

Osteomyelitis - Types
Acute Hematogenous Osteomyelitis
This is seen commonly in children (boys more often than girls) involving metaphysis or epiphysis of long bones most often in the lower extremity. The anatomy of metaphysical region explains the clinical localization of the disease. Non-anastamotic capillary ends of nutrient artery make sharp loops and enter a system of venous sinusoids with low and turbulent blood flow. These capillaries of the nutrient artery are essentially end arteries. Any capillary obstruction therefore causes avascular necrosis. In addition, the metaphysial capillaries and sinusoidal veins lack phagocytes. The cumulative effects of these factors when preceded by minor trauma in an infant or a child produce a small hematoma, vascular occlusion and bone necrosis. This can be invaded by blood-borne pathogen leading to a focus of medullary osteomyelitis.
Bacteria responsible for hematogenous osteomyelitis in infants include S. aureus, Streptococcus agalactiae and Escherichia coli. Whereas in children over the age of 1 to 4 years staphylococcus aureus, Streptococcus pyogenes and H. influenzae are most commonly isolated. With the recent immunization program, the overall incidence of H. influenza osteomyelitis has markedly decreased. In adults with hematogenous osteomyelitis, Staphylococcus aureus is the most common organism isolated. Pseudomonas osteomyelitis is seen in drug addicts and has predilection for spine (Table 1).
Clinical features include chills, fever, malaise, pain and local swelling of the affected limb. Children may present with abrupt fever, irritability, and lethargy along with inability to move the affected extremity and pain or passive movement. In approximately 50% of the cases there may be minimal to no fever, vague complaints including pain of the involved extremity over several months. In general, the outcome of acute osteomyelitis in children is good if antibiotic therapy is directed at the responsible pathogen within seven to ten days of the onset of illness.
Table 1: Microorganisms in Acute Hematogenous Osteomyelitis
| Organism | Patient Category |
|
Staphylococcus aureus Group B Streptococcus Escherichia coli |
Infants |
|
Staphylococcus aureus Streptococcus pyogenes Hemophilus influenzae |
Children (up to 4 years of age) |
|
Staphylococcus aureus |
21 years or older |
|
Gram negative rods |
Elderly |
|
Candida spp. |
Patients with intravascular devices or history of candidemia |
|
Staphylococcus aureus Pseudomonas aeruginosa |
IV drug abuse |
|
Tuberculosis |
HIV, IV drug
abuse, alcoholism, homelessness, immunosupression,
history of a positive PPD and former residence in an endemic area
|
Vertebral Osteomyelitis
Vertebral osteomyelitis is usually of hematogenous origin rather than retrograde
spread via Batsons’s venous plexus. It involves lower dorsal and lumbar spine
with an occasional involvement of the cervical spine. The infection leads to a
thrombosis of one of the segmental arteries supplying two adjacent vertebrae and
the intervertebral disc resulting in Ischemia, infarction and bone necrosis.
Posterior extension can lead to epidural abscess and meningitis
![]() . Staphylococcus aureus is the most common organism although pseudomonas is seen in drug addicts.
A variety of other unusual organisms such as
Actinobacillus
actinomycetemcomitans, Kingella spp., and
Moraxella catarrhalis have been
implicated in vertebral osteomyelitis in rare occasions.
. Staphylococcus aureus is the most common organism although pseudomonas is seen in drug addicts.
A variety of other unusual organisms such as
Actinobacillus
actinomycetemcomitans, Kingella spp., and
Moraxella catarrhalis have been
implicated in vertebral osteomyelitis in rare occasions.
|
62 year old male with 1
week history of right arm weakness admitted for mental status
changes. CSF consistent with bacterial
meningitis but CSF cultures
were negative. C-spine MRI revealed C3-4 osteomyelitis and epidural
abscess. Operative cultures were positive for Group B Streptococcus. |
||
|
|
|
|
Disease usually presents with intractable back pain. Localized tenderness of the
involved segment is present in majority of the patients. Fever and leucocytosis
may be seen in 50% of the patients. Erythrocyte sedimentation rate is usually
elevated and may be used as a prognostic guide during treatment. Radiographs are
usually normal early in the disease course. Magnetic resonance imaging
![]() is the
most sensitive and specific to diagnose disc space infection. Complications of
vertebral osteomyelitis include epidural abscess, spinal cord compression,
paravertebral and retroperitoneal abscess. Motor and sensory deficits occur in
six to fifteen percent of patients.
is the
most sensitive and specific to diagnose disc space infection. Complications of
vertebral osteomyelitis include epidural abscess, spinal cord compression,
paravertebral and retroperitoneal abscess. Motor and sensory deficits occur in
six to fifteen percent of patients.
|
68 year old female
developed Candida glabrata candidemia as a complication of TPN. Was
treated with antifungal but returned 6 months later with back pain
and imaging consistent with vertebral osteomyelitis. Surgical
cultures were positive for Candida glabrata. |
||
|
|
|
|
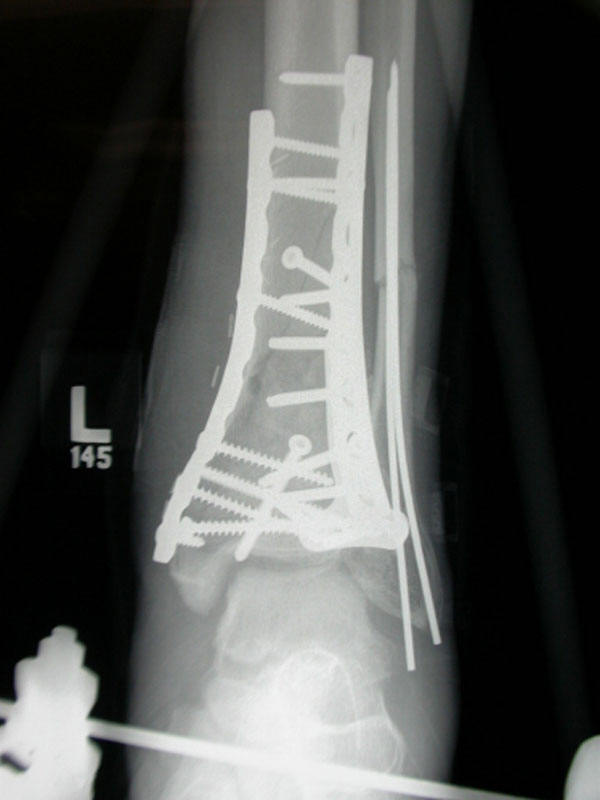
Osteomyelitis Secondary to Contiguous Infection
Contiguous osteomyelitis occurs when the microorganisms are introduced into bone
by trauma, nosocomial contamination following surgical procedure ![]() (a)and extension
from adjacent soft tissue infection. Predisposing factors include open
fractures, internal fixation devices
(a)and extension
from adjacent soft tissue infection. Predisposing factors include open
fractures, internal fixation devices
![]() (b), prosthetic devices
(b), prosthetic devices
![]() (c) and chronic soft tissue
infection
(c) and chronic soft tissue
infection
![]() (d). Multiple organisms are usually isolated from the bone although
staphylococcus aureus and staphylococcus epidermidis are the most prevalent
pathogens.
(d). Multiple organisms are usually isolated from the bone although
staphylococcus aureus and staphylococcus epidermidis are the most prevalent
pathogens.
| a.) 65 year old female with sternal osteomyelitis following a CABG. Photo is taken at bedside following extensive sternal debridement. Definitive closure with a muscle flap had yet to be performed. | d.) 46 year old cirrhotic with chronic osteomyelitis secondary to an open tibial fracture. Pt subsequently developed a complicated skin and soft tissue infection with a draining sinus tract. Cultures were positive for E. coli and MRSA.46 year old cirrhotic with chronic osteomyelitis secondary to an open tibial fracture. Pt subsequently developed a complicated skin and soft tissue infection with a draining sinus tract. Cultures were positive for E. coli and MRSA. | |
|
|
|
|
| c.) Prosthetic devices | ||
|
|
|
|
| b.) internal fixation devices | ||||
 |
|
|
|
|
| b.) Expoosed orthopedic hardware with associated chronic osteomyelitis. | b.) Antibiotic beads inserted into an area of osteomyelitis. |
|
|
|
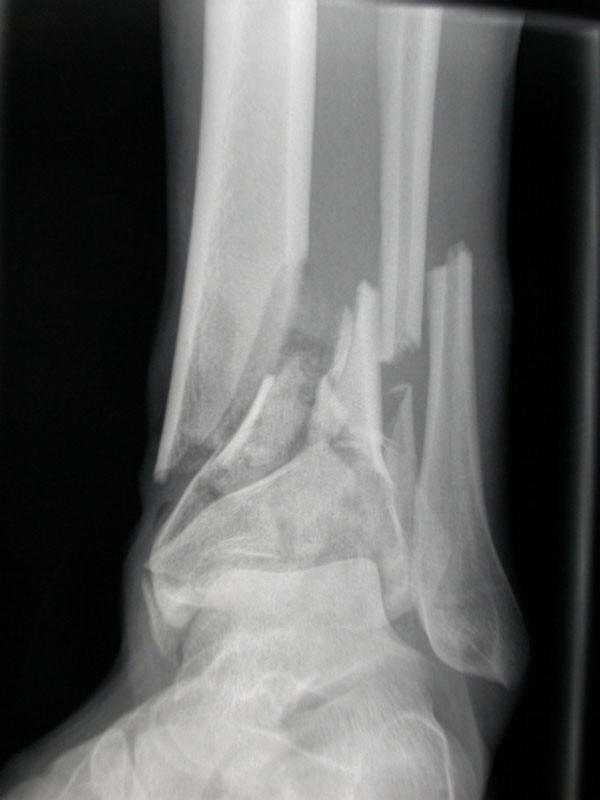
Osteomyelitis Secondary to Vascular Insufficiency
This is a special category seen predominantly in patients with diabetes and
peripheral vascular disease and is localized almost exclusively to lower
extremities. Neuropathy, Ischemia
![]() (a) and biomechanical dysfunction lead to foot
ulcers
(a) and biomechanical dysfunction lead to foot
ulcers
![]() (b). Infection is seen as a consequence of ulcer, which progressively burrows
its way into small bones of the feet. Multiple organisms are usually isolated
although S. aureus and S. agalactiae still predominate. Patients may present
with non-healing foot ulcer, cellulitis
(b). Infection is seen as a consequence of ulcer, which progressively burrows
its way into small bones of the feet. Multiple organisms are usually isolated
although S. aureus and S. agalactiae still predominate. Patients may present
with non-healing foot ulcer, cellulitis
![]() (c) or deep abscess
(c) or deep abscess
![]() (d). Fever and systemic
toxicity are often absent unless there is severe limb threatening infection with
gangrene and fasciitis. Pain may be absent in patients with severe neuropathy.
Osteomyelitis should be suspected when bone is exposed before or after
debridement or probing the ulcer with a stainless steel probe, bone is
encountered. Resection of the infected bone is almost always necessary for a
favorable outcome.
(d). Fever and systemic
toxicity are often absent unless there is severe limb threatening infection with
gangrene and fasciitis. Pain may be absent in patients with severe neuropathy.
Osteomyelitis should be suspected when bone is exposed before or after
debridement or probing the ulcer with a stainless steel probe, bone is
encountered. Resection of the infected bone is almost always necessary for a
favorable outcome.
| a.) Necrotic toe | a.) Moderate-to-severe diabetic foot infection with gangrene of the great toe. | b.) Calcaneal and midfoot diabetic foot ulcer. | b.) Osteomyelitis demonstrated clinically and radiographically. |
|
|
|
|
|
| b.) Mild diabetic foot infection associated with Pseudomonas. | b.) Debridement of an infected foot ulcer. | b.) Patient with Staphylococcus aureus osteomyelitis and lymphangitis | b.) Patient with Staphylococcus aureus osteomyelitis and lymphangitis |
|
|
|
|
|
| c.) Severe diabetic foot infection. | c.) Polymicrobial diabetic foot infection. | d.) Plantar abscess in a diabetic | d.) Lateral foot abscess in a diabetic patient. | d.) Soft tissue abscess of 1st MTP area. |
|
|
|
|
|
|
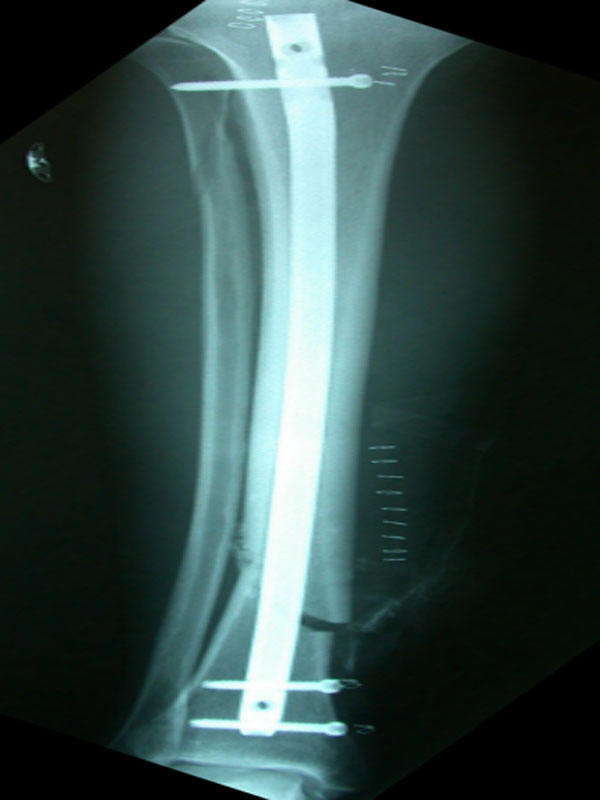
Acute Posttraumatic Osteomyelitis
Posttraumatic osteomyelitis results from pathogenic organisms that proliferate
in traumatized tissue. Traumatized tissue also results in compromised blood
supply, leading to tissue and bone necrosis, which promotes infection. Moreover,
the fixation devices
![]() that are required in the management of fractures serve as
additional foci for bacterial colonization. Other factors that contribute to the
development of osteomyelitis are the presence of hypotension, inadequate
debridement of the fracture site, malnutrition, alcoholism, and smoking. Trauma
has been reported to delay the inflammatory response to bacteria, to depress
cell-mediated immunity, and to impair function of PMNs, including chemotaxis,
superoxide production, and microbial killing.
that are required in the management of fractures serve as
additional foci for bacterial colonization. Other factors that contribute to the
development of osteomyelitis are the presence of hypotension, inadequate
debridement of the fracture site, malnutrition, alcoholism, and smoking. Trauma
has been reported to delay the inflammatory response to bacteria, to depress
cell-mediated immunity, and to impair function of PMNs, including chemotaxis,
superoxide production, and microbial killing.
| fixation devices (see above pictures - internal fixation and prosthetic devices) | |||
|
|
|
 |
 |
Open Fractures and Trauma
The presence of bacteria in an open wound is not sufficient to cause infection. Approximately 60% to 70% of open fractures are contaminated by bacteria, but a much smaller percentage develops infection and the risk of subsequent infection is highly correlated with the degree of soft tissue injury (Table 2). With type IIIB open fractures, those in which extensive soft tissue stripping does not allow for adequate coverage over the site, infection can occur in up to 40% of cases. Moreover, the bacteria recovered from clinical infections are most likely to be hospital acquired pathogens such as S. aureus or gram-negative bacilli (including Pseudomonas aeruginosa). Clinical studies indicate that open fractures that are properly debrided and covered definitively within 10-14 days have a much lower incidence of late osteomyelitis. This supports the contention that many infections are hospital acquired. However, other bacteria should be considered, depending on the environment, specifically, C. perfringens when there is soil contamination, pseudomonas and aeromonas hydrophilia following fresh water injury, and vibrio and erysipelothrix in salt water injury.
Overall, the incidence of infection in Grade I open fractures is nearly zero, that of grade II open fractures is below 5% and depending on the degree of contamination, in Grade III fractures, the infection rate is between 5-40%. Again, the hallmark of treatment is adequate debridement and soft tissue coverage. With recent advance such as vacuum assisted closure (V.A.C.), definitive free tissue transfer may not always be necessary. Furthermore, with complex bone regeneration techniques, an osteo-myo-cutaneous flap can be slowly transported into the defect. In the end, open fractures remain one of the most challenging problems to overcome and treat.
Table 2: Classification of Open Fractures
|
· Minor / Grade I - small punctate wound less than 1 centimeter associated with low velocity trauma. Minimal soft tissue injury. No crushing or comminution. · Moderate / Grade II - wounds which are extensive in length and width but with relatively little soft tissue damage, and only moderate crushing or comminution. · Major / Grade III - wounds of moderate or massive size with considerable soft tissue injury and/or foreign body contamination: o III A - sufficient soft tissue to cover the fracture o III B - insufficient tissue to cover the fracture; also periosteal stripping and severe comminution o III C - arterial damage requiring repair. Degree of soft tissue damage not considered |
Chronic Osteomyelitis
Acute osteomyelitis that is inappropriately treated can become chronic osteomyelitis. Chronic osteomyelitis resulting from acute osteomyelitis is often caused by S. aureus; however, chronic osteomyelitis occurring after a fracture can be poly-microbial. Gram-negative organisms are now seen in about 50% of all cases of chronic osteomyelitis. The fundamental problem in chronic osteomyelitis is devascularization of bone, leaving protected pockets of necrotic material to support bacterial growth in relative seclusion from systemic antibiotic therapy. This collection of necrotic tissue, bone and bacteria is what is termed the sequestrum and the body’s attempt to wall off the offending material with a reactive and inflammatory tissue (either bone or soft-tissue) is termed involucrum. It should be noted that this involucrum can be highly vascular and potentially viable and structural, which should be taken into consideration during surgical debridement.
Chronic Sclerosing Osteomyelitis
Chronic sclerosing osteomyelitis primarily involves the diaphyseal bones of the adolescents. Typical features include intense proliferation of the periosteum leading to bony deposits and progressive sclerosis on the radiographs. Localized pain and tenderness are the hallmarks. In all cases malignancy must be ruled out.
Brodie’s Abscess
Brodie’s abscess is characterized by local pain and low-grade fever. Radiographic changes include central destruction surrounded by sclerosis usually in the metaphysis of the long bones. Majority of cases occur in the lower extremities. The differential diagnosis includes Ewing’s sarcoma and chondroblastoma especially when localized to epiphysis.
ss="MsoNormal">ht: 150%; margin-top: 8px; margin-bottom: 8px"> Tuberculosis Osteomyelitis
The incidence of tuberculosis osteomyelitis varies based on the geographic region and patient risk factors for the disease. While it can involve any bone due to its hematogenous dissemination, it does have a predilection for affecting the spine (Pott’s disease). Clinical presentation includes a subacute onset and often time’s consideration for the disease occurs in patients with negative bacterial cultures with risk factors for tuberculosis. Risk factors include: HIV, IV drug abuse, alcoholism, homelessness, immunosupression, history of a positive PPD and former residence in an endemic area. Evaluation for TB associated osteomyelitis includes acid-fast bone cultures and ziehl-neelsen (or Kinyoun) stains, histopathology (granulomatous inflammation), chest radiograph (looking for active or evidence of previous TB) and either PPD or QuantiFERON testing. Treatment involves an extended course of multiple anti-mycobacterial agents and should be tailored based on sensitivity testing.
Osteomyelitis In A Specific Clinical Setting
Based upon a certain clinical setting, specific organisms as well as bone sites may be involved (Table 3).
Table 3. Osteomyelitis In A Special Clinical Setting
Risk Factors
Organism
Site
I.V. drug abuse
S. aureus
P. aeruginosa
Serratia
Axial skeleton
Sterno-clavicular joint
Sacro-ileac joint
Hemoglobinopathy
Salmonella
Long bones
Tooth abscess
Anaerobes
Mandible
Diabetic foot abscess
Polymicrobial
Small bones of hands and feet
Human bite
Eikenella Corrodens
Staph Aureus
Hands
Animal Bite
Pasteurella Multocida
Hands, feet, face
Puncture wound of foot
P. aeruginosa
Calcaneus
Median sternotomy
S. aureus
S. epidermidis
Sternum
Meat handlers
Brucella Flat bones
Fishermen
M. marinum Small bones of hand
Hemodialysis
S. aureus
S. epidermidis
Axial skeleton
Positive PPD
M. tuberculosis
Thoracic spine
Exposure to TB
Resident in endemic setting
Prosthetic devices
S. aureus
S. epidermidis
Extremities and joints
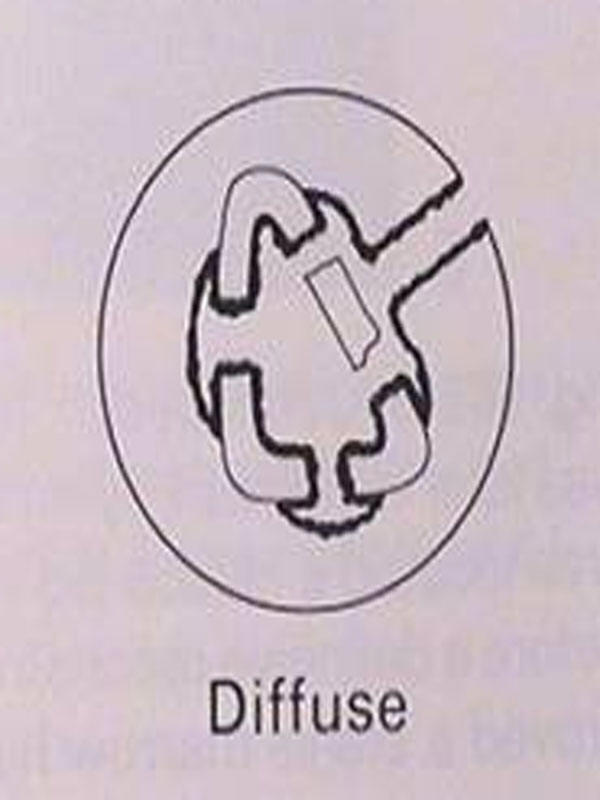
CLASSIFICATION
Historically, osteomyelitis was classified as either acute or chronic depending
on the duration of symptoms. Several classification systems have been proposed
but the most widely used system is that of Cierny and Mader (Table 4)
![]() , which identifies the extent of infection as well as the condition of the
host. This system is based on four factors: the degree of osseous involvement,
the site of involvement, the degree of impairment caused by the disease, and the
general condition of the host. Type I is medullary osteomyelitis
, which identifies the extent of infection as well as the condition of the
host. This system is based on four factors: the degree of osseous involvement,
the site of involvement, the degree of impairment caused by the disease, and the
general condition of the host. Type I is medullary osteomyelitis
![]() (a) (examples of
which include hematogenous osteomyelitis and infections of IM nails). Type II is
superficial osteomyelitis
(a) (examples of
which include hematogenous osteomyelitis and infections of IM nails). Type II is
superficial osteomyelitis
![]() (b) confined to the bone surface. Type III is localized
osteomyelitis
(b) confined to the bone surface. Type III is localized
osteomyelitis
![]() (c) involving the full thickness of the cortex but without the loss of
axial stability. Type IV is diffuse osteomyelitis
(c) involving the full thickness of the cortex but without the loss of
axial stability. Type IV is diffuse osteomyelitis
![]() (d) involving the circumference of
the cortex and loss of axial stability. The general condition of the patient is
based on those factors that affect the response to infection and treatment.
Class A patients have normal systemic defenses, metabolic capabilities, and
vascular supply to the limb. Class B patients have a local (trauma, prior
surgery, local inflammation) or systemic (immunosuppressed, on corticosteroids,
peripheral vascular disease) deficiency. Class C patients are those in whom the
treatment of the disease (the infection) is worse than the infection itself. In
other words, there is greater potential morbidity or mortality from treatment,
than from the infection (or non curative treatment such as suppression). Pairing
the four types of osteomyelitis with the three host classes, Cierny et al
proposed a detailed treatment regimen, which defined optimal treatment
modalities for each stage. As one would expect, class A hosts fared the best
with success rates of 98% were achieved, even in type IV osteomyelitis. For
compromised, class B hosts, success rates were far lower. Depending on anatomic
type, success ranged from 79% to 92%.
(d) involving the circumference of
the cortex and loss of axial stability. The general condition of the patient is
based on those factors that affect the response to infection and treatment.
Class A patients have normal systemic defenses, metabolic capabilities, and
vascular supply to the limb. Class B patients have a local (trauma, prior
surgery, local inflammation) or systemic (immunosuppressed, on corticosteroids,
peripheral vascular disease) deficiency. Class C patients are those in whom the
treatment of the disease (the infection) is worse than the infection itself. In
other words, there is greater potential morbidity or mortality from treatment,
than from the infection (or non curative treatment such as suppression). Pairing
the four types of osteomyelitis with the three host classes, Cierny et al
proposed a detailed treatment regimen, which defined optimal treatment
modalities for each stage. As one would expect, class A hosts fared the best
with success rates of 98% were achieved, even in type IV osteomyelitis. For
compromised, class B hosts, success rates were far lower. Depending on anatomic
type, success ranged from 79% to 92%.
a.) Stage 1: medullary b.) Stage 2: superficial c.) Stage 3: localized d.) Stage 4: diffuse Infection limited to the intramedullary surfaces of bone (i.e. infected intramedullary rod) Contiguous focus of infection (i.e. exposed surface of bone at the base of a soft tissue wound) Full-thickness cortical sequestration that can be removed without compromising bony stability Through and through infection requiring intercalary resection of bone and loss of stability
Host Class Description
A host Normal host B host Systemic compromise (Bs)
- Malnutrition
- Renal, hepatic failure
- Diabetes mellitus
- Chronic hypoxia
- Immune disease
- Malignancy
- Extremes of age
Immunosuppression or Immune deficiency
Local compromise (Bl)
Chronic lymphedema
Venous stasis
Major vessel compromise
Arteritis
Extensive scarring
Radiation fibrosis
Small vessel disease
C host Systemic and local compromise (Bsl) Treatment worse than disease