Parvovirus B19 Infection in Transplant Recipients
Authors: Albert Eid, M.D.
Virology
Parvovirus B19 is a small non-enveloped ubiquitous virus measuring 22 to 24 nm in diameter. It is a member of the family Parvoviridae, subfamily parvivirinae, genus Erythrovirus. The genome consists of single-stranded linear DNA encoding two structural proteins, VP1 and VP2, and a major non-structural protein, NS1. The virus is classified into three different genotypes: genotype 1, Au and Wi strains; genotype 2, LaLi and A6 strains; and genotype 3, V9 strains. Parvovirus B19 uses the P-antigen, a globoside member of the P blood group system, in addition to other coreceptors to enter the cell. Hence, its predilection for erythroid progenitor cells.
Epidemiology
Parvovirus B19 is easily transmitted among children and susceptible adults caring for them (i.e. day-care workers, teachers, and mothers) through the respiratory route. The secondary attack rate for exposed household members is around 50%. Therefore, most people get infected during childhood and the rate of seropositivity among adults is as high as 87% (1). Even though less common, vertical transmission and transmission via blood products and transplanted organs have also been reported. Once infected with Parvovirus B19, immunocompetent individuals are considered immune. However, reinfection was suspected in some cases (1). Furthermore, the detection of Parvovirus B19 DNA in the bone marrow and various tissue specimens (2, 3) long after the initial infection of some patients raised the question of viral reactivation, especially in the context of immunosuppression. Clinical data supporting this hypothesis are lacking, however. Transplant recipients can get infected via droplet inhalation, blood-products transfusion (4) and allograft tissue (5, 6). Nosocomial outbreaks in transplant units have been reported (7). The incubation period of Parvovirus B19 in immunocompetent individuals ranges from 4 to 21 days. Typically, patients are contagious during the week preceding the development of symptoms. Once symptomatic, the risk of transmission decreases significantly, which limits the effectiveness of infection control measures aimed at reducing the risk of transmission to immunosuppressed patients. On the other hand, due to immunosuppression, transplant recipients may shed the virus in respiratory secretions for prolonged period of time. Therefore, those patients should be placed on standard and droplet isolation for the entire duration of hospitalization.
Clinical Manifestations Pertinent to Transplant Recipients
Among solid organ transplant and hematopoietic stem cell transplant recipients, Parvovirus B19 infection occurs on average 7 weeks (range, 1 week - 8 years) after transplantation. Up to 65% of transplant recipients with Parvovirus B19 infection present within the first 3 months following transplantation. Anemia is a constant finding in both solid organ transplant and hematopoietic stem cell transplant recipients. Leucopenia and thrombocytopenia are seen in 33% and 18% of solid organ transplant recipients and 62% and 38% of hematopoietic stem cell transplant recipients, respectively. Fever is present in only one out of four patients. Arthralgia occurs in 6% of transplant recipients while rash is seen more frequently in hematopoietic stem cell transplant as opposed to solid organ transplant recipients (33% vs. 6%) (8). Tissue-invasive disease was reported in 11% of transplant recipients and consisted of hepatitis, myocarditis, pneumonitis, collapsing glomerulopathy, encephalitis, or vasculitis (9, 10, 11, 12, 13, 14, 15). Allograft rejection, dysfunction, or loss in solid organ transplant recipients and failure of stem cell engraftment in relation to Parvovirus B19 infection was seen in 10% of patients (6, 16). The manifestations of Parvovirus B19 infection in both solid organ transplant and hematopoietic stem cell transplant recipients are summarized in Table 1. In transplant recipients, ineffective humoral or cellular immunity allow sustained or recurrent viremia (17). Therefore, relapse of symptomatic infection is not uncommon.
Laboratory Diagnosis
Parvovirus B19 can be detected directly via molecular techniques or indirectly through measurement of viral antibodies. In transplant recipients, Parvovirus B19 serology is not reliable due to inadequate or delayed humoral response to infection (18, 19). Parvovirus B19 IgM antibody was present in only 71% of solid organ transplant and hematopoietic stem cell transplant recipients at the time of diagnosis. In addition, the presence of Parvovirus B19 IgG antibody alone is uncommonly seen among transplant recipients with active Parvovirus B19 infection (7% of patients) and is rather consistent with remote infection (8). The limitations of serological testing in the transplant population mandate the use of molecular techniques in order to improve sensitivity. Parvovirus B19 DNA can be detected in specimens such as blood, bone marrow and tissue from infected organs (i.e. liver, lung, and kidney). Assays using polymerase chain reaction (PCR) have high sensitivity and specificity (20); however, some of them do not detect or poorly detect non-B19 strains (genotypes 2 and 3) (21, 22, 23, 24). The detection of Parvovirus B19 DNA in transplant recipients should be interpreted with caution because it was reportedly detected in the serum and tissue samples (3, 11, 25) from asymptomatic individuals long after the initial infection. However, the detection of viral DNA in transplant recipients in presence of clinical findings suggestive of active infection (i.e. anemia, pancytopenia, fever, and rash) is likely to represent active infection. When the diagnosis is in doubt or when Parvovirus B19 serology and PCR are negative but the clinical suspicion is high, the examination of bone marrow specimen and the use of immunohistochemical staining or in situ hybridization could be very helpful in establishing the diagnosis (8). Typical bone marrow findings include overall hypercellularity, presence of giant pronormoblasts, and absence of late normoblasts.
ANTIVIRAL THERAPY
Specific antiviral agents to treat Parvovirus B19 infection are not available.
ADJUNCTIVE THERAPY
Reduction of immunosuppression The dose of immunosuppressive drugs should be reduced if at all possible when symptomatic Parvovirus B19 infection is diagnosed in transplant recipients.
Intravenous immunoglobulin (IVIG) Resolution of Parvoviremia is typically seen when patients develop specific viral antibodies. This observation led to the common practice of using IVIG in patients with symptomatic parvovirus B19 infection. Many transplant recipients were clinically improved after receiving IVIG (8, 26, 27, 28). However, some others experienced long-lasting resolution of the infection without receiving IVIG (8). The optimal dose of IVIG and the schedule of administration are not known. Most physicians recommend 400 mg/kg/day for 5 days. This is supported by the fact that transplant recipients who received a total dose of ≤ 2 gm/kg did not experience more relapse compared to those who received > 2 gm/kg. Despite therapy, up to 28% of SOT recipients and 9.5% of hematopoietic stem cell transplant recipients experienced relapse after receiving IVIG (8). In case of Parvovirus B19 infection relapse, patients can be successfully treated with additional courses of IVIG (29, 30, 31).
ENDPOINTS FOR MONITORING THERAPY
The use of PCR to monitor the response to therapy is not recommended since Parvoviremia may persist for months in asymptomatic patients following primary infection (29, 30). It is recommended, however, to follow the hemoglobin concentration and consider obtaining Parvovirus B19 PCR in case of recurrence of anemia.
VACCINES
No Parvovirus B19 vaccine is currently approved or commercially available for clinical use. However, the development of two recombinant human Parvovirus B19 vaccines (MEDI-491 and VAI-VP705) is underway. Both vaccines are composed of viral capsid proteins VP1 and VP2. The results of a study using MEDI-491 were promising 32 while another phase I/II randomized, controlled, double-blind clinical trial using VAI-VP705 is still in progress.
ANTIVIRAL PROPHYLAXIS
Primary antiviral prophylaxis is not recommended for solid organ transplant and hematopoietic stem cell transplant recipients to prevent Parvovirus B19 infection.
INFECTION CONTROL MEASURES
Since patients with Parvovirus B19 are contagious prior to becoming symptomatic, efforts aimed at containing the virus are unlikely to be effective. Therefore, transplant recipients should not be asked to avoid exposure to children and adults susceptible to or already symptomatic with Parvovirus B19 infection. The low incidence of this infection among transplant recipients, especially pediatric transplant recipients, does not support such recommendation. Furthermore, Parvovirus B19 serostatus of donors and recipients is not routinely determined. When Parvovirus B19 infection is suspected or confirmed in hospitalized transplant recipients, standard and droplet precautions should be implemented to avoid nosocomial transmission. Specifically, pregnant health-care workers should avoid contact with those patients if they have not been previously infected. Since immunosuppressed patients shed the virus for a prolonged period of time, precautions should be implemented for the entire duration of their hospital stay.
REFERENCES
1. Heegaard ED, Petersen BL, Heilmann CJ, Hornsleth A. Prevalence of parvovirus B19 and parvovirus V9 DNA and antibodies in paired bone marrow and serum samples from healthy individuals. J Clin Microbiol 2002;40:933-6. [PubMed]
2. Corcioli F, Zakrzewska K, Rinieri A, Fanci R, Innocenti M, Civinini, R, De Giorgi V, Di Lollo S, Azzi A. Tissue persistence of parvovirus B19 genotypes in asymptomatic persons. J Med Virol 2008;80:2005-11. [PubMed]
3. Cassinotti P, Siegl G, Michel BA, Bruhlmann P. Presence and significance of human parvovirus B19 DNA in synovial membranes and bone marrow from patients with arthritis of unknown origin. J Med Virol 1998;56:199-204. [PubMed]
4. Parsyan A, Candotti D. Human erythrovirus B19 and blood transfusion - an update. Transfus Med 2007;17:263-78. [PubMed]
5. Heegaard ED, Laub Petersen B. Parvovirus B19 transmitted by bone marrow. Br J Haematol 2000;111:659-61. [PubMed]
6. Yango A Jr., Morrissey P, Gohh R, Wahbeh A. Donor-transmitted parvovirus infection in a kidney transplant recipient presenting as pancytopenia and allograft dysfunction. Transpl Infect Dis 2002;4:163-6. [PubMed]
7. Lui SL, Luk WK, Cheung CY, Chan TM, Lai KN, Peiris JS. Nosocomial outbreak of parvovirus B19 infection in a renal transplant unit. Transplantation 2001;71:59-64. [PubMed]
8. Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis 2006;43:40-8. [PubMed]
9. Jonetzko P, Graziadei I, Nachbaur K, Vogel W, Pankuweit S, Zwick R, Pachinger O, Poelzl G. Fatal course of parvovirus B19-associated myocarditis in a female liver transplant recipient. Liver Transpl 2005;11:463-6. [PubMed]
10. Klumpen HJ, Petersen EJ, Verdonck LF. Severe multiorgan failure after parvovirus B19 infection in an allogeneic stem cell transplant recipient. Bone Marrow Transplant 2004;34:469-70. [PubMed]
11. Moudgil A, Shidban H, Nast CC, Bagga A, Aswad S, Graham SL, Mendez R, Jordan SC. Parvovirus B19 infection-related complications in renal transplant recipients: treatment with intravenous immunoglobulin. Transplantation 1997;64:1847-50. [PubMed]
12. Laurenz M, Winkelmann B, Roigas J, Zimmering M, Querfeld U, Muller D. Severe parvovirus B19 encephalitis after renal transplantation. Pediatr Transplant 2006;10:978-81. [PubMed]
13. Lee PC, Hung CJ, Lin YJ, Wang JR, Jan MS, Lei HY. A role for chronic parvovirus B19 infection in liver dysfunction in renal transplant recipients? Transplantation 2002;73:1635-9. [PubMed]
14. Liefeldt L, Plentz A, Klempa B, Kershaw O, Endres AS, Raab U, Neumayer HH, Meisel H, Modrow S. Recurrent high level parvovirus B19/genotype 2 viremia in a renal transplant recipient analyzed by real-time PCR for simultaneous detection of genotypes 1 to 3. J Med Virol 2005;75:161-9. [PubMed]
15. Waldman M, Kopp JB. Parvovirus B19 and the kidney. Clin J Am Soc Nephrol 2007;2 Suppl 1:S47-56. [PubMed]
16. Solano C, Juan O, Gimeno C, Garcia-Conde J. Engraftment failure associated with peripheral blood stem cell transplantation after B19 parvovirus infection. Blood 1996;88:1515-7. [PubMed]
17. Kurtzman GJ, Cohen BJ, Field AM, Oseas R, Blaese RM, Young NS. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J Clin Invest 1989;84:1114-23. [PubMed]
18. Kurtzman GJ, Ozawa K, Cohen B, Hanson G, Oseas R, Young NS. Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med 1987;317:287-94. [PubMed]
19. Broliden K. Parvovirus B19 infection in pediatric solid-organ and bone marrow transplantation. Pediatr Transplant 2001;5:320-30. [PubMed]
20. Manaresi E, Gallinella G, Zuffi E, Bonvicini F, Zerbini M, Musiani M. Diagnosis and quantitative evaluation of parvovirus B19 infections by real-time PCR in the clinical laboratory. J Med Virol 2002;67:275-81. [PubMed]
21. Baylis SA, Shah N, Minor PD. Evaluation of different assays for the detection of parvovirus B19 DNA in human plasma. J Virol Methods 2004;121:7-16. [PubMed]
22. Hokynar K, Norja P, Laitinen H, Palomaki P, Garbarg-Chenon A, Ranki A, Hedman K, Soderlund-Venermo M. Detection and differentiation of human parvovirus variants by commercial quantitative real-time PCR tests. J Clin Microbiol 2004;42:2013-9. [PubMed]
23. Braham S, Gandhi J, Beard S, Cohen B. Evaluation of the Roche LightCycler parvovirus B19 quantification kit for the diagnosis of parvovirus B19 infections. J Clin Virol 2004;31:5-10. [PubMed]
24. Harder TC, Hufnagel M, Zahn K, Beutel K, Schmitt HJ, Ullmann U, Rautenberg P. New LightCycler PCR for rapid and sensitive quantification of parvovirus B19 DNA guides therapeutic decision-making in relapsing infections. J Clin Microbiol 2001;39:4413-9. [PubMed]
25. Cassinotti P, Siegl G. Quantitative evidence for persistence of human parvovirus B19 DNA in an immunocompetent individual. Eur J Clin Microbiol Infect Dis 2000;19:886-7. [PubMed]
26. Bergen GA, Sakalosky PE, Sinnott JT. Transient aplastic anemia caused by parvovirus B19 infection in a heart transplant recipient. J Heart Lung Transplant 1996;15:843-5. [PubMed]
27. Chang FY, Singh N, Gayowski T, Marino IR. Parvovirus B19 infection in a liver transplant recipient: case report and review in organ transplant recipients. Clin Transplant 1996;10:243-7. [PubMed]
28. Janner D, Bork J, Baum M, Chinnock R. Severe pneumonia after heart transplantation as a result of human parvovirus B19. J Heart Lung Transplant 1994;13:336-8. [PubMed]
29. Kumar J, Shaver MJ, Abul-Ezz S. Long-term remission of recurrent parvovirus-B associated anemia in a renal transplant recipient induced by treatment with immunoglobulin and positive seroconversion. Transpl Infect Dis 2005;7:30-3. [PubMed]
30. Liang TB, Li DL, Yu J, Bai XL, Liang L, Xu SG, Wang WL, Shen Y, Zhang M, Zheng SS. Pure red cell aplasia due to parvovirus B19 infection after liver transplantation: a case report and review of the literature. World J Gastroenterol 2007;13:2007-10. [PubMed]
31. Renoult E, Bachelet C, Krier-Coudert MJ, Diarrassouba A, Andre JL, Kessler M. Recurrent anemia in kidney transplant recipients with parvovirus B19 infection. Transplant Proc 2006;38:2321-3. [PubMed]
32. Ballou WR, Reed JL, Noble W, Young NS, Koenig S. Safety and immunogenicity of a recombinant parvovirus B19 vaccine formulated with MF59C.1. J Infect Dis 2003;187:675-8. [PubMed]
Table 1: Manifestations of Parvovirus B19 Infection in Solid Organ Transplant or Hematopoietic Stem Cell Transplant Recipients
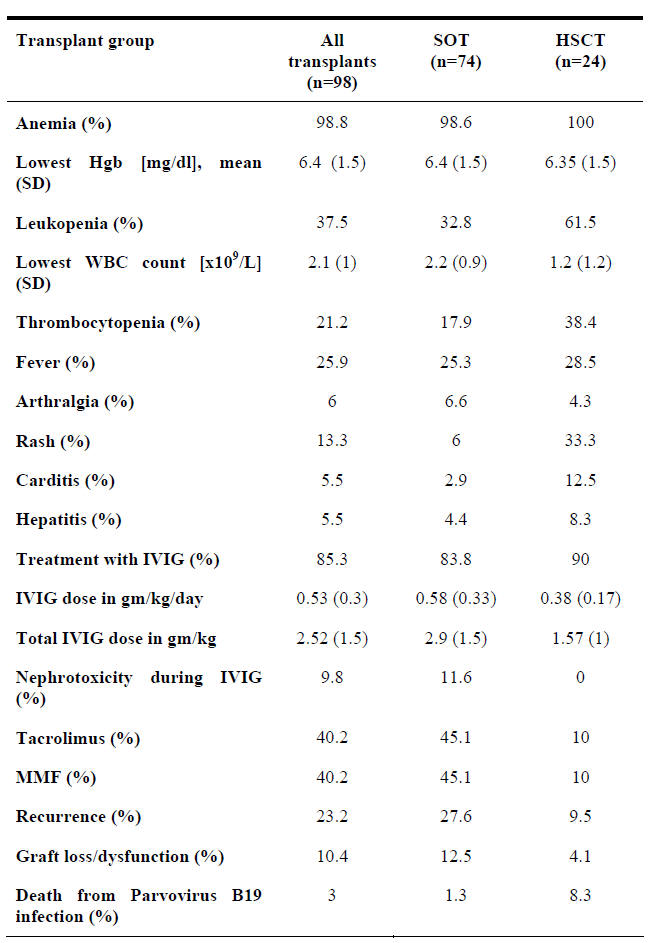
SOT=Solid organ transplant
HSCT = Hematopoietic stem cell transplant
MMF = Mycophenolate mofetil
PVB19 = Parvovirus B19
Hgb = Hemoglobin
IVIG = Intravenous immunoglobulin
SD = Standard deviation.
What's New?
Douvoyiannis, M. Neurologic Manifestations Associated with Parvovirus B19 Infection. Clin Infect Dis 2009;48:1713-1723.
GUIDED MEDLINE SEARCH FOR
review artcles
Bonvicini F, Musiani M, Zerbini M. Human Parvovirus
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
History
Bassols, AC. Parvovirus B19 and the New Century. Clin Infect Dis. 2008 Feb 15;46(4):537-9.
