Bartonella Species
Authors: Jane E. Koehler, M.D., David A. Relman, M.D. Updated October, 2010
Microbiology
Bacteria belonging to the Bartonella genus are extremely fastidious, slow-growing gram-negative rods that are dependent on blood or hemin for growth. In 1992, the Bartonella genus was comprised of a single species (B. bacilliformis). Bartonella henselae, B. quintana, B. vinsonii and B. elizabethae were previously classified in the genus Rochalimaea and considered rickettsiae, but were moved to the genus Bartonella in 1993 (5). Currently, there are twenty-nine officially recognized species of Bartonella (24, 26), and at least six additional Bartonella species for which isolates exist, but that have not yet been officially recognized (B. phoceensis, B. rattimassiliensis, B. tamiae, B. washoensis, B. australis, and B. rochalimae). Eight Bartonella species have been isolated from humans: B. henselae, B. quintana, B. elizabethae, B. bacilliformis, B. rochalimae, B. washoensis, B. tamiae, and B. vinsonii subsp. arupensis. Of these, B. quintana, B. bacilliformis, and B. henselae have been isolated most frequently from humans. The remaining five species, B. elizabethae (13),B. rochalimae (19), B. washoensis (41, 59), B. tamiae (40) and B. vinsonii subsp. arupensis (86) have been isolated only from three or fewer humans. AdditionalBartonella species have been associated with human infection by DNA amplification from human tissue and fluids.
Epidemiology
Contemporary Bartonella infections were first identified in the US in 1983, early in the AIDS epidemic, when immunosuppressed HIV-infected patients developed striking cutaneous and hepatosplenic vascular lesions (10, 44, 79). These lesions were named bacillary angiomatosis (in skin and lymph nodes), and bacillary peliosis in liver and spleen (43) (see Figures). The causative organisms remained unknown until bacterial DNA was amplified (66) and cultured from human blood (75) and tissues (37). Initial epidemiological investigation revealed that patients who developed bacillary angiomatosis lesions were statistically more likely to have had contact with cats (83). A more detailed molecular epidemiology study determined that although bacillary angiomatosis lesions caused by B. henselae were associated with cat owners, the other agent of bacillary angiomatosis, B. quintana, was associated with homeless patients who had exposure to body lice (38). Bartonella henselae was subsequently identified as the agent of cat scratch disease in immunocompetent patients, and the domestic cat as the reservoir and vector for transmission of the bacterium to humans (17; see review 62). The prevalence of B. henselae bacteremia in domestic cats is surprisingly high, e.g., 41% in the San Francisco Bay area (35). Of note, although 35 Bartonella species have been identified, only B. quintana and B. henselae have been isolated from bacillary angiomatosis lesions of HIV-infected patients.
Bartonellae alternate between two niches: the gut of obligately hematophagous arthropod vectors and the bloodstream of the mammalian reservoir (34).Bartonella species usually have both arthropod and mammalian host specificity, e.g., the domestic cat is the mammalian host for B. henselae and the cat flea is the arthropod vector that infects cats (9). Humans are believed to be the definitive reservoir for B. quintana and B. bacilliformis, and the body louse and sand fly, respectively, are their arthropod vectors. Indeed, the specificity between a Bartonella species and its vector is illustrated by B. bacilliformis, which infects humans only in the restricted geographic region where the transmitting sand fly vector is found: in the South American Andes mountains (48). It is now evident that numerous mammalian species have persistent bloodstream infection with their own cognate Bartonella species (7). Although B. quintana, B. henselae, and B. bacilliformishave been isolated repeatedly from humans, the association of other Bartonella species with human infection often reflects the unique exposure of those individuals to the mammalian host and/or its arthropod vector: e.g., a woman who developed B. alsatica lymphadenitis following rabbit exposure (3); another woman diagnosed withB. rochalimae bacteremia after receiving insect bites while traveling in Peru (19); a cattle rancher with B. vinsonii subsp. arupensis bacteremia (86); and a person living in rural Nevada who developed B. washoensis infection (41).
Clinical Manifestations
The spectrum of human disease caused by Bartonella species includes cat scratch disease (B. henselae), bacillary angiomatosis (B. henselae and B. quintana), bacillary peliosis (B. henselae), endocarditis (B. henselae, B. quintana, B. elizabethae, B. alsatica, B. koehlerae, B. vinsonii subsp. berkhoffii, andCandidatus Bartonella mayotimonensis), relapsing bacteremia (B. henselae and B. quintana), bacteremia (B. quintana, B. vinsonii subsp. arupensis), trench fever (B. quintana), Oroya fever (B. bacilliformis) and verruga peruana (B. bacilliformis) (48). Infections occur in both immunocompromised and immunocompetent individuals, but the manifestations and response to treatment are dramatically different, depending on the degree of immunosuppression of the patient. The Bartonellainfections most frequently encountered by physicians in the United States and Europe are culture-negative endocarditis and cat scratch disease (the latter is by far the more common, with greater than 22,000 cases annually in the United States (28). The incidence of bacillary angiomatosis has decreased since the introduction of highly active antiretroviral therapy (HAART). Currently, the majority of bacillary angiomatosis cases in AIDS patients are attributable to B. quintana, in homeless individuals who have limited access to HAART.
Cat scratch disease, a benign, self-limited granulomatous lymphadenitis caused by B. henselae, occurs in immunocompetent individuals. The primary cat scratch disease lesion occurs at the site of a cat scratch, 3-10 days after the inoculation. Lymphadenopathy develops two weeks after the scratch and regresses spontaneously over the ensuing 2-4 months (50). Occasionally, complications of cat scratch disease occur in the immunocompetent host such as retinitis (69), or encephalitis (45). Bartonella species are an important cause of culture-negative endocarditis in the United States and Europe; indeed, Bartonella may be the most common cause of culture-negative endocarditis in the U.S. At the French National Reference Center, Houpikian and Raoult found that Bartonella was the second most common cause of culture-negative endocarditis from 1983-2001, after Coxiella (25). Of the Bartonella species that have been associated with culture-negative endocarditis, B. quintana is by far the most common, but a number of cases due to B. henselae have also been reported. A number of other Bartonella species have been identified as the cause of endocarditis, each in single patients, including B. vinsonii subsp. arupensis and subsp. berkhoffii, B. koehlerae, B. alsatica, B. elizabethae, and Candidatus Bartonella mayotimonensis. Bartonella infection is also a substantial cause of fever of unknown origin (FUO) in children and in patients with late-stage HIV infection. In a prospective evaluation of 146 children with fever of unknown origin, bartonellosis was the third most common cause, after EBV infection and osteomyelitis (29). In a prospective study of 382 HIV-infected patients presenting with fever of undetermined cause, 18% had evidence ofBartonella infection (33).
Bacillary angiomatosis and bacillary peliosis are vascular proliferative manifestations of Bartonella infection that occur in immunocompromised patients (58,65), especially those co-infected with HIV. Bacillary angiomatosis infections occur most commonly late in HIV infection; in one series, the median CD4 cell count of patients at the time of bacillary angiomatosis diagnosis was 22/mm3 (55). B. quintana and B. henselae cause bacillary angiomatosis; these lesions are usually noted in the skin, but also can occur in the brain, bones, subcutaneous tissues, lymph nodes, gastrointestinal, and respiratory tracts. Peliosis is a closely related vascular proliferative lesion caused by B. henselae infection in the liver and spleen of immunocompromised patients (58). More than half of patients with focal bacillary angiomatosis or peliosis are bacteremic with the corresponding Bartonella species, emphasizing the systemic nature of this disease (37).
Bacillary angiomatosis or severe CSD also have been reported in immunosuppressed patients who have undergone solid organ transplantation (16, 32, 67).Bartonella infection in transplant patients often presents with fever and persistent granulomatous (or, less often, angiomatous) cutaneous and/or hepatic lesions. Several renal transplant patients developed acute transplant rejection simultaneously with their Bartonella infection (16). B. bacilliformis infections occur exclusively in South America, most commonly in immunocompetent people living in the Peruvian Andes at an altitude between 2,500 and 8,000 feet (85). The acute infection, Oroya fever, can cause a severe, potentially fatal hemolytic anemia that often is followed by a chronic phase known as verruga peruana, or Peruvian warts (48). These cutaneous lesions can be clinically indistinguishable from those of bacillary angiomatosis and persist for months to years, causing few symptoms.
Laboratory DIAGNOSIS
Diagnosis of Bartonella infections remains challenging. Focal Bartonella infection, such as bacillary angiomatosis, can be diagnosed from biopsied tissue samples using the Warthin-Starry silver stain. Although Bartonella bacilli are abundant in bacillary angiomatosis lesions, they are very difficult to visualize in the tissue except with the Warthin-Starry stain (43). In contrast to bacillary angiomatosis, few if any bacilli are present in cat scratch disease lymph nodes. Instead of the vascular proliferative changes in the tissue observed in bacillary angiomatosis, there is often stellate pattern of necrosis present in cat scratch disease lymph nodes. Because chronic drainage can result from incisional biopsy of cat scratch disease lymph nodes, the favored approach to obtaining tissue for diagnosis of cat scratch disease is with fine needle aspirate (FNA). The cellular material obtained from FNA should be sent for cytology, mycobacterial, fungal, and bacterial cultures. Performing an adequate FNA is critical for ruling out other infectious causes or malignancy as the cause of the lymphadenopathy.
Bartonella species can be isolated from human blood collected in either EDTA (6) or Wampole isolator tubes (75). Pelleted blood cells or homogenized tissue can be inoculated onto solid agar containing blood (37, 75). Blood-containing agars that are permissive for growth of Bartonella species include chocolate agar (37) and heart infusion agar with 5% rabbit blood (75). Co-cultivation of biopsied tissue with endothelial cells has good sensitivity for isolation of Bartonella (37, 42); however, this method is too laborious for most diagnostic microbiology laboratories.
The indirect fluorescence antibody (IFA) test (12) provides a more accessible means of diagnosing Bartonella infections. This test is performed at the U.S. Centers for Disease Control and Prevention and at some U.S. State Public Health laboratories, and is the principal serological test in the US for which the sensitivity and specificity have been determined using culture-confirmed sera. Submission of sera for IFA testing at the CDC is done through, or on approval of, the respective state labs. A number of commercial laboratories in the U.S. use variations of an IFA test to quantify Bartonella antibodies in the serum. In Europe, Bartonellaserological testing is available at the Unité des Rickettsies directed by Professeur Didier Raoult.
Note that there is extensive cross-reactivity between B. quintana and B. henselae with IFA tests, and patients with culture-proven B. henselae often have higher IFA titers to B. quintana than B. henselae (33). In addition, 25% of AIDS patients with culture-positive Bartonella infection remain seronegative by the IFA test (33). The IFA test on sera from patients with endocarditis usually reveals very high titers of anti-Bartonella antibodies. Using their microimmunofluorescence assay, a titer of 1:1600 has a positive predictive value for Bartonella endocarditis of 88% (60). After treatment, elevated IFA titers usually decrease; following IFA titers may be useful, especially in HIV-infected patients who are at risk for relapse, and those with Bartonella endocarditis (21).
Pathogenesis
Bartonella organisms are incapable of living free in the environment (except perhaps in the excreted feces of the arthropod vector). Bartonellae gain entry to the mammalian host when the infected arthropod bites and deposits feces at the site of the bite (e.g., B. quintana); the human then self-inoculates when scratching the bite. For B. henselae infection of humans, inoculation presumably occurs after being scratched by a cat whose claws are contaminated with infected flea feces.
The bloodstream phase of Bartonella infection has been studied in the rat model, with the rat strain of Bartonella, B. tribocorum (73). B. tribocorumadheres to, and enters, red blood cells (RBC) and multiplies until a critical density is achieved, without rupturing the RBC. The lifespan of the RBC is not affected by the presence of B. tribocorum (73). The mechanism of RBC entry and persistence has not been determined.
Several virulence factors of bartonellae have been identified and characterized: type IV secretion systems (71, 72), a family of hemin binding protein (Hbp) outer membrane proteins (54), a family of B. quintana outer membrane adhesions (Vomp) that undergo phase variation, and their ortholog in B. henselae, BadA (68,89). The mechanism of angiogenesis and endothelial cell proliferation in the bacillary angiomatosis lesions of immunocompromised patients is not fully understood. However, expression of BadA and the Vomp have been shown to activate hypoxia-inducible factor 1 in host cells in vitro, with subsequent secretion of proangiogenic cytokines (e.g., vascular endothelial growth factor) (68, 74). Bartonellae have been shown to interact with endothelial cells, although these interactions differ depending on whether the infection is in vivo (localization is exclusively extracellular to endothelial cells) (43) or in vitro (localization is intracellular in endothelial cells) (14). The study of Bartonella pathogenesis has been facilitated by publication of the genome sequences for B. quintana and B. henselae (1).
SUSCEPTIBILITY IN VITRO AND IN VIVO
Single Drug
In Vitro Susceptibilities
As for many other fastidious organisms, in vitro susceptibility testing for Bartonella species is not standardized. Additionally, there is a striking discrepancy between some of the in vitro and in vivo antibiotic susceptibility patterns obtained for Bartonella species. This discrepancy is especially notable for antibiotics that affect cell wall synthesis, e.g., penicillins. Most in vitro susceptibility testing reveals MIC90 values of approximately <0.05 µg/ml for penicillin (51), yet clinical failures and even dramatic disease progression have been frequently noted during treatment with this drug (36, 39). The clinical utility of MICs derived from in vitrosusceptibility testing therefore has not been established, and we do not recommend routine susceptibility testing of Bartonella isolates to guide patient therapy.
The earliest in vitro susceptibility testing for Bartonella species was reported by Myers, et al. in 1984 (56). They tested two B. quintana strains, the Fuller strain (type strain ATCC VR358) and Heliodoro strain using the agar dilution method (antibiotics diluted with agar composed of GC agar base with IsoVitaleX supplementation). They found a MIC50/MIC90 for penicillin of 0.024/0.035 µg/ml and 0.024/0.044 µg/ml for the 2 strains, respectively. The Fuller and Heliodoro strains were susceptible to erythromycin (MIC50/MIC90 0.026/0.036 µg/ml and 0.033/0.040 µg/ml, respectively), doxycycline (MIC50/MIC90 0.021/0.036 µg/ml and 0.095/0.115 µg/ml) and tetracycline (MIC50/MIC90 0.040/0.068 µg/ml and 0.35/0.82 µg/ml). Maurin and colleagues (51) reported MICs for 28 antibiotics with 14Bartonella isolates, also using an agar dilution method, but using Columbia agar supplemented with 5% horse blood. They also observed susceptibility to penicillin G (MIC90 range of 0.015-0.06 µg/ml) for all Bartonella species: B. quintana (9 strains), B. vinsonii (1 strain), B. elizabethae (1 strain) and B. henselae (3 strains). All 14 Bartonella species and strains tested were susceptible to erythromycin, doxycycline and rifampin. Additionally, they noted susceptibility of all Bartonella isolates to azithromycin and clarithromycin. Sobraques, et al. (76) tested the in vitro susceptibility of four strains of B. bacilliformis and found susceptibilities that were similar to other Bartonella species, including susceptibility to erythromycin (MIC 0.06 µg/ml), azithromycin (MIC 0.015 µg/ml), clarithromycin (MIC 0.015 to 0.03 µg/ml), doxycycline (MIC 0.03 to 0.06 µg/ml), rifampin (MIC 0.003 µg/ml), and streptomycin (MIC 4 µg/ml). In another study, the susceptibility of B. henselae and B. quintana to five macrolides was tested using in vitro cultivation with Vero cell monolayers: all five macrolide antibiotics demonstrated activity against both theseBartonella species (27). All human Bartonella isolates tested to date have been susceptible in vitro to erythromycin and tetracycline except one that was resistant to erythromycin on one testing (MIC >256 µg/ml), but on retesting was susceptible (MIC 0.06 µg/ml) (11). A subsequent relapse isolate from the same patient also was susceptible at an MIC of 0.06 µg/ml.
Fluoroquinolone susceptibility testing of Bartonella isolates revealed heterogeneity of susceptibility among strains (2), and B. bacilliformis isolates were found to be intrinsically resistant to quinolones, even isolates obtained prior to introduction of quinolone antibiotics (15). Both groups of researchers consequently recommend against use of fluoroquinolones for treatment of Bartonella infections.
The E-test (AB Biodisk, Solna, Sweden) was utilized to test the erythromycin, azithromycin, doxycycline, ciprofloxacin, rifampin and vancomycin susceptibilities of 10 B. henselae isolates grown on chocolate agar (87). The susceptibilities for doxycycline, erythromycin, azithromycin, and rifampin correlated with those from agar dilution methods. Two additional groups determined MICs using the E-test and similarly found susceptibility to macrolides and tetracyclines in multipleB. henselae isolates (57, 84). Dörbecker, et al. (18), compared the E-test with agar dilution for 21 isolates of B. henselae and 10 other strains of Bartonella, and also found good correlation between the two methods. Use of E-test strips with Bartonella isolates streaked on fresh chocolate agar is the susceptibility testing method most accessible to a clinical microbiology lab, and may be useful in the specific situation of comparing initial and relapse isolates for changes in susceptibility.
In Vivo Susceptibilities
Animal data substantiate the role of macrolides or tetracyclines in the treatment of Bartonella infections. Regnery and colleagues experimentally infected 25 cats with B. henselae and then treated groups of 5 cats for two weeks with one of four different antibiotics: tetracycline, amoxicillin, erythromycin or enrofloxacin, a fluoroquinolone (64). They cultured the blood of each animal at intervals after experimental infection, and determined that only tetracycline or erythromycin treatment resulted in a significant decrease in the titer of B. henselae bacilli in the blood at any time during the period after infection. There were no significant differences among the four antibiotics with regard to the apparent frequency at which bacteremia resolved. However, the duration of bacteremia did differ: bacteremia resolved at day 71 for tetracycline- and erythromycin-treated cats, at day 83 for control cats and at days 98 and 127 for those treated with enrofloxacin and amoxicillin, respectively.
Combination Drugs
In vitro susceptibility testing with a combination of drugs has not been performed.
ANTIMICROBIAL THERAPY
The aggregate of anecdotal cases of cat scratch disease treatment do not demonstrate clearly that treatment affects outcome, and virtually all patients recover fully regardless of whether they receive antibiotic treatment or not. In contrast, bacillary angiomatosis and bacillary peliosis can be fatal if not treated (10), and the response to antibiotic treatment is often dramatic. This difference in treatment response may be due to the number of Bartonella bacilli present in lesions (fewer in cat scratch disease, more numerous in bacillary angiomatosis) or the immune status of the infected individual, or both. Because of the differences in Bartonella infections in the immunocompromised and immunocompetent patients, the approach to treatment of Bartonella infections in these two groups is addressed separately below. Of note, consensus guidelines for the treatment of Bartonella infections in humans have been published (70).
Antimicrobial Therapy for Bartonella Infection in the Immunocompromised Patient
Drug of Choice in the Immunocompromised Patient
Bacillary Angiomatosis and Bacillary Peliosis: Treatment of bacillary angiomatosis has not been studied prospectively or systematically, and recommendations are based on the aggregate of anecdotal cases and small series. Stoler, et al. described the first patient with bacillary angiomatosis in 1983, and although the disease had neither been named nor the infecting bacillus identified, they treated the patient successfully with erythromycin (79). In 1987, we treated the cutaneous and osseous bacillary angiomatosis lesions of the first prospectively identified patient at San Francisco General Hospital with erythromycin; the lesions resolved completely (36). We have now treated more than 65 patients with biopsy-proven bacillary angiomatosis, and from our experience and from reviewing the published literature on treatment of anecdotal bacillary angiomatosis cases (summarized in 39 and 52), either one of two drugs can be confidently recommended for first line therapy: erythromycin or doxycycline. The response to treatment appears to be equivalent whether erythromycin or doxycycline is prescribed and whether the bacillary angiomatosis is caused by B. henselae or B. quintana, although it is interesting to consider that erythromycin may have an antiangiogenic effect on B. quintana in vitro (53).
For treatment of Bartonella infection in the immunocompromised patient, the drug of first choice is either erythromycin 500 mg Q6H PO or IV, or doxycycline 100 mg Q12H PO or IV. We usually initiate therapy for bacillary angiomatosis with oral doxycycline, and specifically favor oral doxycycline over erythromycin in several situations: 1) when poor patient compliance dictates twice daily dosing; 2) when it is important to achieve maximal CNS antibiotic delivery for focal CNS Bartonella infection; or 3) when severe gastrointestinal symptoms are present that could be exacerbated by oral erythromycin therapy. Rifampin also appears to have in vivo activity in patients with bacillary angiomatosis lesions, but because in general, bacteria spontaneously develop rifampin resistance at a high rate, we do not recommend use of rifampin alone.
Combination therapy, with the addition of rifampin to either erythromycin or doxycycline is recommended for immunocompromised patients with acute, life-threatening Bartonella infection, including bacillary peliosis or CNS disease. Failure to respond to antibiotic treatment after 7-10 days should prompt change of drug therapy to the other first line drug, addition of rifampin, change of route of antibiotic administration to intravenous, or all of these. The intravenous route is especially important in those patients with severe Bartonella infection of the gastrointestinal tract who may have inadequate absorption of the antibiotic and symptom exacerbation by oral erythromycin therapy.
Penicillins and first generation cephalosporins lack efficacy and should not be used to treat Bartonella infection in immunocompromised patients (36, 39). Although some anecdotal reports describe a response of Bartonella infection to ciprofloxacin, we have observed progression of bacillary angiomatosis lesions in patients treated with ciprofloxacin (81). Reports of in vitro intrinsic resistance associated with gyrA mutations (2, 15) corroborate the lack of in vivo efficacy that we have observed. We also have isolated Bartonella species from immunocompromised patients treated with gentamicin or trimethoprim/sulfamethoxazole and would therefore not recommend treatment of patients with bacillary angiomatosis infection with either of these antibiotics.
It is evident that the majority of patients with cutaneous bacillary angiomatosis have systemic disease, and a short duration of treatment is frequently associated with relapse. At least three months antibiotic treatment is recommended for patients with cutaneous bacillary angiomatosis, and four months for patients with bacillary peliosis (39). For patients with bacillary angiomatosis of the bone or CNS, treatment should continue for at least four months. After discontinuation of antibiotic therapy, patients should be monitored carefully for relapse of Bartonella infection in the same organ or at a new site. If relapse occurs, the patient should receive treatment and then secondary prophylaxis with either doxycycline or a macrolide, as long as the CD4+ count remains <200 cells/µL (30).
Special Infections in the Immunocompromised Patient
For patients with bacillary angiomatosis of the bone or CNS, treatment should continue for at least four months. Combination therapy, with the addition of rifampin to either erythromycin 500 mg IV or PO Q6H or doxycycline 100 mg IV or PO Q12H is recommended for immunocompromised patients with acute, life-threatening Bartonella infection, including bacillary peliosis or CNS disease. Failure to respond to antibiotic treatment after 7-10 days should prompt change of drug therapy to the other first line drug, addition of rifampin 300 mg PO Q12H, change of route of antibiotic administration to intravenous, or all of these. The intravenous route is especially important in those patients with severe Bartonella infection of the gastrointestinal tract who may have inadequate absorption of the antibiotic and symptom exacerbation by oral erythromycin therapy.
Underlying Diseases in the Immunocompromised Patient
Immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients has not been described in association with Bartonella infection, but patients with Bartonella CNS or ophthalmic lesions should probably be treated with doxycycline and rifampin for 2-4 weeks before instituting ART (30).
Alternative Therapy in the Immunocompromised Patient
For patients unable to tolerate erythromycin or doxycycline, alternative antibiotics include minocycline (100 mg PO Q12H), used to treat bacillary angiomatosis successfully in an immunocompetent adult (82) or tetracycline (500 mg PO Q6H), used to treat an HIV-infected patient with bacillary angiomatosis successfully (37). There also is limited experience with azithromycin; this antibiotic could be considered if a patient is incapable of complying with BID doxycycline. Azithromycin (500 mg PO Q24H) for 28-90 days was used to treat five of the ten immunocompetent patients with B. quintana bacteremia reported by Spach, et al. (78), and one immunocompromised patient with bacillary angiomatosis was successfully treated with 1 gm PO Q24H (23).
Combination Therapy in the Immunocompromised Patient
Combination therapy, with the addition of rifampin to either erythromycin or doxycycline, is recommended for immunocompromised patients with acute, life-threatening Bartonella infection, including bacillary peliosis or CNS disease.
Antimicrobial Therapy for Bartonella Infections in the Immunocompetent Patient
Drug of Choice in the Immunocompetent Patient
Uncomplicated Cat Scratch Disease: The only prospective, double-blind, placebo-controlled study of treatment of immunocompetent patients with uncomplicated cat scratch disease was reported by Bass, et al. (4). They treated patients with 5 days of azithromycin or placebo and found no statistically significant difference between the two groups in the duration of lymphadenopathy. However, they did find a statistically significant difference between the numbers of patients achieving 80% reduction of lymph node volume (by sonography) at 30 days after initiation of treatment in the azithromycin group.
Because the natural course of cat scratch disease is relatively benign, self-limited and extremely variable, the data for treating uncomplicated CSD are not currently compelling. More important, because antibiotic resistance of many bacteria is increasing dramatically, treatment of uncomplicated cat scratch disease does not appear to be justified either from the standpoint of the individual patient who is likely to experience little benefit yet would be exposed to potential antibiotic side effects, or from a public health standpoint.
Special Infections in the Immunocompetent Patient Complicated Cat Scratch Disease Including Retinitis, CNS Infection and Granulomatous Hepatitis:Treatment of complicated cat scratch disease, including retinitis, granulomatous hepatitis and encephalitis with a number of different antibiotics has been reported by a number of authors. As with cat scratch disease, however, it is not clear that antibiotics have any efficacy in the treatment of these manifestations in immunocompetent patients. Wong, et al. (88) report one patient with stellate neuroretinitis from whose blood B. henselae was isolated. However, despite receiving doxycycline and rifampin for one month and experiencing resolution of ophthalmologic symptoms, B. henselae could still be cultured from the blood of this patient.
Many clinicians elect to administer oral antibiotics to patients with visual impairment or to give intravenous antibiotics to those ill enough to require hospitalization. Clinical experience with doxycycline 100 mg PO BID and rifampin 300 mg PO BID in immunocompromised patients makes this combination a reasonable choice if treatment of these immunocompetent patients is elected.
Relapsing Bacteremia: Clinical experience with treatment of isolated Bartonella bacteremia also is limited. Historically, soldiers with B. quintana relapsing bacteremia (trench fever) during World War I cleared the infection in the absence of antibiotic treatment. In contemporary times, one immunocompetent patient with B. henselae bacteremia and meningitis was treated with amoxicillin for five days, then doxycycline for two weeks, and finally ceftriaxone for ten days, but had positive blood cultures for the subsequent six weeks, despite resolution of symptoms (46). Although treatment did not eliminate the bacteremia in this patient, patients withBartonella bacteremia should be treated, probably with doxycycline for 2-4 weeks. An important caveat should be kept in mind: the patient must first be evaluated carefully for endocarditis because this will change the duration and follow-up of antibiotic treatment.
Edocarditis: Spach, et al. (77) described four patients with Bartonella endocarditis, all of whom received antibiotic therapy with multiple drugs, three of whom had valve replacement and all of whom were cured. The one patient who did not require valve replacement received treatment with ceftriaxone, doxycycline and erythromycin. The other three patients who required valve replacement did not receive treatment with a first line antibiotic against Bartonella species prior to valve replacement, and whether surgery was required due to ineffective antibiotic treatment or the natural course of Bartonella endocarditis in these three patients is not known. In these three patients, valve replacement may have been curative.
In a series of 33 Bartonella endocarditis patients (60), only one surviving patient did not require valve replacement, and this patient was treated with ceftriaxone and doxycycline. The remainder of the patients received numerous other antibiotics before and after valvular surgery. B. henselae was isolated from one patient after completion of a course of amoxicillin and gentamicin, and we have isolated Bartonella species from a patient receiving similar treatment. Thus, the culture-negative endocarditis regimen using gentamicin plus penicillin or amoxicillin is unlikely to treat Bartonella endocarditis adequately. In a retrospective study of patients with Bartonella endocarditis, patients receiving an aminoglycoside were more likely to have a better outcome (61).
For culture-negative endocarditis in which Bartonella is a potential cause, a treatment regimen of ceftriaxone 2 gm IV QD with gentamicin in the first two weeks of therapy, with or without doxycycline 100 mg PO Q12H, has been recommended (70). Patients with proven Bartonella endocarditis should receive six weeks of antibiotic therapy with two drugs. In our experience treating patients with Bartonella endocarditis, we administer doxycycline 100 mg IV or PO Q12H plus rifampin 300 mg PO Q12H for 6 weeks. We have observed that nearly every patient with documented Bartonella endocarditis has had significant renal insufficiency that prevented use of gentamicin; we therefore favor a doxycycline plus rifampin regimen for 6 weeks.
Oroya Fever and Verruga Peruana: Antibiotic treatment of patients infected with B. bacilliformis has been documented to decrease the morbidity and mortality associated with both acute and chronic stages of infection with this pathogen. Mortality due to Oroya fever was estimated to be 40% prior to the era of antibiotics and is now estimated to be approximately 8% (22). Some patients who survive the acute anemia succumb to intercurrent infection with intracellular pathogens such asSalmonella, Toxoplasma and Mycobacterium because of an immunodeficient state that may develop during Oroya fever (22). Thus, although penicillin treatment can attenuate the lysis of erythrocytes during the acute phase, patients in the endemic regions of South America often are treated with chloramphenicol 500 mg IV or PO QID for 14 days plus another antibiotic (preferably a beta-lactam) (47, 70) to reduce the mortality from intercurrent Salmonella infection.
Antibiotic treatment of Oroya fever may not prevent subsequent development of verrugae (85), and once verruga peruana develops, the currently favored treatment is streptomycin at 15-20 mg/kg/day IM for 10 days (47, 70, 76). Macrolides have also been used successfully, but with much less experience (76).
Bartonella elizabethae: Only one human infection with B. elizabethae has been reported, in a patient with endocarditis (13). This patient defervesced during treatment with nafcillin and gentamicin but developed progressive congestive heart failure and required valve replacement.
Bartonella vinsonii subsp. arupensis: This species has been isolated from only one human, a cattle rancher, with bacteremia and fever (86). The patient defervesced after a single dose of ceftriaxone and did not appear to have endocarditis by cardiac echocardiography.
Alternative Therapy in the Immunocompetent Patient
For patients unable to tolerate erythromycin or doxycycline, alternative antibiotics include minocycline (100 mg PO Q12H), used to treat bacillary angiomatosis successfully in an immunocompetent adult (82) or tetracycline (500 mg PO Q6H), used to treat an HIV-infected patient with bacillary angiomatosis successfully (37). There also is limited experience with azithromycin; this antibiotic could be considered if a patient is incapable of complying with BID doxycycline. Azithromycin (500 mg PO Q24H) for 28-90 days was used to treat five of the ten immunocompetent patients with B. quintana bacteremia reported by Spach, et al. (78), and one immunocompromised patient with bacillary angiomatosis was successfully treated with 1 gm PO Q24H (23).
Combination Therapy in the Immunocompetent Patient
For culture-negative endocarditis in which Bartonella is a potential cause, a treatment regimen of ceftriaxone 2 gm IV QD with gentamicin in the first two weeks of therapy, with or without doxycycline 100 mg PO Q12H, has been recommended (70). Patients with proven Bartonella endocarditis should receive six weeks of antibiotic therapy with two drugs. In our experience treating patients with Bartonella endocarditis, we administer doxycycline 100 mg IV or PO Q12H plus rifampin 300 mg PO Q12H for 6 weeks. We have observed that virtually every patient with documented Bartonella endocarditis has had significant renal insufficiency that prevented use of gentamicin.
ADJUNCTIVE THERAPy
The majority of patients with Bartonella endocarditis require valve replacement, in addition to antimicrobial therapy. However, the contribution of valve replacement to the cure of Bartonella infection has not been studied prospectively, and most patients reported in the literature received no treatment or were treated with an inadequate regimen before diagnosis of Bartonellaendocarditis.
ENDPOINTS FOR MONITORING THERAPY
Patients with cutaneous bacillary angiomatosis lesions should be evaluated for presence of Bartonella infection at other sites that would alter therapy duration, e.g., for osseous lesions or endocarditis. Cutaneous lesions usually improve in the first several weeks of antibiotic therapy and resolve completely in one to two months, depending on the size and number of lesions. Patients with peliosis hepatitis can be monitored by abdominal CT scanning, and those with osseous lesions by 99mtechnetium bone scans. Patients in whom antibiotic therapy has been stopped should be followed closely for recurrence of Bartonella infection at the original site as well as at new sites, e.g., a patient treated for osteomyelitis may recur with bacteremia (37).
A Jarisch-Herxheimer-like reaction has been described in immunocompromised patients after receiving the first several doses of antibiotics (37). Physicians should advise patients of this possible treatment complication, and patients with severe respiratory and/or cardiovascular compromise should be monitored carefully following institution of antimicrobial therapy. This reaction has occasionally been mistaken for an adverse drug reaction.
An additional concern is the propensity for doxycycline to cause pill-associated ulcerative esophagitis (31). This complication is most frequently reported when a dose is taken with only a small amount of liquid or at night just before retiring, and can be prevented by taking doxycycline several hours before bedtime with copious amounts of water.
VACCINES
There are no vaccines available for prevention of Bartonella infection in either humans or animals.
PREVENTION OR INFECTION CONTROL MEASURES
Prevention of Bartonella Infection
Bartonella species are vector borne, and control of the associated vector may help decrease incidence of these infections. The cat flea efficiently transmits B. henselae from cat to cat (9), and this is hypothesized to facilitate creation of the large reservoir of infected cats (as many as 41% of cats are infected in the San Francisco Bay area (35)). Fleas could potentially transmit B. henselae directly to humans, but this has not been documented to date, and the vast majority of human B. henselae infections occur following a cat scratch or bite (49). Control of cat flea infestation is recommended, especially for the pets of immunocompromised patients (63). This strategy may reduce the transmission of B. henselae by decreasing feline infection, reducing contamination of cat claws due to scratching, and reducing the potential of direct transmission to humans via fleas.
Additional recommendations for decreasing risk of B. henselae infection include acquiring a mature cat, which is less likely to scratch and less likely to be bacteremic (8, 35), washing cat wounds immediately with soap and water and avoiding rough play with the cat (30, 63).
Antibiotic treatment of cats belonging to immunocompromised individuals has been proposed, but it is not evident that Bartonella infection can be permanently eradicated from the feline reservoir (64). In addition, treatment usually involves orally force-feeding antibiotics to the cat, which incurs substantial risk of cat scratches and bites and is likely to increase risk of transmission of B. henselae to the humans involved.
Bartonella quintana is transmitted from human to human by the body louse (80); homeless individuals are at increased risk for infestation with the body louse and thus for infection with B. quintana. Avoiding infestation with and exposure to the body louse is the only current recommendation for prevention of B. quintana infection. For B. bacilliformis, the substantial decrease of infection in endemic regions of the Peruvian Andes has been attributed not only to antibiotic treatment of humans but also to reduction of the sand fly vector for this Bartonella species (85). For B. elizabethae and B. vinsonii subsp. arupensis, the reservoirs are apparently rodents (86), but the vectors are as yet unknown. There currently are no recommendations about prevention of infection with these two species.
Antibiotic Prophylaxis
Antibiotics of the macrolide, tetracycline and rifamycin classes have shown good in vivo activity against Bartonella species. Treatment with macrolide antibiotics was protective against B. henselae and B. quintana infection in HIV-infected patients in one study (38). It is therefore likely that Mycobacterium aviumcomplex prophylaxis or treatment regimens that include an antibiotic of the macrolide, rifamycin or tetracycline class will provide adequate prophylaxis againstBartonella infection.
Infection Control
There are no situations in which infection control is necessary for patients infected with Bartonella.
REFERENCES
1. Alsmark CM, Frank AC, Karlberg EO, Legault BA, Ardell DH, Canback B, Eriksson AS, Naslund AK, Handley SA, Huvet M, La Scola B, Holmberg M, Andersson SG. The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. Proc Natl Acad Sci U S A 2004;101(26):9716-21. [PubMed]
2. Angelakis E, Biswas S, Taylor C, Raoult D, Rolain J. Heterogeneity of susceptibility to fluoroquinolones in Bartonella isolates from Australia reveals a natural mutation in gyrA. J Antimicrob Chemother 2008;61:1252-5. [PDF] [PubMed]
3. Angelakis E, Lepidi H, Canel A, Rispal P, Perraudeau F, Barre I, Rolain J, Raoult D. Human case of Bartonella alsatica lymphadenitis. Emerg Infect Dis 2008;14:1951-3. [PubMed]
4. Bass JW, Freitas BC, Freitas AD, Sisler CL, Chan DS, Vincent JM, Person DA, Claybaugh JR, Wittler RR, Weisse ME, Regnery RL, Slater LN. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatric Infectious Disease Journal 1998;17:447-52.[PubMed]
5. Brenner DJ, O'Connor SP, Winkler HH, Steigerwalt AG. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol 1993;43(4):777-86. [PubMed]
6. Brenner SA, Rooney JA, Manzewitsch P, Regnery RL. Isolation of Bartonella (Rochalimaea) henselae: effects of methods of blood collection and handling. J Clin Microbiol 1997;35(3):544-7. [PubMed]
7. Chomel B, Boulouis H, Breitschwerdt E, Kasten R, Vayssier-Taussat M, Birtles R, Koehler J, Dehio C. Ecological fitness and strategies of adaptation ofBartonella species to their hosts and vectors. Vet Res 2009;40:29. [PubMed]
8. Chomel BB, Abbott RC, Kasten RW, Floyd-Hawkins KA, Kass PH, Glaser CA, Pedersen NC, Koehler JE. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol 1995;33(9):2445-50. [PubMed]
9. Chomel BB, Kasten RW, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield AN, Abbott RC, Pedersen NC, Koehler JE. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol 1996;34:1952-6. [PubMed]
10. Cockerell CJ, Whitlow MA, Webster GF, Friedman-Kien AE. Epithelioid angiomatosis: A distinct vascular disorder in patients with the acquired immunodeficiency syndrome or AIDS-related complex. Lancet 1987;2:654-6. [PubMed]
11. Colson P, Lebrun L, Drancourt M, Boue F, Raoult D, Nordmann P. Multiple recurrent bacillary angiomatosis due to Bartonella quintana in an HIV-infected patient. Eur J Clin Microbiol Infect Dis 1996;15(2):178-80. [PubMed]
12. Dalton MJ, Robinson LE, Cooper J, Regnery RL, Olson JG, Childs JE. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national referral center. Arch Intern Med 1995;155(15):1670-6.[PubMed]
13. Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, Weyant RS, Steigerwalt AG, Weaver RE, Daneshvar MI, O'Connor SP. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol 1993;31(4):872-81. [PubMed]
14. Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci 1997;110:2141-54. [PubMed]
15. del Valle L, Flores L, Vargas M, García-de-la-Guarda R, Quispe R, Ibañez Z, Alvarado D, Ramírez P, Ruiz J. Bartonella bacilliformis, endemic pathogen of the Andean region, is intrinsically resistant to quinolones. Int J Infect Dis 2010;14:e506-10. [PubMed]
16. Dharnidharka VR, Richard GA, Neiberger RE, Fennell RS, 3rd. Cat scratch disease and acute rejection after pediatric renal transplantation. Pediatr Transplant 2002;6(4):327-31. [PubMed]
17. Dolan MJ, Wong MT, Regnery RL, Jorgensen JH, Garcia M, Peters J, Drehner D. Syndrome of Rochalimaea henselae adenitis suggesting cat scratch disease. Ann Intern Med 1993;118(5):331-6. [PubMed]
18. Dörbecker C, Sander A, Oberle K, Schülin-Casonato T. In vitro susceptibility of Bartonella species to 17 antimicrobial compounds: comparison of Etest and agar dilution. J Antimicrob Chemother 2006;58:784-8. [PubMed]
19. Eremeeva M, Gerns H, Lydy S, Goo J, Ryan E, Mathew S, Ferraro M, Holden J, Nicholson W, Dasch G, Koehler J. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med 2007;356:2381-7. [PubMed]
20. Fournier PE, Lelievre H, Eykyn SJ, Mainardi JL, Marrie TJ, Bruneel F, Roure C, Nash J, Clave D, James E, Benoit-Lemercier C, Deforges L, Tissot-Dupont H, Raoult D. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 2001;80(4):245-51. [PubMed]
21. Fournier PE, Mainardi JL, Raoult D. Value of microimmunofluorescence for diagnosis and follow-up of Bartonella endocarditis. Clin Diagn Lab Immunol 2002;9(4):795-801. [PubMed]
22. Garcia-Caceres U, Garcia FU. Bartonellosis: An immunodepressive disease and the life of Daniel Alcides Carrion. Am J Clin Pathol 1991;95 (Suppl. 1):S58-66.[PubMed]
23. Guerra LG, Neira CJ, Boman D, Ho H, Casner PR, Zuckerman M, Verghese A. Rapid response of AIDS-related bacillary angiomatosis to azithromycin. Clin Infect Dis 1993;17(2):264-6. [PubMed]
24. Gundi V, Taylor C, Raoult D, La Scola B. Bartonella rattaustraliani sp. nov., Bartonella queenslandensis sp. nov. and Bartonella coopersplainsensis sp. nov., identified in Australian rats. Int J Syst Evol Microbiol 2009;59:2956-61. [PubMed]
25. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005;84:162-73.[PubMed]
26. Inoue K, Kabeya H, hiratori H, Ueda K, Kosoy M, Chomel B, Boulouis H, Maruyama S. Bartonella japonica sp. nov. and Bartonella silvatica sp. nov., isolated from Apodemus mice. Int J Syst Evol Microbiol 2010;60:759-63. [PubMed]
27. Ives TJ, Manzewitsch P, Regnery RL, Butts JD, Kebede M. In vitro susceptibilities of Bartonella henselae, B. quintana, B. elizabethae, Rickettsia rickettsii, R. conorii, R. akari, and R. prowazekii to macrolide antibiotics as determined by immunofluorescent-antibody analysis of infected Vero cell monolayers. Antimicrob Agents Chemother 1997;41:578-82. [PubMed]
28. Jackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health 1993;83(12):1707-11.[PubMed]
29. Jacobs RF, Schutze GE. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis 1998;26:80-4. [PubMed]
30. Kaplan J, Benson C, Holmes K, Brooks J, Pau A, Masur H. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009;58(RR-4):1-207. [PubMed]
31. Kikendall JW, Friedman AC, Oyewole MA, Fleischer D, Johnson LF. Pill-induced esophageal injury. Case reports and review of the medical literature. Dig Dis and Sci 1983;28:174-82. [PubMed]
32. Koehler J, Duncan L. Case 30-2005: A 56-Year-Old Man with Fever and Axillary Lymphadenopathy. N Engl J Med 2005;353:1387-94. [PubMed]
33. Koehler J, Sanchez M, Tye S, Garrido-Rowland C, Chen F, Maurer T, Cooper J, Olson J, Reingold A, Hadley W, Regnery R, Tappero J. Prevalence ofBartonella infection among Human Immunodeficiency Virus-infected patients with fever. Clinical Infectious Diseases 2003;37:559-66. [PubMed]
34. Koehler JE. Bartonella Species. In: Nataro JP, Blaser MJ, Cunningham-Rundles S, eds. Persistent Bacterial Infections. Washington, D.C.: ASM Press; 2000:339-53. [PubMed]
35. Koehler JE, Glaser CA, Tappero JW. Rochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. JAMA 1994;271(7):531-5. [PubMed]
36. Koehler JE, LeBoit PE, Egbert BM, Berger TG. Cutaneous vascular lesions and disseminated cat-scratch disease in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. Ann Intern Med 1988;109(6):449-55. [PubMed]
37. Koehler JE, Quinn FD, Berger TG, LeBoit PE, Tappero JW. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med 1992;327(23):1625-31. [PubMed]
38. Koehler JE, Sanchez MA, Garrido CS, Whitfeld MJ, Chen FM, Berger TG, Rodriguez-Barradas MC, LeBoit PE, Tappero JW. Molecular epidemiology ofbartonella infections in patients with bacillary angiomatosis-peliosis. N Engl J Med 1997;337(26):1876-83. [PubMed]
39. Koehler JE, Tappero JW. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus. Clin Infect Dis 1993;17(4):612-24.[PubMed]
40. Kosoy M, Morway C, Sheff K, Bai Y, Colborn J, Chalcraft L, Dowell S, Peruski L, Maloney S, Baggett H, Sutthirattana S, Sidhirat A, Maruyama S, Kabeya H, Chomel B, Kasten R, Popov V, Robinson J, Kruglov A, Petersen L. Bartonella tamiae sp. nov., a newly recognized pathogen isolated from three human patients from Thailand. J Clin Microbiol 2008;46:772-5. [PubMed]
41. Kosoy M, Murray M, Gilmore RD, Jr., Bai Y, Gage KL. Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. J Clin Microbiol 2003;41(2):645-50. [PubMed]
42. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol 1999;37(6):1899-905. [PubMed]
43. LeBoit PE, Berger TG, Egbert BM, Beckstead JH, Yen TSB, Stoler MH. Bacillary angiomatosis: The histopathology and differential diagnosis of a pseudoneoplastic infection in patients with human immunodeficiency virus disease. Am J Surg Pathol 1989;13:909-20. [PubMed]
44. LeBoit PE, Berger TG, Egbert BM, Yen TSB, Stoler MH, Bonfiglio TA, Strauchen JA, English CK, Wear DJ. Epithelioid haemangioma-like vascular proliferation in AIDS: manifestation of cat-scratch disease bacillus infection? Lancet 1988;i:960-3. [PubMed]
45. Lewis P, Glaser CA. Encephalitis. Pediatr Rev 2005;26:353-63. [PubMed]
46. Lucey D, Dolan MJ, Moss CW, Garcia M, Hollis DG, Wegner S, Morgan G, Almeida R, Leong D, Greisen KS, et al. Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations. Clin Infect Dis 1992;14(3):683-8. [PubMed]
47. Maguiña C, Gotuzzo E. Bartonellosis. New and old. Infect Dis Clin North Am 2000;14:1-22. [PubMed]
48. Maguiña C, Guerra H, Ventosilla P. Bartonellosis. Clinics in Dermatology 2009;27:271-80. [PubMed]
49. Margileth AM. Cat scratch disease: a therapeutic dilemma. Vet Clin N Am 1987;17:91-103. [PubMed]
50. Margileth AM. Recent Advances in Diagnosis and Treatment of Cat Scratch Disease. Curr Infect Dis Rep 2000;2:141-6. [PubMed]
51. Maurin M, Gasquet S, Ducco C, Raoult D. MICs of 28 antibiotic compounds for 14 Bartonella (formerly Rochalimaea) isolates. Antimicrob Agents Chemotherapy 1995;39:2387-91. [PubMed]
52. Maurin M, Raoult D. Antimicrobial susceptibility of Rochalimaea quintana, Rochalimaea vinsonii, and the newly recognized Rochalimaea henselae. J Antimicrob Chemother 1993;32:587-94. [PubMed]
53. Meghari S, Rolain J, Grau G, Platt E, Barrassi L, Mege J, Raoult D. Antiangiogenic effect of erythromycin: an in vitro model of Bartonella quintana infection. J Infect Dis 2006;193:380-6. [PubMed]
54. Minnick MF, Sappington KN, Smitherman LS, Andersson SG, Karlberg O, Carroll JA. Five-member gene family of Bartonella quintana. Infect Immun 2003;71(2):814-21. [PubMed]
55. Mohle-Boetani JC, Koehler JE, Berger TG, LeBoit PE, Kemper CA, Reingold AL, Plikaytis BD, Wenger JD, Tappero JW. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus: Clinical characteristics in a case-control study. Clin Infect Dis 1996;22:794-800. [PubMed]
56. Myers WF, Grossman DM, Wisseman CLJ. Antibiotic susceptibility patterns in Rochalimaea quintana, the agent of trench fever. Antimicrob Agents Chemother 1984;25:690-3. [PubMed]
57. Pendle S, Ginn A, Iredell J. Antimicrobial susceptibility of Bartonella henselae using Etest methodology. J Antimicrob Chemother 2006;57:761-3. [PubMed]
58. Perkocha LA, Geaghan SM, Yen TSB, Nishimura SL, Chan SP, Garcia-Kennedy R, Honda G, Stoloff AC, Klein HZ, Goldman RL, Van Meter S, Ferrell LD, LeBoit PE. Clinical and pathological features of bacillary peliosis hepatis in association with human immunodeficiency virus infection. N Engl J Med 1990;323:1581-6. [PubMed]
59. Probert W, Louie J, Tucker J, Longoria R, Hogue R, Moler S, Graves M, Palmer H, Cassady J, Fritz C. Meningitis due to a "Bartonella washoensis"-like human pathogen. J Clin Microbiol 2009;47:2332-5. [PubMed]
60. Raoult D, Fournier PE, Drancourt M, Marrie TJ, Etienne J, Cosserat J, Cacoub P, Poinsignon Y, Leclercq P, Sefton AM. Diagnosis of 22 new cases ofBartonella endocarditis. Ann Intern Med 1996;125:646-52. [PubMed]
61. Raoult D, Fournier PE, Vandenesch F, Mainardi JL, Eykyn SJ, Nash J, James E, Benoit-Lemercier C, Marrie TJ. Outcome and treatment of Bartonellaendocarditis. Arch Intern Med 2003;163(2):226-30. [PubMed]
62. Regnery R, Tappero J. Unraveling mysteries associated with cat-scratch disease, bacillary angiomatosis, and related syndromes. Emerg Infect Dis 1995;1:16-21.[PubMed]
63. Regnery RL, Childs JE, Koehler JE. Infections associated with Bartonella species in persons infected with human immunodeficiency virus. Clin Infect Dis 1995;21 Suppl 1:S94-8. [PubMed]
64. Regnery RL, Rooney JA, Johnson AM, Nesby SL, Manzewitsch P, Beaver K, Olson JG. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. AJVR 1996;57(12):1714-9. [PubMed]
65. Relman DA, Falkow S, LeBoit PE. The organism causing bacillary angiomatosis, peliosis hepatis, and fever and bacteremia in immunocompromised patients. N Engl J Med [letter] 1991;324:1514. [PubMed]
66. Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. The agent of bacillary angiomatosis: An approach to the identification of uncultured pathogens. N Engl J Med 1990;323:1573-80. [PubMed]
67. Rheault M, van Burik J-A, Mauer M, Ingulli E, Ferrieri P, Jessurun J, Chavers B. Cat-scratch disease relapse in a kidney transplant recipient. Pediatr Transplantation 2007;11:105-9. [PubMed]
68. Riess T, Andersson S, Lupas A, Schaller M, Schafer A, Kyme P, Martin J, Walzlein J, Ehehalt U, Lindroos H, Schirle M, Nordheim A, Autenrieth I, Kempf V.Bartonella adhesin A mediates a proangiogenic host cell response. J Exp Med 2004;200:1267-78. [PubMed]
69. Roe R, Jumper J, Fu A, Johnson R, McDonald H, Cunningham E. Ocular bartonella infections. Int Ophthalmol Clin 2008;48:93-105. [PubMed]
70. Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrobial Agents and Chemotherapy 2004;48:1921-33. [PubMed]
71. Schmiederer M, Anderson B. Cloning, sequencing, and expression of three Bartonella henselae genes homologous to the Agrobacterium tumefaciens VirB region. DNA Cell Biol 2000;19(3):141-7. [PubMed]
72. Schroder G, Dehio C. Virulence-associated type IV secretion systems of Bartonella. Trends Microbiol 2005;13(7):336-42.[PubMed]
73. Schulein R, Seubert A, Gille C, Lanz C, Hansmann Y, Piemont Y, Dehio C. Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J Exp Med 2001;193(9):1077-86. [PubMed]
74. Schulte B, Linke D, Klumpp S, Schaller M, Riess T, Autenrieth I, Kempf V. Bartonella quintana variably expressed outer membrane proteins mediate vascular endothelial growth factor secretion but not host cell adherence. Infect Immun 2006;74:5003-13. [PubMed]
75. Slater LN, Welch DF, Hensel D, Coody DW. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N Engl J Med 1990;323:1587-93. [PubMed]
76. Sobraques M, Maurin M, Birtles RJ, Raoult D. In vitro susceptibilities of four Bartonella bacilliformis strains to 30 antibiotic compounds. Antimicrob Agents Chemother 1999;43(8):2090-2. [PubMed]
77. Spach DH, Kanter AS, Daniels NA, Nowowiejski DJ, Larson AM, Schmidt RA, Swaminathan B, Brenner DJ. Bartonella (Rochalimaea) species as a cause of apparent "culture-negative" endocarditis. Clin Infect Dis 1995;20(4):1044-7. [PubMed]
78. Spach DH, Kanter AS, Dougherty MJ, Larson AM, Coyle MB, Brenner DJ, Swaminathan B, Matar GM, Welch DF, Root RK, et al. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med 1995;332(7):424-8. [PubMed]
79. Stoler MH, Bonfiglio TA, Steigbigel RT, Pereira M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am J Clin Pathol 1983;80:714-8. [PubMed]
80. Strong RPl. Trench Fever: Report of Commission, Medical Research Committee, American Red Cross. Oxford: Oxford University Press; 1918. [PubMed]
81. Tappero JW, Koehler JE. Cat scratch disease and bacillary angiomatosis [letter]. JAMA 1991;266:1938-9. [PubMed]
82. Tappero JW, Koehler JE, Berger TG, Cockerell CJ, Lee T-H, Busch MP, Stites DP, Mohle-Boetani J, Reingold AL, LeBoit PE. Bacillary angiomatosis and bacillary splenitis in immunocompetent adults. Ann Intern Med 1993;118(5):363-5. [PubMed]
83. Tappero JW, Mohle-Boetani J, Koehler JE, Swaminathan B, Berger TG, LeBoit PE, Smith LL, Wenger JD, Pinner RW, Kemper CA, et al. The epidemiology of bacillary angiomatosis and bacillary peliosis. JAMA 1993;269(6):770-5. [PubMed]
84. Tsuneoka H, Yanagihara M, Nojima J, Ichihara K. Antimicrobial susceptibility by Etest of Bartonella henselae isolated from cats and human in Japan. J Infect Chemother 2010;Epub ahead of print [PubMed]
85. Weinman D, Kreier JP. Bartonella and Grahamella. In: Kreier JP, ed. Parasitic Protozoa. New York: Academic Press, Inc.; 1977:197-233. [PubMed]
86. Welch DF, Carroll KC, Hofmeister EK, Persing DH, Robison DA, Steigerwalt AG, Brenner DJ. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol 1999;37(8):2598-601. [PubMed]
87. Wolfson C, Branley J, Gottlieb T. The Etest for antimicrobial susceptibility testing of Bartonella henselae. J Antimicrob Chemother 1996;38:963-8. [PubMed]
88. Wong MT, Dolan MJ, Lattuada CP, Jr., Regnery RL, Garcia ML, Mokulis EC, LaBarre RA, Ascher DP, Delmar JA, Kelly JW, et al. Neuroretinitis, aseptic meningitis, and lymphadenitis associated with Bartonella (Rochalimaea) henselae infection in immunocompetent patients and patients infected with human immunodeficiency virus type 1. Clin Infect Dis 1995;21(2):352-60. [PubMed]
89. Zhang P, Chomel BB, Schau MK, Goo JS, Droz S, Kelminson KL, George SS, Lerche NW, Koehler JE. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proceedings of the National Academy of Sciences USA 2004;101:13630-5.[PubMed]
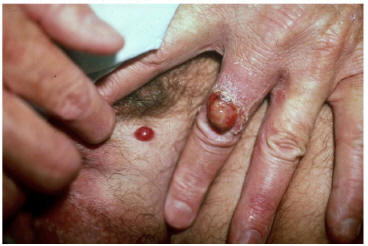
A friable, exophytic angiomatous BA nodule of the finger and an evolving dome-shaped vascular papule in the same patient
(Figure 2, From CID 1993;17:612-624)
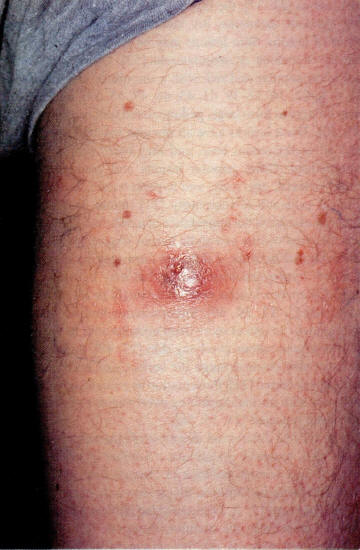
Unusual-appearing erythematous, dry, scaling plaque of cutaneous BA mimicking staphylococcal pyoderma: R. quintana was cultured from this lesion (Figure 3, From CID 1993;17:612-624)
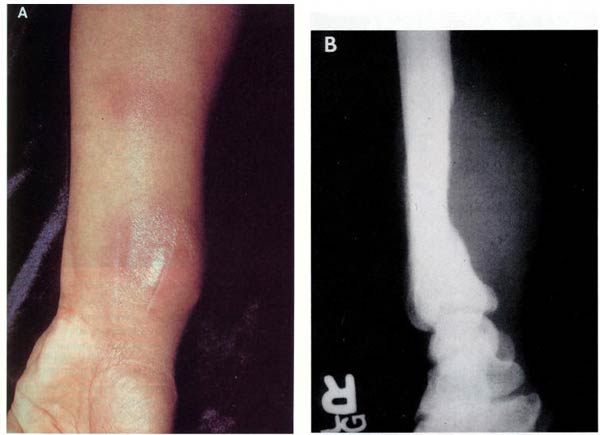
A: tense, firm, erythematous wrist mass due to BA.
B: a roentgenogram of the wrist of the same patinet demonstrates cortical bone erosion of the radius with active periostitis, adjacent to the vascular soft-tissue mass
(Figure 4, From CID 1993;17:612-624)
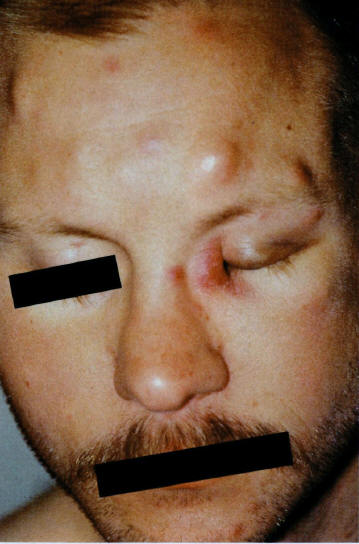
Multiple subcutaneous BA nodules in a patient with concomitant KS of the medial left-eye canthus
(Figure 5, From CID 1993;17:612-624)
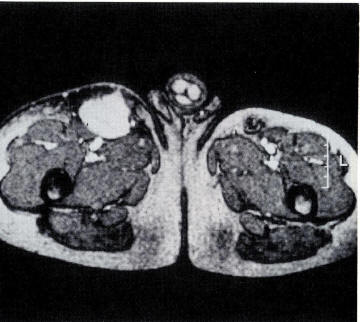
A magnetic resonance image shows a deep, highly vascular subcutaneous soft-tissue mass of BA in the anterior right thigh.
(Figure 6, From CID 1993;17:612-624)
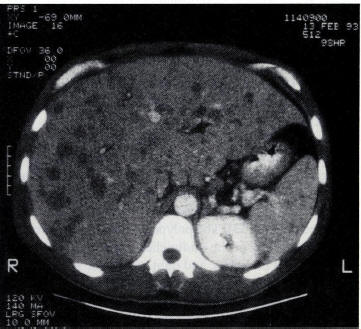
A computed tomogram of the abdomen shows hepatosplenomegaly with numerous low-density hepatic parenchymal lesions in addition to pelvic ascites and pulmonary effusions. Peliosis hepatis was demonstrated on histopathologic examination of a liver specimen obtained by percutaneous liver biopsy.
(Figure 7, From CID 1993;17:612-624)
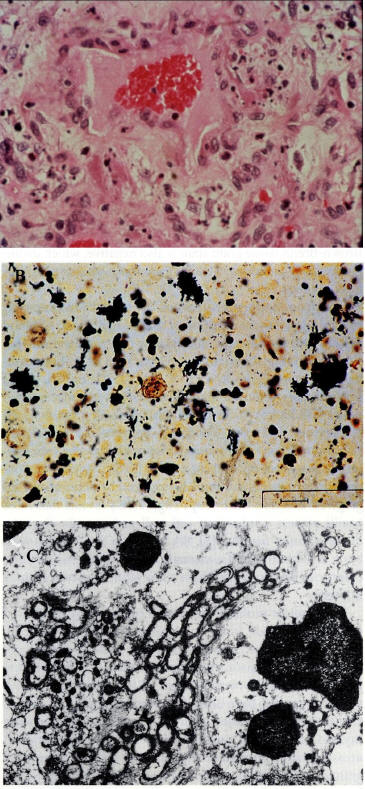
A: hematoxylin and eosin staining of a biopsied cutaneous BA lesion demonstrated a dermal vessel. The vessel is lined with protuberant endothelial cells surrounded by myxoid connective tissue containing neutrophils and amphophilic granular material in close proximity to the vascular lumen (original magnification x240).
B: Warthin-Starry staining of cutaneous BA tissue reveals multiple clumps of tangled, dark silver-straining bacillary organisms (bar=10μm).
C: a transmission electron micrograph of cutaneous tissue shows multiple trilaminar cell-walled bacillary organisms
(Figure 8, From CID 1993;17:612-624)
What's New
Maman E, et al. Musculoskeletal Manifestations of Cat Scratch Disease. Clin Infect Dis. 2007 Dec 15;45:1535-40.
Breitschwerdt EB, et al. Bartonella sp. Bacteremia in Patients with Neurological and Neurocognitive Dysfunction. J Clin Microbiol 2008;46:2856-2861.
Angelakis E, et al. Human Case of Bartonella alsatica lymphadenitis. Emerg Infect Dis 2008;14:1951-1952.
GUIDED MEDLINE SEARCH FOR:
Review articles
Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The Two faces of interleukin 10 in human infectious diseases. Lancet Infectious Diseases 2006:7;557-569.
Rolain JM, et al. Recommendations for Treatment of Human Infections Caused by Bartonella species. Antimicrobial Agents Chemother 2004;48(6):1921-1933.
Kaplan JE, et al. Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America, 2009.
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
History
Berger S. Emergence of Infectious Diseases into the 21st Century, 2008.
Cadena J, et al. Carrion's Disease, 2008.
Raoult D. From Cat Scratch Disease to Bartonella henselae Infection. Clin Infect Dis. 2007 Dec 15;45:1541-2.
Schultz, M. Daniel Alcides Carrion. Emerg Infect Dis. 2010 June,16:1025-7.
GUIDED MEDLINE SEARCH FOR HISTORICAL ASPECTS
Table of Contents
- Microbiology
- Epidemiology
- Clinical Manifestations
- Laboratory Diagnosis
- Pathogenesis
- Susceptibility in Vitro and in Vivo
- Antimicrobial Therapy
- Antimicrobial Therapy for Bartonella Infection in the Immunocompromised Patient
- Antimicrobial Therapy for Bartonella Infections in the Immunocompetent Patient
- Drug of Choice in the Immunocompetent Patient
- Uncomplicated Cat Scratch Disease
- Special Infections in the Immunocompe
tent Patient Complicated Cat Scratch Disease Including Retinitis, CNS Infection and Granulomatous Hepatitis - Relapsing Bacteremia
- Edocarditis
- Oroya Fever and Verruga Peruana
- Bartonella elizabethae
- Bartonella vinsonii subsp.
arupensis
- Alternative Therapy in the Immunocompetent Patient
- Combination Therapy in the Immunocompetent Patient
- Drug of Choice in the Immunocompetent Patient
- Adjunctive Therapy
- Endpoints for Monitering Therapy
- Vaccines
- Prevention
- References