

Osteomyelitis - Diagnosis
CLINICAL MANIFESTATIONS
A history of infection or inter-current illness as well as remote surgery or
trauma should raise the clinical suspicion for osteomyelitis. Normal signs of
inflammation (rubor, calor, dolor, tumor) may be absent and thus the diagnosis
of infection when clinical signs are masked can be difficult. Patients can often
have a history of infection of another site, such as the lungs, bladder, or
skin, especially with a history of trauma. They usually complain of substantial
pain in the affected area. Moreover, reduced activity, malaise, and anorexia may
be exhibited. Local findings include swelling and warmth, occasional redness,
tenderness to palpation, drainage
![]() , and restricted range of motion of adjacent
joints. With a history of trauma, clinical risk factors for infection include a
history of open fracture, severe soft tissue injury, a history of substance
abuse and smoking, inadequate previous treatment, and an immunocompromised
state.
, and restricted range of motion of adjacent
joints. With a history of trauma, clinical risk factors for infection include a
history of open fracture, severe soft tissue injury, a history of substance
abuse and smoking, inadequate previous treatment, and an immunocompromised
state.
| 46 year old cirrhotic with chronic osteomyelitis secondary to an open tibial fracture. Pt subsequently developed a complicated skin and soft tissue infection with a draining sinus tract. Cultures were positive for E. coli and MRSA. | 65 year old with orthopedic hardware in the left ankle presented with redness of the leg and consitutional symptoms. Examination revealed a sinus tract (arrow) draining purulent material that was positive for a coagulase negative staphylococcus. Patient is currently on long term suppression with oral antibiotics waiting for hardware to be removed. | |
|
|
|
|
DIAGNOSIS
Laboratory Findings
Routine blood cultures are of little help unless the patients have manifestations of systemic disease, such as in hematogenous osteomyelitis (Cierny and Mader Type I). Blood cultures are positive in about 50% to 75% in such cases. Laboratory changes suggestive of infection include elevations in the white blood cell (WBC) count, and elevations in the C-reactive protein (CRP) and Erythrocyte Sedimentation Rate (ESR) levels. The erythrocyte sedimentation rate may be normal in the first 48 hours but rises to levels above 100 mm/h, and may remain elevated for weeks. The CRP/ESR can be an excellent screening tool to measure response to treatment. (Figure 2)
Figure 2: Graph of ESR/CRP response to surgical and antibiotic treatment in chronic osteomyelitis. Note drop of both CRP (top) and ESR (bottom) after surgical intervention red arrow. Antibiotics alone were initiated at black arrow.
Radiographic Imaging
Radiographic changes may not be seen for at least 10 days. When present, it
usually signifies trabecular bone destruction
![]() . If the infection spreads to the
cortex (usually within 3 to 6 weeks), a periosteal reaction may be evident on
plain radiographs. Unfortunately, radiologic findings in the initial
presentation of acute osteomyelitis are often normal. The most common
radiographic sign of bone infection is rarefaction, representing diffuse
demineralization secondary to inflammatory hyperemia soft tissue swelling with
obliteration of tissue planes, trabecular destruction, lysis and cortical
permeation, periosteal reactions and involucrum formation. One study reported
that in cases of eventually proven osteomyelitis, 5% of radiographs were
abnormal initially, 33% were abnormal by 1 week, and 90% were abnormal by 4
week.
. If the infection spreads to the
cortex (usually within 3 to 6 weeks), a periosteal reaction may be evident on
plain radiographs. Unfortunately, radiologic findings in the initial
presentation of acute osteomyelitis are often normal. The most common
radiographic sign of bone infection is rarefaction, representing diffuse
demineralization secondary to inflammatory hyperemia soft tissue swelling with
obliteration of tissue planes, trabecular destruction, lysis and cortical
permeation, periosteal reactions and involucrum formation. One study reported
that in cases of eventually proven osteomyelitis, 5% of radiographs were
abnormal initially, 33% were abnormal by 1 week, and 90% were abnormal by 4
week.
| Xray of great toe demonstrating reabsorption and bone destruction. | Osteomyelitis demonstrated clinically and radiographically. | Xray demonstrating great toe regeneration after antibiotic treatment. | Sequential radiographic demonstration of 1st metatarsal destruction. |
|
|
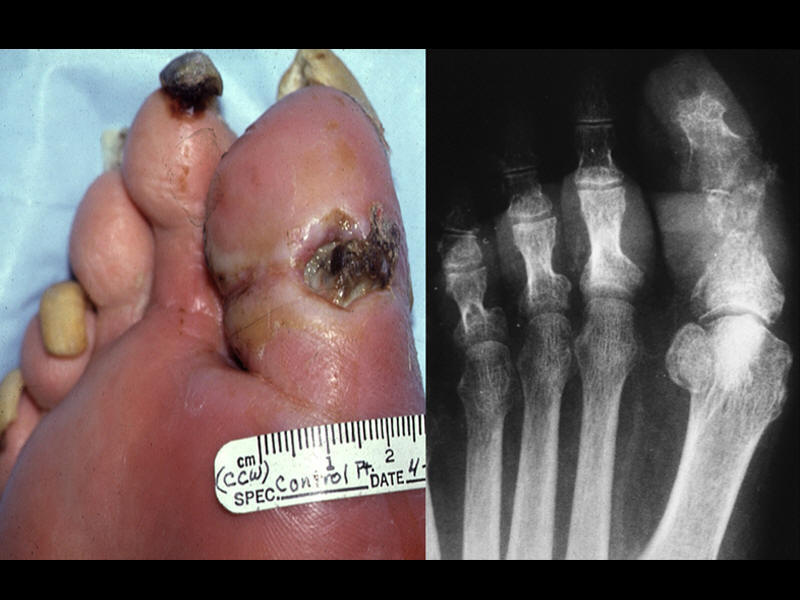 |
|
|
Nuclear Medicine
Radionuclide Scintigraphy is a very useful diagnostic tool and can be performed
with various algorithms. Technetium-99m is the principal radioisotope employed
in most bone scans. After intravenous injection, there is rapid distribution of
this agent throughout the extra cellular fluid. Within several hours, more than
half the dose will accumulate in bone, while the remainder is excreted in the
urine. There is evidence to suggest that the technetium phosphates bind to both
the organic and the inorganic matrix. There is preferential incorporation into
metabolically active bone. Following the initial injection, dynamic images are
captured over the specified region. These are followed by static images at later
time points. The first phase represents the blood flow phase, the second phase
immediately post-injection represents the bone pooling phase, and the third
phase is a delayed image made at 3 hours when there is decreased soft tissue
activity. Classically, osteomyelitis presents as a region of increased blood
flow and should appear “hot” on all phases, with focal uptake in the third
phase. Other processes such as a healing fracture, a loose prosthesis, and
degeneration do not appear hot
![]() in the early phase despite a hot appearance in
the delayed phase. Reported sensitivities of bone scintigraphy for the detection
of osteomyelitis vary considerably from 32% to 100%. Reported specificities have
ranged from 0% to 100%.
in the early phase despite a hot appearance in
the delayed phase. Reported sensitivities of bone scintigraphy for the detection
of osteomyelitis vary considerably from 32% to 100%. Reported specificities have
ranged from 0% to 100%.
| Bone scan demonstrating osteomyelitis | Positive Bone scan for osteomyelitis | Bone scan demostrating osteomyelitis |
|
|
|
|
Gallium-67 citrate binds rapidly to serum proteins, particularly transferrin. There is uptake in the blood, especially by leukocytes. Gallium has been used in conjunction with technetium-99 to increase the specificity of the bone scan. Several mechanisms have been postulated to explain the increased activity at sites of inflammation. Bacteria have high iron requirements and thus avidly take up gallium. Gallium is strongly bound to bacterial siderophores and leukocyte lactoferins. In a typical study, gallium is injected intravenously and delayed images are acquired (at 48 to 72 hours). The hallmark of osteomyelitis is focal increased uptake of gallium. Unfortunately, gallium's non-specific bone uptake can be problematic since any processes causing reactive new bone formation will “light up.” In the case of patients with fractures or prosthesis, osteomyelitis cannot be diagnosed with gallium alone. Most authors will interpret gallium images along with bone scans. The reported sensitivities and specificities for the diagnosis of osteomyelitis range from 22% to 100% and 0% to 100%, respectively.
Indium-111 or 99mTC-HMPAO (Ceretec) labeled leukocyte scan is useful as a confirmatory test following a positive Tc-RBC bone scan. The leukocytes migrate to the region of active infection so that the scan can confirm the presence of an active inflammatory reaction. The use of a combined red cell and white cell scan increases both the sensitivity and specificity significantly, and now represents the gold standard of radionuclide testing for infection. Leukocytes are labeled and then re-injected, where they redistribute in the intravascular space. Immediate images thereafter show activity in the lungs, liver, spleen, and blood pool. The half-life is about 7 hours. After 24 hours, only the liver, spleen, and bone marrow show activity. Normal-healing wounds and fully treated infections show no increase in uptake. Leukocytes that migrate to an area of active bone infection will show increased uptake. Most results show improved sensitivity (80–100%) and specificity (50–100%) for the diagnosis of osteomyelitis. Indium-labeled WBC scans are generally superior to bone scans and gallium scans in the detection of infection. In one study, evaluating the diagnostic utility of indium scans in 39 patients with suspected osteomyelitis, confirmed by bone biopsy, Indium scans were 97% sensitive and 82% specific for osteomyelitis. The few false-positive results occurred in patients with overlying soft tissue infections. An accompanying bone scan can help to differentiate bone infection from soft tissue infection. In these situations, the indium scan should be performed before the bone scan to avoid false-positive results (from the remaining technetium uptake). With both tests, the sensitivities and specificities are in excess of 90%. Until recently, a clinician investigating for the site of infectious foci using nuclear medicine had a choice between 67Ga-citrate imaging and 111In-oxine leukocyte imaging, but scientific advances (especially in nuclear medicine) have increased these choices considerably, and continue to increase them. Several techniques in nuclear medicine significantly aid infection diagnosis, including imaging with 99mTc-hexamethylpropyleneamine oxime (99mTc-HMPAO (Ceretec) and 99mTc-stannous fluoride colloid-labelled leukocytes. Each radiopharmaceutical has specific advantages and disadvantages that make it suitable to diagnose different infectious processes (e.g., soft-tissue sepsis, osteomyelitis, abscesses, and infections commonly found in immunocompromised patients).
Marrow scanning
![]() is also increasingly used for diagnosis of infection. With use
of microcolloid bone marrow scans, more information is available to determine
whether there is truly an infection. There is the possibility of leukocyte
accumulation with certain inflammatory conditions that could result in a false
positive indium scan. An infection will tend to suppress marrow activity and
thus render the marrow scan cold, while the white cell scan will still be hot.
If the white cell scan is hot as is the marrow scan, it is possible that an
infection may not be present. One study examined technetium labeled white cell
scans (Tc-HMPAO) versus Tc microcolloid marrow scans in total joints. They found
that in 77 patients, the white cell scans had a sensitivity of 96% and
specificity or 30% by itself. When the colloid scan was added, the sensitivity
went down to 93% but the specificity went up to 98%. The addition of a regular
red cell scan was not helpful. In another study, an indium white cell scan was
compared to technetium sulphur colloid scans to differentiate infection from
Charcot arthropathies. They found that white cell scans were positive in 4 out
of 20 cases, of which three were infected. In the sixteen negative white cell
scans, the marrow scan was also negative. However, in the four positive cases,
the marrow scan was positive in two cases which were confirmed to be infected.
They concluded that white cell scans can be positive in hematopoetically active
bones which can occur in the absence of infection and that marrow scans should
be used to confirm the diagnosis.
is also increasingly used for diagnosis of infection. With use
of microcolloid bone marrow scans, more information is available to determine
whether there is truly an infection. There is the possibility of leukocyte
accumulation with certain inflammatory conditions that could result in a false
positive indium scan. An infection will tend to suppress marrow activity and
thus render the marrow scan cold, while the white cell scan will still be hot.
If the white cell scan is hot as is the marrow scan, it is possible that an
infection may not be present. One study examined technetium labeled white cell
scans (Tc-HMPAO) versus Tc microcolloid marrow scans in total joints. They found
that in 77 patients, the white cell scans had a sensitivity of 96% and
specificity or 30% by itself. When the colloid scan was added, the sensitivity
went down to 93% but the specificity went up to 98%. The addition of a regular
red cell scan was not helpful. In another study, an indium white cell scan was
compared to technetium sulphur colloid scans to differentiate infection from
Charcot arthropathies. They found that white cell scans were positive in 4 out
of 20 cases, of which three were infected. In the sixteen negative white cell
scans, the marrow scan was also negative. However, in the four positive cases,
the marrow scan was positive in two cases which were confirmed to be infected.
They concluded that white cell scans can be positive in hematopoetically active
bones which can occur in the absence of infection and that marrow scans should
be used to confirm the diagnosis.
Abnormal marrow scan suggesting osteomyelitis
In general, a total body bone scan (RBC) alone is of little value. The best potential for determining infection is to add the white cell scan as well as the marrow scan. It is even more important that the radiologist know the clinical history, view the radiographs and interpret the scintigraphs in such context. Otherwise, a rather vague and generic reading will be obtained that has little clinical value to the general practitioner. Ongoing data are being presented that may find the regular total body bone scan to be of lesser value when compared to the pair if marrow scan and white cell scanning. Generally speaking however, all three test should be order and will take approximately one week to obtain.
Magnetic Resonance Imaging
Magnetic resonance imaging continues to play an important role in the evaluation
of musculoskeletal infections. The sensitivity and specificity of Magnetic
Resonance Imaging (MRI) imaging for osteomyelitis range from 60% to 100% and 50%
to 90% respectively. It has the spatial resolution necessary to evaluate
accurately the extent of the infection in preparation for surgical treatment and
localizes any abscess cavities. T1 and T2 weighted imaging is usually
sufficient, fat suppression and STIR (Short TI Inversion Recovery) sequences may
be added to better image bone marrow and soft tissue abnormalities. It also has
the ability to differentiate between infected bone and involved adjacent soft
tissue structures. Images can be acquired in any orientation and there is no
radiation exposure. Gadolinium enhancement should be obtained in the
postoperative population to better differentiate post surgical artifact from
infection-related bone marrow edema patterns. Gadolinium may better
differentiate abscess formation from diffuse inflammatory changes and
non-infectious fluid collections. Characteristically, active osteomyelitis
displays a decreased signal on T1-weighted images ![]() and appears bright on
T2-weighted images
and appears bright on
T2-weighted images
![]() . The process represents the replacement of marrow fat with
water from edema, exudate, hyperemia, and ischemia. The MRI signal
characteristics that reflect osteomyelitis are intrinsically non-specific:
tumors and fractures can also increase the marrow water content. In patients
without prior complications, MRI has been found to be sensitive (but not
specific) for osteomyelitis. When a fracture or prior surgery is evident, MRI is
less specific in the diagnosis of infection. Furthermore, in the presence of
metallic implants, the artifact makes it difficult to comment on areas near the
implant, which may be of primary interest. When no metallic implants exist, an
MRI should be used to help stage the infection, to help determine the extent of
both bone and soft tissue infection, and to differentiate between non-specific
marrow changes and infection.
. The process represents the replacement of marrow fat with
water from edema, exudate, hyperemia, and ischemia. The MRI signal
characteristics that reflect osteomyelitis are intrinsically non-specific:
tumors and fractures can also increase the marrow water content. In patients
without prior complications, MRI has been found to be sensitive (but not
specific) for osteomyelitis. When a fracture or prior surgery is evident, MRI is
less specific in the diagnosis of infection. Furthermore, in the presence of
metallic implants, the artifact makes it difficult to comment on areas near the
implant, which may be of primary interest. When no metallic implants exist, an
MRI should be used to help stage the infection, to help determine the extent of
both bone and soft tissue infection, and to differentiate between non-specific
marrow changes and infection.
| 62 year old male with 1 week history of right arm weakness admitted for mental status changes. CSF consistent with bacterial meningitis but CSF cultures were negative. C-spine MRI revealed C3-4 osteomyelitis and epidural abscess. Operative cultures were positive for Group B Streptococcus. | ||
|
|
|
|
CT, PET, SPECT, Biopsy, Culture, and Molecular Diagnostics
Computed tomography has assumed a lesser role in the evaluation of osteomyelitis with the widespread use of MRI. It remains unsurpassed, however, in the imaging of cortical bone. It is especially useful in delineating the cortical details in chronic osteomyelitis, such as sequestra and foreign bodies. It also is useful in evaluating the adequacy of cortical debridement in the staged treatment of chronic osteomyelitis. Thus, it can help differentiate between type III and type IV infections. It should be used with fine cuts and can be ordered with contrast to help with surgical planning. It is most useful when trying to establish the structural integrity or permeation of the infection into the bone. It is not useful when there is a lot of metal implant present unless special software manipulations are done to minimize the artifact.
The use of fluorine-18 labeled 2-fluoro-2-deoxy- d-glucose positron emission tomography FDG (PET) scanning enables non-invasive detection and demonstration of the extent of chronic osteomyelitis with 97% accuracy. Positron emission tomography is especially accurate in the central skeleton within active bone marrow. While it is not yet in widespread use, it remains an adjunctive tool if other methods fail. The overall accuracy of FDG-PET in evaluating infection involving orthopaedic hardware was 96.2% for hip prosthesis, 81% for knee prosthesis, and 100% in 15 patients with other orthopaedic devices. Among the patients having chronic osteomyelitis, the accuracy is 91%. FDG- PET appears to be a sensitive and specific method for the detection of infectious foci due to metallic implants in patients with trauma. Sensitivity, specificity, and accuracy were 100%, 93.3%, and 97%, respectively, for all PET data; 100%, 100%, and 100%, respectively, for the central skeleton; and 100%, 87.5%, and 95%, respectively, for the peripheral skeleton. 18F-FDG-PET used to investigate infection in postoperative spine holds promise to become the standard imaging technique in this difficult patient population, with sensitivity, specificity and accuracy at 100%, 81%, and 86%, respectively, in a recent study. The accurate differentiation between synovitis, loosening or infection is often difficult with conventional X-rays, arthrography, or bone scintigraphy. Results suggest that FDG PET could be a useful tool for differentiating between infected and loose orthopaedic prostheses as well as for detecting only inflammatory tissue such as synovitis. Unfortunately, the study is expensive and not covered by insurance carriers. Ongoing studies may find it to be more efficacious than the sequence of scintigraphy, but until such studies demonstrate cost efficacy, it may be of less value to the clinician.
Single Photon Emission Computed Tomography (SPECT) provides a qualitative and quantitative look at the volume distribution of biologically significant radiotracers after injection into the human body. SPECT has been used to diagnose spinal prosthetic infection. The overall sensitivity of Tc-99m HMPAO leukocyte scan with SPECT to detect bone infection was 92%, with a specificity rate of 85%. The bone biopsy/culture is another important method to identify the bacteria and determine the appropriate antibiotic. The procedure can usually be done under fluoroscopic guidance. While sinus tract cultures can be helpful, they should not be the sole guide for antibiotic treatment. In a prospective study, Mousa found that 88.7% of deep sinus tract isolates were identical to operative specimens in 55 patients with chronic bone infection. These results were dependent on aspiration of material by syringe from the depths of an active flowing sinus and immediate inoculation on culture media. Bone biopsy remains the preferred diagnostic procedure in chronic osteomyelitis. Histological and microbiologic evaluation of percutaneous biopsy samples should be combined in cases of suspected osteomyelitis. The sensitivity of culture in the diagnosis of osteomyelitis could be improved from 42% to 84% by the addition of histological evaluation.
Molecular Diagnostics are being developed for diagnosis in osteomyelitis because some infections remain without an identified pathogen, when using standard techniques. The most commonly used method for the diagnosis of orthopaedic infections is the polymerase chain reaction. Sequences within bacterial 16S ribosomal RNA have served as targets for amplification and detection. The Polymerized Chain Reaction has been used to identify very small remnants of bacteria by identifying their nuclear contents. Unfortunately, it cannot easily delineate between nuclear materials from living or dead bacteria, thus increasing the likelihood of false positive studies. Recent studies have found that bacterial typing and assays that target other characteristics of bacteria, such as RNA, may help differentiate between viable and necrotic bacterial elements. Further investigations are required before these techniques can be widely used as they lack sufficient sensitivity and specificity, but their use remains promising.