation 2009;87(10):1530-7. [PubMed]
46. Griffin JJ, Meduri GU. New approaches in the diagnosis of nosocomial pneumonia. Med Clin North Am 1994;78(5):1091-122. [PubMed]
47. Grim SA, Pham T, Thielke J, Sankary H, Oberholzer J, Benedetti E, Clark NM. Infectious complications associated with the use of rituximab for ABO-incompatible and positive cross-match renal transplant recipients. Clin Transplant 2007;21(5):628-32. [PubMed]
48. Guilinger RA, Paradis IL, Dauber JH, Yousem SA, Williams PA, Keenan RJ, Griffith BP. The importance of bronchoscopy with transbronchial biopsy and bronchoalveolar lavage in the management of lung transplant recipients. Am J Respir Crit Care Med 1995;152(6 Pt 1):2037-43. [PubMed]
49. Hachem RR, Chakinala MM, Yusen RD, Lynch JP, Aloush AA, Patterson GA, Trulock EP. A comparison of basiliximab and anti-thymocyte globulin as induction agents after lung transplantation. J Heart Lung Transplant 2005;24(9):1320-6. [PubMed]
50. Halloran PF, Leung S. Approved immunosuppressants. In: Norman EJ, Suki WN, editors. Primer on Transplantation. Thorofare, NJ: American Society of Transplant Physicians; 1998. [PubMed]
51. Hebart H, Kanz L, Jahn G, Einsele H. Management of cytomegalovirus infection after solid-organ or stem-cell transplantation: current guidelines and future prospects. Drugs 1998;55(1):59-72. [PubMed]
52. Hershberger RE, Starling RC, Eisen HJ, Bergh CH, Kormos RL, Love RB, Van Bakel A, Gordon RD, Popat R, Cockey L, Mamelok RD. Daclizumab to prevent rejection after cardiac transplantation. N Engl J Med 2005;352(26):2705-13. [PubMed]
53. Hertz MI, Jordan C, Savik SK, Fox JMK, Park S, Bolman RM, Dosland-Mullan BM. Randomized trial of daily versus three-times weekly prophylactic ganciclovir after lung and heart-lung transplantation. J Heart Lung Transplant 1998;17:913-920. [PubMed]
54. Hodges TN, Torres FP, Zamora MR. Treatment of respiratory syncytial viral and parainfluenza lower respiratory tract infection in lung transplant patients. J Heart Lung Transplant 2001;20(2):170. [PubMed]
55. Holt ND, Gould FK, Taylor CE, Harwood JF, Freeman R, Healy MD, Corris PA, Dark JH. Incidence and significance of noncytomegalovirus viral respiratory infection after adult lung transplantation. J Heart Lung Transplant 1997;16:416-419. [PubMed]
56. Hong SK, Hwang S, Lee SG, Lee LS, Ahn CS, Kim KH, Moon DB, Ha TY. Pulmonary complications following adult liver transplantation. Transplant Proc 2006;38(9):2979-81. [PubMed]
57. Hopkins P, McNeil K, Kermeen F, Musk M, McQueen E, Mackay I, Sloots T, Nissen M. Human Metapneumovirus in Lung Transplant Recipients and Comparison to Respiratory Syncytial Virus. Am. J. Respir. Crit. Care Med. 2008;178(8):876-881. [PubMed]
58. Husain S, Alexander BD, Munoz P, Avery RK, Houston S, Pruett T, Jacobs R, Dominguez EA, Tollemar JG, Baumgarten K, Yu CM, Wagener MM, Linden P, Kusne S, Singh N. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis 2003;37(2):221-9. [PubMed]
59. Husain S, Paterson DL, Studer SM, Crespo M, Pilewski J, Durkin M, Wheat JL, Johnson B, McLaughlin L, Bentsen C, McCurry KR, Singh N. Aspergillus Galactomannan Antigen in the Bronchoalveolar Lavage Fluid for the Diagnosis of Invasive Aspergillosis in Lung Transplant Recipients. [Article]. 2007. [PubMed]
60. Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis 2006;43(3):331-9. [PubMed]
61. Iversen M, Burton CM, Vand S, Skovfoged L, Carlsen J, Milman N, Andersen CB, Rasmussen M, Tvede M. Aspergillus infection in lung transplant patients: incidence and prognosis. Eur J Clin Microbiol Infect Dis 2007;26(12):879-86. [PubMed]
62. Joos L, Chhajed PN, Wallner J, Battegay M, Steiger J, Gratwohl A, Tamm M. Pulmonary infections diagnosed by BAL: a 12-year experience in 1066 immunocompromised patients. Respir Med 2007;101(1):93-7. [PubMed]
63. Jurawan R, de Almeida M, Smith A, Weilert F. Swine H1N1 influenza in a post liver transplant patient. N Z Med J 2009;122(1304):107-11. [PubMed]
64. Kahan BD, Rajagopalan PR, Hall M. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation 1999;67(2):276-84. [PubMed]
65. Kesten S, Chaparro C. Mycobacterial infections in lung transplant recipients. Chest 1999;115:741-745. [PubMed]
66. Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, Mohanakumar T, Trulock EP, Walter MJ. Respiratory Viral Infections Are a Distinct Risk for Bronchiolitis Obliterans Syndrome and Death. Am. J. Respir. Crit. Care Med. 2004;170(2):181-187. [PubMed]
67. Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170(1):22-48. [PubMed]
68. Kramer MR, Stoehr C, Lewiston NJ, Starnes VA, Theodore J. Trimethoprim-sulfamethoxazole prophylaxis for Pneumocystis carinii infections in heart-lung and lung transplantation--how effective and for how long? Transplantation 1992;53(3):586-9. [PubMed]
69. Krinzman S, Basgoz N, Kradin R, Shepard JAO, Flieder DB, Wright CD, Wain JC, Ginns LC. Respiratory syncytial virus-associated infections in adult recipients of solid organ transplants. J Heart Lung Transplant 1998;17:202-210. [PubMed]
70. Kumar D, Morris MI, Kotton CN, Fischer SA, Michaels MG, Allen U, Blumberg EA, Green M, Humar A, Ison MG. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant;10(1):18-25. [PubMed]
71. Kutinova A, Woodward RS, Ricci JF, Brennan DC. The incidence and costs of sepsis and pneumonia before and after renal transplantation in the United States. Am J Transplant 2006;6(1):129-39. [PubMed]
72. Larcher C, Geltner C, Fischer H, Nachbaur D, Muller LC, Huemer HP. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant 2005;24(11):1891-901. [PubMed]
73. Lehto JT, Anttila VJ, Lommi J, Nieminen MS, Harjula A, Taskinen E, Tukiainen P, Halme M. Clinical usefulness of bronchoalveolar lavage in heart transplant recipients with suspected lower respiratory tract infection. J Heart Lung Transplant 2004;23(5):570-6. [PubMed]
74. Lenner R, Padilla ML, Teirstein AS, Gass A, Schilero GJ. Pulmonary complications in cardiac transplant recipients. Chest 2001;120(2):508-13. [PubMed]
75. Linden P, Williams P, Chan KM. Efficacy and safety of amphotericin B lipid complex injection (ABLC) in solid-organ transplant recipients with invasive fungal infections. Clin Transplant 2000;14(4 Pt 1):329-39. [PubMed]
76. Liu V, Dhillon GS, Weill D. A multi-drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart–lung transplant recipients. Transplant Infectious Disease 2009;12(1):38-44. [PubMed]
77. Lopez-Montes A, Gallego E, Lopez E, Perez J, Lorenzo I, Llamas F, Serrano A, Andres E, Illescas L, Gomez C. Treatment of tuberculosis with rifabutin in a renal transplant recipient. Am J Kidney Dis 2004;44(4):e59-63. [PubMed]
78. Malouf MA, Glanville AR. The spectrum of mycobacterial infection after lung transplantation. Am J Respir Crit Care Med 1999;160(5 Pt 1):1611-6. [PubMed]
79. Mehrad B, Paciocco G, Martinez FJ, Clark Ojo T, Iannettoni MD, Lynch JP, III. Spectrum of Aspergillus Infection in Lung Transplant Recipients : Case Series and Review of the Literature. Chest 2001;119(1):169-175. [PubMed]
80. Michaelides A, Liolios L, Glare EM, Spelman DW, Bailey MJ, Walters EH, Williams TJ, Snell GI, Kotsimbos TC. Increased human cytomegalovirus (HCMV) DNA load in peripheral blood leukocytes after lung transplantation correlates with HCMV pneumonitis. Transplantation 2001;72(1):141-7. [PubMed]
81. Monforte V, Roman A, Gavalda J, Bravo C, Tenorio L, Ferrer A, Maestre J, Morell F. Nebulized amphotericin B prophylaxis for Aspergillus infection in lung transplantation: study of risk factors. J Heart Lung Transplant 2001;20(12):1274-81. [PubMed]
82. Murray S, Charbeneau J, Marshall BC, LiPuma JJ. Impact of Burkholderia Infection on Lung Transplantation in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2008;178(4):363-371. [PubMed]
83. Myerowitz RL, Stalder H, Oxman MN, Levin MJ, Moore M, Leith JD, Gantz NM, Hierholzer JC. Fatal disseminated adenovirus infection in a renal transplant recipient. Am J Med 1975;59(4):591-8. [PubMed]
84. Network OPaT. Transplants by Donor Type. In: Organ Procurement and Transplant Network; 2010. [PubMed]
85. Novick RJ, Moreno-Cabral CE, Stinson EB, Oyer PE, Starnes VA, Hunt SA, Shumway NE. Nontuberculous mycobacterial infections in heart transplant recipients: a seventeen-year experience. J Heart Transplant 1990;9(4):357-63. [PubMed]
86. O'Brien JD, Ettinger NA, Shevlin D, Kollef MH. Safety and yield of transbronchial biopsy in mechanically ventilated patients. Crit Care Med 1997;25(3):440-6. [PubMed]
87. Palmer SM, Henshaw NG, Howell DN, Miller SE, Davis RD, Tapson VF. Community respiratory viral infection in adult lung transplant recipients. Chest 1998;113(944-950). [PubMed]
88. Papin TA, Grum CM, Weg JG. Transbronchial biopsy during mechanical ventilation. Chest 1986;89(2):168-70. [PubMed]
89. Paradowski LJ. Saprophytic fungal infections and lung transplantation-revisited. J Heart Lung Transplant 1997;16:524-531. [PubMed]
90. Patel R, Roberts GD, Keating MR, Paya CV. Infections due to nontuberculous mycobacteria in kidney, heart, and liver transplant recipients. Clin Infect Dis 1994;19(2):263-73. [PubMed]
91. Paya CV. Fungal infections in solid-organ transplantation. Clinical Infectious Diseases 1993;16:677-688. [PubMed]
92. Pelaez A, Lyon GM, Force SD, Ramirez AM, Neujahr DC, Foster M, Naik PM, Gal AA, Mitchell PO, Lawrence EC. Efficacy of Oral Ribavirin in Lung Transplant Patients With Respiratory Syncytial Virus Lower Respiratory Tract Infection. The Journal of Heart and Lung Transplantation 2009;28(1):67-71. [PubMed]
93. Pirat A, Ozgur S, Torgay A, Candan S, Zeyneloglu P, Arslan G. Risk factors for postoperative respiratory complications in adult liver transplant recipients. Transplant Proc 2004;36(1):218-20. [PubMed]
94. Pugliese F, Ruberto F, Cappannoli A, Perrella SM, Bruno K, Martelli S, Marcellino V, D'Alio A, Diso D, Rossi M, Corradini SG, Morabito V, Rolla M, Ferretti G, Venuta F, Berloco PB, Coloni GF, Pietropaoli P. Incidence of Fungal Infections in a Solid Organ Recipients Dedicated Intensive Care Unit. Transplantation Proceedings 2007;39(6):2005-2007. [PubMed]
95. Raj R, Cerdan M, Yepeshurtado A, Kimbrough R, Nugent K. Novel swine-origin (S-OIV) H1N1 influenza A pneumonia in a lung transplant patient: a case report and review of the literature on performance characteristics of rapid screening tests for the S-OIV. Am J Med Sci 2009;338(6):506-8. [PubMed]
96. Reddy AJ, Zaas AK, Hanson KE, Palmer SM. A single-center experience with ganciclovir-resistant cytomegalovirus in lung transplant recipients: treatment and outcome. J Heart Lung Transplant 2007;26(12):1286-92. [PubMed]
97. Reichenberger F, Dickenmann M, Binet I, Soler M, Bolliger C, Steiger J, Brunner F, Thiel G, Tamm M. Diagnostic yield of bronchoalveolar lavage following renal transplantation. Transpl Infect Dis 2001;3(1):2-7. [PubMed]
98. Richardson CM, Pointon KS, Manhire AR, Macfarlane JT. Percutaneous lung biopsies: a survey of UK practice based on 5444 biopsies. Br J Radiol 2002;75(897):731-5. [PubMed]
99. Ridgeway AL, Warner GS, Phillips P, Forshag MS, McGiffin DC, Harden JW, Harris RH, Benjamin WH, Zorn GL, Dunlap NE. Transmission of Mycobacterium tuberculosis to recipients of single lung transplants from the same donor. Am J Respir Crit Care Med 1996;153:1166-1168. [PubMed]
100. Sanchez JL, Kruger RM, Paranjothi S, Trulock EP, Lynch JP, Hicks C, Shannon WD, Storch GA. Relationship of cytomegalovirus viral load in blood to pneumonitis in lung transplant recipients. Transplantation 2001;72(4):733-5. [PubMed]
101. Schulman LL, Scully B, McGregor CC, Austin JH. Pulmonary tuberculosis after lung transplantation. Chest 1997;111(5):1459-62. [PubMed]
102. Shanahan A, Malani PN, Kaul DR. Relapsing cytomegalovirus infection in solid organ transplant recipients. Transpl Infect Dis 2009;11(6):513-8. [PubMed]
103. Shelhamer JH, Gill VJ, Quinn TC, Crawford SW, Kovacs JA, Masur H, Ognibene FP. The laboratory evaluation of opportunistic pulmonary infections. Ann Intern Med 1996;124(6):585-99. [PubMed]
104. Shelhammer JH, Toews GB, Masur H, Surrfredini AF, Pizzo PA, Walsh TJ, Henderson DK. Respiratory disease in the immunosuppressed patient. Annals of Internal Medicine 1992;117:415-431. [PubMed]
105. Shelton E, Yong M, Cohney S. Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology (Carlton) 2009;14(7):696-9. [PubMed]
106. Shreeniwas R, Schulman LL, Berkmen YM, McGregor CC, Austin JHM. Opportunistic bronchopulmonary infections after lung transplantation: clinical and radiographic findings. Radiology 1996;200:349-356. [PubMed]
107. Sileri P, Pursell KJ, Coady NT, Giacomoni A, Berliti S, Tzoracoleftherakis E, Testa G, Benedetti E. A standardized protocol for the treatment of severe pneumonia in kidney transplant recipients. Clin Transplant 2002;16(6):450-4. [PubMed]
108. Simsir A, Greenebaum E, Nuovo G, Schulman LL. Late fatal adenovirus pneumonitis in a lung transplant recipient. Transplantation 1998;65(4):592-4. [PubMed]
109. Singh N, Gayowski T, Wagener M, Marino IR, Yu VL. Pulmonary infections in liver transplant recipients receiving tacrolimus. Changing pattern of microbial etiologies. Transplantation 1996;61(3):396-401. [PubMed]
110. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis 1998;27(5):1266-77. [PubMed]
111. Sleiman CH, Roue C, Mal H, Raffy O, Mangiapan G, Groussard O, Baldeyrou P, Fournier M, Pariente R. Pespiratory syncytial virus(RSV) infection in single lung transplant recipients(abstract). American Journal of Respiratory and Critical Care Medicine 1996;153 (4; Part 2):A263. [PubMed]
112. Smyth RL, Scott JP, Borysiewicz LK, Sharples LD, Stewart S, Wreghitt TG, Gray JJ, Higenbottam TW, Wallwork J. Cytomegalovirus infection in heart-lung transplant recipients: risk factors, clinical associations, and response to treatment. J Infect Dis 1991;164(6):1045-50. [PubMed]
113. Snell GI, Levvey BJ, Chin W, Kotsimbos AT, Whitford H, Williams TJ, Richardson M. Rescue therapy: a role for sirolimus in lung and heart transplant recipients. Transplant Proc 2001;33(1-2):1084-5. [PubMed]
114. Solé A, Salavert M. Fungal infections after lung transplantation. Transplantation Reviews 2008;22(2):89-104. [PubMed]
115. Stratta RJ, Pietrangeli C, Baillie GM. Defining the risks for cytomegalovirus infection and disease after solid organ transplantation. Pharmacotherapy 2010;30(2):144-57. [PubMed]
116. Taylor JL, Palmer SM. Mycobacterium abscessus Chest Wall and Pulmonary Infection in a Cystic Fibrosis Lung Transplant Recipient. The Journal of Heart and Lung Transplantation 2006;25(8):985-988. [PubMed]
117. Tveit DJ, Hypolite IO, Poropatich RK, Hshieh P, Cruess D, Hawkes CA, Agodoa LY, Abbott KC. Hospitalizations for bacterial pneumonia after renal transplantation in the United States. J Nephrol 2002;15(3):255-62. [PubMed]
118. Wahidi MM, Rocha AT, Hollingsworth JW, Govert JA, Feller-Kopman D, Ernst A. Contraindications and safety of transbronchial lung biopsy via flexible bronchoscopy. A survey of pulmonologists and review of the literature. Respiration 2005;72(3):285-95. [PubMed]
119. Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, Yanovich S, Stiff P, Greenberg R, Donowitz G, Schuster M, Reboli A, Wingard J, Arndt C, Reinhardt J, Hadley S, Finberg R, Laverdiere M, Perfect J, Garber G, Fioritoni G, Anaissie E, Lee J. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 2002;346(4):225-34. [PubMed]
120. Weinberg A, Zamora MR, Li S, Torres F, Hodges TN. The value of polymerase chain reaction for the diagnosis of viral respiratory tract infections in lung transplant recipients. Journal of Clinical Virology 2002;25(2):171-175. [PubMed]
121. Wendt CH, Fox JMK, Hertz MI. Paramyxovirus infection in lung transplant recipients. Journal of Heart and Lung Transplantation 1995;14:479-485. [PubMed]
122. West P, Schmiedeskamp M, Neeley H, Oberholzer J, Benedetti E, Kaplan B. Use of high-dose ganciclovir for a resistant cytomegalovirus infection due to UL97 mutation. Transpl Infect Dis 2008;10(2):129-32. [PubMed]
123. Westney GE, Kesten S, De Hoyos A, Chapparro C, Winton T, Maurer JR. Aspergillus infection in single and double lung transplant recipients. Transplantation 1996;61:915-919. [PubMed]
124. Wheat LJ, Walsh TJ. Diagnosis of invasive aspergillosis by galactomannan antigenemia detection using an enzyme immunoassay. Eur J Clin Microbiol Infect Dis 2008;27(4):245-51. [PubMed]
125. Wiesmayr S, Stelzmueller I, Tabarelli W, Bargehr D, Graziadei I, Freund M, Ladurner R, Steurer W, Geltner C, Mark W, Margreiter R, Bonatti H. Nocardiosis following solid organ transplantation: a single-centre experience. Transpl Int 2005;18(9):1048-53. [PubMed]
126. Winkler M, Pratschke J, Schulz U, Zheng S, Zhang M, Li W, Lu M, Sgarabotto D, Sganga G, Kaskel P, Chandwani S, Ma L, Petrovic J, Shivaprakash M. Caspofungin for post solid organ transplant invasive fungal disease: results of a retrospective observational study. Transpl Infect Dis 2010. [PubMed]
127. Winthrop KL, Kubak BM, Pegues DA, Hufana C, Costamagna P, Desmond E, Sanders C, Shen P, Flores-Ibarra L, Osborne E, Bruckner D, Flood J. Transmission of mycobacterium tuberculosis via lung transplantation. Am J Transplant 2004;4(9):1529-33. [PubMed]
128. Yazaki H, Goto N, Uchida K, Kobayashi T, Gatanaga H, Oka S. Outbreak of Pneumocystis jiroveci pneumonia in renal transplant recipients: P. jiroveci is contagious to the susceptible host. Transplantation 2009;88(3):380-5. [PubMed]
129. Zaidi S, Elidemir O, Heinle JS, McKenzie ED, Schecter MG, Kaplan SL, Dishop MK, Kearney DL, Mallory GB. Mycobacterium abscessus in cystic fibrosis lung transplant recipients: report of 2 cases and risk for recurrence. Transplant Infectious Disease 2009;11(3):243-248. [PubMed]
130. Zavala DC. Pulmonary hemorrhage in fiberoptic transbronchial biopsy. Chest 1976;70(5):584-8. [PubMed]
131. Zavala DC. Pulmonary hemorrhage in fiberoptic transbronchial biopsy. Chest 1976;70(5):584-8. 131. Zavala DC. Complications following fiberoptic bronchoscopy. The "good news" and the "bad news". Chest 1978;73(6):783-5. [PubMed]
Figure 1
Temporal relationship between infectious etiology and time after transplantation. The risk for bacterial and fungal pneumonia is greatest in the first four weeks and decreases after three months, whereas the risk for CMV infection peaks after the discontinuation of antiviral prophylaxis in at-risk patients. Non-CMV viral infection is typically community acquired and develops more than 6 months after surgery.
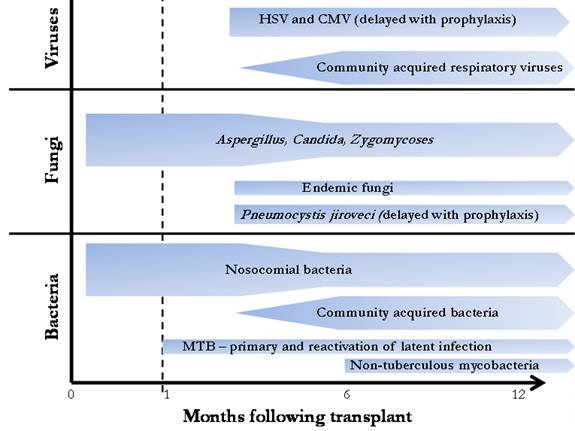
Figure 2
Suggested approach to suspected pneumonia in a solid organ transplant recipient. Focal radiographic consolidative changes in conjunction with findings suggestive of bacterial pneumonia lead to empiric antibiotic therapy. Patients presenting > 6 months after transplantation are treated for community acquired pneumonia with observation while those < 6 months post-procedure are treated for nosocomial infection. All patients in the latter category, lung transplant recipients, and those non-responsive to empiric therapy should have bronchoscopy performed. Patients with diffuse or nodular opacities should receive empiric treatment but bronchoscopy should be performed to obtain a diagnosis. In patients without a diagnosis after bronchoscopic evaluation, radiologic percutaneous biopsy or surgical lung biopsy should be considered.
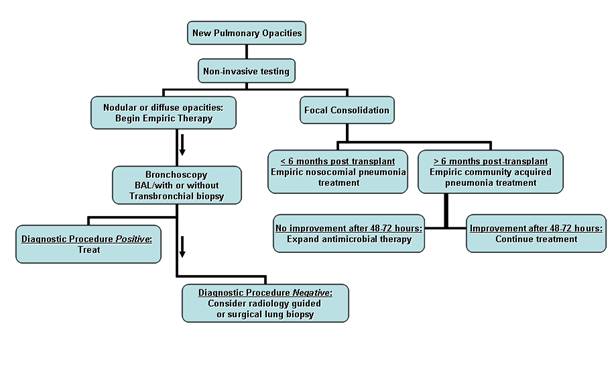
Table 1: Noninvasive testing for obtaining a microbiological diagnosis in solid organ transplant patients with pneumonia
| Sample | Laboratory studies |
|---|---|
| Serum | Blood cultures |
| CMV quantitative viral load by PCR | |
| Aspergillus galactomannen antigen | |
| Histoplasma, Cryptococcus, Blastomycosisantibody titer | |
| Histoplasma, Cryptococcus antigen | |
| Urine | Pneumococcal antigen |
| Legionella antigen | |
| Histoplasmaantigen | |
| Nasopharyngeal swab or sputum | Routine culture and Gram stain |
| Fungal culture and stain | |
| AFB culture and stain | |
| Viral antigen testing for RSV, parainfluenza, influenza, adenovirus |
Table 2: Radiographic signs associated with microbiological diagnoses
| Finding | Suspected pathogen |
|---|---|
| Focal consolidation | Bacterial pathogens |
| "Tree-in-bud opacity" | Atypical pathogens including fungi and mycobacteria |
| Ground glass opacity | P. jiroveci, viral infections including CMV in at-risk patients |
| Nodular opacity | Fungi and mycobacteria |
| "Halo sign" | Aspergillus |
| Pneumothorax | P. jiroveci |
Table 3: Laboratory evaluation of bronchoalveolar lavage (BAL) samples
Essential studies |
|
Optional studies |
|
