Sepsis
Authors: Daniel Sweeney, M.D., Michael J. Cuttica, M.D., Lawrence Osei M.D., Anthony Suffredini, M.D., Henry Masur, M.D.
DEFINITION
The word sepsis is derived from the Greek,sêpsismeaning “decay or “to rotten.” During the last century, this term was used to describe a broad range of systemic infectious disease states independent of the severity of the illness. The absence of precise terminology to describe protean manifestations and the degree of disease hampered clinical trial design and the development of management strategies for sepsis. Clinical experience suggested that the presence of shock and the development of organ failure syndromes were key factors in determining outcome from serious infections yet these characteristics were not delineated by the term sepsis.
To address the need for more precise terminology, a consensus conference standardized the definitions used to describe severe infections and their sequelae (Table 1) (7). These definitions recognized that following the development of an infection or after sustaining an injury (i.e. trauma or pancreatitis), a systemic inflammatory response syndrome (SIRS) occurs that can be manifested by fever, tachycardia, tachypnea and leukocytosis . “Sepsis” was defined as the invasion of sterile tissue by one or more microbial pathogens with a resultant systemic inflammatory response. It is important to note, however, that in 70% of cases of sepsis, the offending pathogen could not be identified although infection seemed to be the only plausible initiating agent. The severity of sepsis was delineated based on responses to fluid resuscitation and the presence of new organ failure syndromes (e.g. coagulopathy or renal insufficiency). “Severe sepsis” compared to “sepsis” was characterized by a more severe systemic inflammatory response to infection resulting in hypotension and organ hypoperfusion (e.g. renal failure, impaired mentation or hypoxemia). “Septic shock” was the more grave consequence of an infection manifested by persistent hypotension despite adequate fluid resuscitation and organ dysfunction.
W hen evaluated prospectively these definitions describe a continuum from systemic inflammatory responses, to sepsis (26% of patients with evidence of systemic inflammatory response), to severe sepsis (18% of septic patients) and ultimately septic shock (4% of severe septic patients) (64). The risk of death increased incrementally with the severity of illness (systemic inflammation 7%, sepsis, 16%, severe sepsis 20% and septic shock 46%) (64). This hierarchical model of the severity of infection and the clinical manifestations of the host response to infection has provided a pragmatic to tool to compare clinical manifestations of infectious syndromes.
EPIDEMIOLOGY
In the United States sepsis causes more than 200,000 deaths annually and is the second most common infectious cause of death in the United States after pneumonia (23). The crude mortality rate is 15%, but increases to 20 and 45% for severe sepsis and septic shock respectively (Table 1) (88). Recent epidemiologic data suggests that the incidence of sepsis is increasing at a rate of 8.7% annually (39). A number of factors are likely to be contributing to this increase: improved recognition and reporting; increased age of the general population;incr eased numbers of patients at risk for sepsis because of compromised immunity (i.e. HIV infection, therapies for malignancies or organ transplant recipients); and increased use of indwelling devices (e.g. vascular access devices) as well as the emergence of newly resistant pathogens in the community. The risk of sepsis is greatest in the very young and the elderly (39, 87). Among pediatric patients, sepsis is more common in infants less than one year of age and affects boys more than girls (87). The incidence of sepsis is higher in men than women and occurs more frequently in minorities (39).
Over the last fifty years, there has been a change in the predominant organisms causing sepsis. In the early 1960s, gram-negative bacilli were the most common cause of bacteremia and sepsis whereas in the last two decades, gram-positive cocci and yeasts have emerged as the major microbial pathogens in sepsis (39). Patients who survive an episode of sepsis have an increased risk of dying during the subsequent five years when compared to controls (62). Whether this is a direct result of the episode of sepsis, or is a marker of co-morbid conditions that predisposed to the development of sepsis is not known (62).
PATHOGENESIS
Sepsis results from a systemic immune response with multiple inflammatory mediators activated by microbial invasion of sterile tissue or the blood stream. These inflammatory responses are necessary to survive an infection, but may also result in host tissue injury (i.e. acute lung injury, hypotension or acute renal failure) (80). Sepsis includes a broad range of syndromes. The clinical picture of a child in the throes of purpura fulminans and an elderly patient with urosepsis are strikingly different, yet there are aspects of host immune response and pathologic mechanisms that are common to both of these septic patients (Figure 1).
Review Article: Van der Poll, T. and Opal, S. Host-pathogen Interactions in Sepsis. The LANCET Infectious Diseases 2008; Vol.8, Issue 1, 32-42.
Innate Immunity: Detection and Early Response to Infection
Infection can initiate a cascade of inflammatory events that results in sepsis. Characteristics of the host’s immunologic integrity (i.e. age, comorbidities, or indwelling hardware) and the pathogen’s virulence (i.e. factors such as pili, polysaccharide capsules and exotoxins) influence the severity of the syndrome and the patient’s outcome. Host survival requires the ability to identify, contain and eradicate a microbial invader. Innate immunity represents the immediate nonpathogen specific host response to infection. Adaptive immunity, on the other hand, is a less rapid (occurring over three to five days) but more precise response to an infection requiring the clonal expansion of lymphocytes and the production of antibody to a specific pathogen (41).
The innate immune system engages pathogens via pattern recognition receptors (PRR). These receptors are genetically predetermined and are believed to have evolved through natural selection (41). The PRRs bind to molecular structures termed pathogen-associated molecular patterns (PAMPs) which are shared across microbial species (e.g. endotoxin or peptidoglycan). Host pattern recognition receptors can be divided into three groups: secreted, endocytic and signaling (41). Mannose-binding lectin and C-reactive protein, are prototypical secreted PRRs that bind to microbial cell membrane components leading to complement activation. Endocytic PRRs such as macrophage mannose receptor are located on the surface of phagocytes and facilitate the phagocytosis of pathogens(37). Signaling PRRs, include Toll-like receptors (TLR) and the nucleotide-binding oligomerization (NOD) proteins, NOD1 and NOD2. Ten human TLRs have been discovered on the surface of various cells including phagocytes. Unlike TLRs which are cell membrane bound, NOD1 and NOD2 are intracellular proteins found in epithelial, monocytes, dendritic cells and granulocytes where they bind to bacterial peptidoglycan moieties (28). The diversity of ligands for TLRs and NODs permit the detection and subsequent response to a broad range of infections (35). And both TLRs and NODs have attracted interest as potential targets for therapeutic blockade (42).
Cytokines and Other Mediators: Signal Amplification and Coordination of the Immune Response
Both TLR and NOD pathways lead to the activation of nuclear factor kappa binding (NF-κB) transcription factor and amplification of the initiating signal via the production of cytokines. In general, cytokines are signaling molecules which can be classified as either pro or anti-inflammatory. Activation of NF-κB leads to the synthesis and release of pro-inflammatory cytokines tumor necrosis factor (TNF), and interleukin-1 (IL-1) by cells of the innate immune system including monocytes and macrophages (55, 76). These signaling molecules augment a series of local and systemic inflammatory responses and engage various components of the immune and coagulation systems including: acting on the hypothalamus to induce fever; stimulation of bone marrow to release neutrophils; activation of endothelium to promote extravasation of phagocytes and complement to the site of infection; triggering of hepatocytes to synthesize C-reactive protein which binds cell wall components of bacteria, fungi and parasites; and the promotion of platelet-endothelial adhesion with clot formation (29). In addition to IL-1 and TNF other prominent pro-inflammatory cytokines and immune modulators involved in the immune response to infection include IL-8, IL-12 and interferon gamma. Absolute pro-inflammatory cytokine deficiencies can predispose the host to overwhelming infection and death while excessive amounts can cause damage to the host. Elevated levels of pro-inflammatory cytokines are also associated with increased mortality and experimental infusions of TNF produce a clinical syndrome identical to sepsis which can be reversed with antibody therapy specific to TNF (4, 65, 81, 86). Thus, the systemic effects of cytokines including vasodilatory shock and disseminated intravascular coagulation have been implicated in the pathogenesis of sepsis. Nonetheless, while clinical trials aimed at improving survival in septic shock have targeted these pro-inflammatory cytokines, no trial inhibiting a single cytokine has shown clinical benefit (17, 54, 90).
Immunosuppression: Confining Inflammation to the Site of Infection or Predisposing the Host to Sepsis
Paradoxically, infection induces host production of anti-inflammatory mediators. Traditionally, this immunosuppressive response was thought to curb inflammation during the recovery phase of infection. More recent evidence suggests that normally pro and anti-inflammatory signaling pathways are activated simultaneously and serve to concentrate the immune response at the site of insult all the while limiting systemic immune activation (48). Circulating IL-10 which inhibits phagocyte activation and cortisol which inhibits cytokine synthesis by monocytes are both increased in the setting of infection. Similarly, blood levels of both IL-1 receptor agonist and soluble IL-6 receptors are also elevated with infection thereby limiting the systemic effect of their respective pro-inflammatory ligands (48). Inflammatory markers in bronchial washings of patients with Acute Respiratory Distress Syndrome (ARDS) with paired blood samples from the same host are consistent with this dichotomous model of inflammation at the site of infection and systemic immunosuppression (61). Appreciation for the role of anti-inflammatory mechanisms has given rise to the thought that some of the complications of sepsis may in part be a function of an immunosuppressed state (referred to as immune paralysis) in which anti-inflammatory cytokine levels are disproportionately elevated and immune cells either respond poorly to pro-inflammatory stimulation or actually undergoing apoptosis (8, 16, 20, 24, 25, 67).
Septic Shock and Subsequent Organ Dysfunction
Sepsis may progress to cardiovascular collapse and multiple organ dysfunction that culminate in death or residual organ failure. Vascular endothelial dysfunction is central to the development of the hypotension and end organ injury in sepsis. Nitric oxide (NO), a signaling molecule and potent vasodilator, is produced by nitric oxide synthase (NOS) present in vascular endothelium and smooth muscles cells. In sepsis an inducible isoform of NOS (iNOS) is over expressed in response to pro-inflammatory cytokines (71). The resultant increase in NO plays a prominent role in the vasodilatation that is a hallmark of septic shock (34, 44). Administration of NOS inhibitors to septic patients reverses shock, but also increased mortality secondary to widespread tissue ischemia (21). Vasopressin deficiency which is frequently present in septic patients may contribute to septic shock and organ dysfunction at the vascular endothelial level (33).
In addition to systemic hypotension, maldistribution of blood flow between organ systems has also been implicated as a contributory factor in sepsis (2). Microvascular dysfunction secondary to activation of the coagulation system and the formation of micro thrombi further impairs oxygen and nutrient delivery at the cellular level. Even when blood flow is believed to be adequate there is evidence that tissues are unable to optimally extract oxygen. This may in part be due to decreased mitochondrial function (79).
In addition to the direct effect on the vasculature, sepsis directly impairs cardiac performance which further compromises organ perfusion. The usual hemodynamic profile of septic shock includes high cardiac output, low mean arterial pressure and tachycardia. Left ventricular function is depressed as evidenced by a decrease in the ejection fraction. Patients who survive sepsis maintain stroke volume by undergoing acute ventricular dilation which reverses during their recovery phase (50, 57). Elevated levels of troponin may be observed in sepsis; however the cause myocardial dysfunction is not ischemia as coronary blood flow is maintained (11). Circulating immune mediators are likely to contribute to the depressed ventricular function observed in sepsis although no specific agent has been identified (58, 60).
(Printable Version of Definition of Sepsis)
DIFFERENTIAL DIAGNOSIS
The differential diagnosis for SIRS is extremely broad and this is reflected in the fact that more than two-thirds of intensive care unit patients and a substantial number of patients on general medical units at some point during their hospitalization meet the criteria for this diagnosis (37a, 84a). Causes of SIRS include both infectious and noninfectious conditions such as trauma, autoimmune disease, drug reaction, myocardial infarction and pulmonary embolism . When a patient with SIRS develops concomitant shock it imperative that the hypotension be further defined as therapies for various types of shock differ greatly. There are four broad shock states: hypovolemic, distributive, cardiogenic, and extracardiac obstructive shock. The various shock states can be distinguished by characteristic hemodynamic profiles and clinical characteristics. However, as patients have increasing comorbidities, either confounding organ dysfunction or overlapping features can cloud this differential (Tables 2 and 3). Each type of shock will be briefly reviewed in the context of a differential diagnosis for a patient presenting in septic shock.
Hypovolemic shock is caused by a decrease in circulating blood volume. The decrement in blood volume can be either hemorrhagic in origin (e.g. related to trauma or GI bleeding) or non-hemorrhagic (e.g. due to vomiting, diarrhea, burns with increased insensible losses, or polyuric states such as diabetes insipidus or DKA). The patient in hypovolemic shock presents with tachycardia and hypotension. Extremities are cool to touch and cyanotic related to a low cardiac output state. Patients exhibit signs of hypovolemia such as collapsed neck veins and oliguria or anuria. Similar findings can be seen in early septic shock when a vasodilatory response to overwhelming infection leads to a relatively hypovolemic picture.
Distributive shock is characterized by vasodilatation with either normal or elevated cardiac output. Classically, patients will present with warm extremities and tachycardia although decompensated distributive shock may be notable for extremities which are cool to touch. Organ injury is due to the inadequate cardiac preload, maldistribution of blood flow and may be further worsened by the inability to utilize oxygen adequately on a cellular level (89). Besides sepsis, anaphylaxis or neuralgic injury with loss of autonomic nervous system function can also cause distributive shock; therefore, allergen exposure or spinal cord injury should be considered when a patient presents with a clinical picture consistent with distributive shock.
Cardiogenic shock results from inadequate tissue perfusion due to cardiac dysfunction. It is characterized by a low cardiac output and a high systemic vascular resistance in the setting of normo- or hypervolemia. Cardiac dysfunction can be driven by myocardial issues such as ischemia/infarction and cardiomyopathies, mechanical issues such as valvular disease or conduction issues such as arrhythmias. In the setting of the septic patient it is important to consider primary cardiac issues in the differential of the shock state since underlying heart disease can complicate the management of the septic patient. In addition, septic shock can have primary cardiac depressive effects which will effect the presentation of the patient.
Extracardiac obstructive shock can be divided into two broad categories: increased intrathoracic pressure and intrinsic vascular flow obstruction. The primary effect of both categories can be either pump failure if cardiac output is compromised or loss of preload if blood return to the heart is compromised. As a result, extracardiac obstructive shock can present as a primarily cardiogenic shock type picture when the inciting event leads to primary cardiac failure or it can also present as a hypovolemic picture when preload is lost, or a mixture of the two. Increases in intrathoracic pressure leading to a shock state can be seen in the setting of a tension pneumothorax or as a result of positive pressure ventilation such as in obstructive lung disease with air trapping and the progressive rise of auto peep. Intrinsic vascular flow obstruction can be seen in massive pulmonary embolism, cardiac tamponade, and severe pulmonary hypertension. It is important to consider these issues in the differential diagnosis of the septic patient as some of these are mechanical issues which can be corrected expeditiously leading to rapid hemodynamic improvement.
CLINICAL MANIFESTATIONS
Neurologic Dysfunction
A decline in a patients level of consciousness can be one of the first manifestations of sepsis (6, 56). Focal neurologic deficits and seizures are rare and the brain appears anatomically normal on imaging unless patients have pre-existing disease that enhances the likelihood of ischemia induced focal pathology . Patients who have a decline in mental status related to sepsis have worse outcomes than those who do not (5, 78). The etiology of this impaired level of consciousness is multifactorial and includes decreased cerebral perfusion and oxygen extraction.
Pulmonary Dysfunction
Sepsis is one of the most common causes of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS)(18). The sepsis syndrome accounts for up to 43% of cases of ARDS and leads to a significant increase in ICU mortality (26). How sepsis causes diffuse alveolar epithelial injury is unclear. As lung function and hypoxia worsen tissue perfusion and oxygenation are compromised. ARDS can readily be confused with diffuse pneumonia, or can be complicated by secondary pneumonia, and thus much ARDS is in fact a mixed picture with overlapping pathologies.
Cardiovascular Dysfunction
Global cardiac depression is common in sepsis as described above. And there is no convincing evidence for ischemia as a cause of myocardial dysfunction in sepsis (11, 22). Nonetheless, it is important that clinicians be cognizant of the fact that patients with preexisting coronary artery disease can suffer regional ischemia and infarction in the setting of sepsis (44).
Renal Dysfunction
Oliguria and renal failure are common complications of sepsis. Renal dysfunction is caused by the shunting of blood away from the renal vascular bed to other vital organs. Oliguria may resolve with fluid resuscitation. Renal failure associated with sepsis is usually reversible. However, many other causes of renal dysfunction can complicate clinical presentation and outcome, such as contrast nephropathy, drug toxicity, diabetes, or hypertension.
Gastrointestinal Dysfunction
Hypoperfusion of the GI tract in sepsis can increase intestinal permeability which is associated with the onset of multiple organ dysfunction syndrome (13). Mucosal hypoperfusion can lead to ulceration and increase the risk of GI bleeding. These consequences of sepsis can influence the efficacy of enteral nutrition, and the access of bowel flora to lymphatics and capillaries.
DIAGNOSIS
Patients with sepsis need a careful history and physical examination. While laboratory assessment and monitoring provides invaluable and sometimes essential information for diagnosis and for therapeutic monitoring, failure to assess the history and failure to examine the patient carefully can lead to unnecessary therapies with resulting toxities, and to failure to initiate appropriate therapy promptly, thus reducing the likelihood of a successful outcome.
History can direct diagnosis in many obvious ways. The environmental flora is important: specific pathogens might be suggested if a patient traveled to the Southwest United States (Coccidioides), to Sub-Saharan Africa (malaria or Yellow Fever) or to Martha’s Vineyard (Tularemia, Babesisia, Anaplasma). Specific pathogens might also be suggested by recent food ingestion (peanut butter and salmonella), travel on a cruise ship (Norovirus), or membership on a team with reported cases of MRSA skin disease . Pre existing medical conditions (ureteral calculi), immunosuppression (chronic corticosteroids, recent human stem cell transplant) are examples of the myriad of information that a skilled history can elicit.
Physical examination is also vital to an informed management strategy. The presence of foreign bodies (intravascular catheters or urinary catheters), signs of pulmonary consolidation, skin lesions suggesting infection, or abdominal distention may direct the diagnostic and therapeutic strategies in more focused directions than an unfocused, empiric, “shotgun” approach.
Imaging studies are increasingly important for localizing the source of infection. Computer assisted tomography (CAT scans) and magnetic resonance imaging (MRI) are more and more standardized for patients who can be transported and safely monitored for the procedure. Consideration must also be given to the advantage and toxicities of contrast agents. Such imaging is vital for providing diagnostic clues based on patterns or disease, or providing targets for aspiration or biopsy. Such imaging is also essential for recognizing obstructions of vital structures such as bronchi or ureters, for identifying collections of purulence or foreign bodies that must be removed.
For patients with sepsis, severe sepsis, or septic shock, any potential source of infection should be carefully assessed. Bronchoscopy, lumbar punctures, sinus aspirations, soft tissue aspirations or biopsies, and lymph node biopsies are among the many invasive techniques that are a standard component of an effective search for the causative process and identification of the causative organism. Any purulent discharge needs to be examined by direct microscopy and culture. Similarly, suspicious body fluids, secretions, and excretions should be similarly examined, such as sputum, urine, stool, or CSF. Tissue biopsies of skin, lung, lymph nodes, or aspiration of collections are clearly important. Microbiology laboratories are more and more likely to be distant from the area of patient care. Despite this, clinicians must communicate with the laboratory to assure that specimens are collected and transported properly, and that the optimal stains and cultures are performed. As diagnostic and therapeutic interventions become more feasible for viruses, fungi, fastidious bacteria, optimal management requires increasingly sophisticated diagnostic approaches.
Blood cultures are an important part of a diagnostic evaluation. The likelihood that useful information will be derived from blood cultures depends on selecting patients with a reasonable likelihood of having organisms in their blood stream. A patient with a likely viral infection, or a patient who is stable with a bacterial infection and doing well on antimicrobial therapy, by definition, is unlikely to be bacteremic or fungemic. Obtaining a blood culture on such a patient is more likely to yield a contaminant than a true pathogen, potentially causing unnecessary drugs, unnecessary expense, and unnecessary toxicity. Thus, care must be exercised in choosing which patients to draw blood cultures on, when to draw cultures, and what types of cultures to obtain.
Blood cultures should ideally be obtained before new antimicrobial therapy is started. Obtaining blood cultures prior to starting or switching antibiotics will increase the likelihood that such cultures will identify the causative agent. However, obtaining cultures show not substantially delay the initiation of potentially life saving therapy.
Ablood culture is defined as a single blood draw with inoculation of all bottles used by the laboratory’s culture system. Some blood culture systems will require inoculation of two bottles, but others require one or three bottles for routine use. The suggested quantity of blood should be drawn: suboptimal ratio of media to blood, or suboptimal volume of blood will reduce the yield of cultures. Extra bottles must be inoculated for special pathogens such as viruses, certain fungi, certain zoonoses and mycobacteria. The yield of blood cultures, once a high risk patient and an appropriate time (e.g. before antimicrobial agents are started) are identified, depends on the volume of blood drawn and the processing technique. Current guidelines for evaluating fever in the intensive care unit now recommend three cultures be drawn rather than two cultures based on the relationship between blood culture yield and volume of blood (53). When blood cultures are drawn, at least two sets should be drawn. Having two sets offers several advantages: 1) the volume of blood cultured is double that of a single culture, increasing the yield; 2) if an organism is identified that could be a contaminant, having two positive cultures provides more convincing evidence than a single positive culture; 3) if there is an intravascular catheter in place, drawing one culture percutaneously and one through the catheter (or through the catheter most likely to be infected due to chronology or local signs or symptoms) permits an assessment of “time to positivity” which can provide suggestive information as to whether or not the catheter is the source of any bacteria or yeast cultured from the blood (43, 63, 69). Molecular tests on blood and body fluids are an increasingly important part of the diagnostic armamentarium, especially for viruses and fungi. Gene probes for tuberculosis, rapid tests for influenza or RSV virus, or PCR for hepatitis B or C or for HIV are examples of molecular techniques that are becoming routine aspects of patient evaluation. Serial serologies are useful diagnostically, but results rarely come back in time to influence acute management. Some serologies can be useful for patients with some form of sepsis, such as West Nile IgG and IgM, or Lyme disease ELISA and Western Blot. There has also been interest in surrogate markers for the presence of sepsis.While markers such as CRP, protein C, leptin, tumor necrosis factor and procalcitonin have been studied with intriguing results, there is no clearly established role for such biomarkers in the diagnosis or management of sepsis (10, 36, 74).
Review Article: Gilbert DN. Use of Plasma Procalcitonin Levels as an Adjunct to Clinical Microbiology. J Clin Microbiol 2010;48:2325-2329.
(Printable Version of Causes and Diagnosis of Sepsis)
SOURCE CONTROL
While antimicrobial therapy can be life saving, some patients cannot be successfully treated without removing or draining the site of infection. When patients have septic shock due to an infected catheter, patients are far more likely to have a successful outcome if the catheter is promptly removed. Similarly, if the source of sepsis, severe sepsis, or septic shock is an infected foreign body such as a joint prosthesis, a pacemaker, or an Omaya shunt, that device must be removed to optimize outcome. Foreign bodies such as biliary stints, urinary stints, Foley catheters, chest tubes, or ventriculostomies must usually be removed to optimize outcome. There are, however, some specific syndromes and specific pathogens that can be treated medically if there is an urgent need to do so, without removing the foreign body.
Collections of purulent fluid must be drained in most situations. Urosepsis associated with an obstructed ureter, a pneumonia with an associated empyema, a liver abscess, or a renal abscess are examples of collections that must be drained to optimize outcome. How that drainage is accomplished depends on the location, the infection, and the patient. In some situations needle drainage is adequate; in other situations more extensive surgical drainage is required. In some situations, such as brain abscesses and liver abscesses, medical therapy alone can be successful, but such an approach requires judgment, careful evaluation of the literature, with focus on how ill the patient is and what the risks and benefits of the technical options are.
EMPIRIC ANTIMICROBIAL THERAPY
The urgency of e mpiric therapy depends on the severity of the clinical syndrome and the vulnerability of the host. For some patients, there is no urgency to start therapy until the offending pathogen is identified. For others with severe sepsis or septic shock, empiric therapy is life saving, i.e. antimicrobial therapy must be started before the specific pathogen or, in some cases, before the site of infection, is identified.
Table 4 provides some potential empiric regimens for the therapy of septic shock. Many possible regimens could be used with similar results. The choice in each situation depends on the pathogens likely to cause a specific infectious syndrome, knowledge of organisms the host has been colonized with, consideration of what antibiotics the patient has recently been exposed to, local epidemiologic trends in pathogens and resistance patterns, and patient ability to tolerate specific agents.
When developing regimens, particular attention should be given to MRSA and Candida, in addition to pathogens such as Acinetobacter or Pseudomonas that may be especially prevalent in a given setting. For seriously ill patients, MRSA has become especially common in many areas when skin, soft tissue, catheter, or pulmonary infection s are involved, or when patients present with sepsis of unknown source. Thus, antibiotic coverage for MRSA should be universally considered, i.e. there should usually be a clearly articulated rationale for not including such coverage.
Candida is the fourth most common pathogen causing blood stream infections in intensive care units. Moreover, candidemia is increasingly common among patients with central venous catheters, arterial catheters, neutropenia, and complicated gastrointestinal surgical procedures. Since time to initiation of therapy for Candida correlates with outcome, consideration of an antifungal agent should be part of the initial management algorithm (27, 47, 73). For patients with severe sepsis or septic shock, the initiation of appropriate therapy is urgent. Several observational data bases have demonstrated that outcome become progressively less favorable the longer the interval until appropriate therapy is started (32, 47). Thus, initial regimens need to be broad in order to be certain that the causative agent is treated. The initial regimen can subsequently be narrowed when the causative agent is definitively identified. The initial agent must be infused into the patient promptly, and not merely ordered in the computer: the system involved in transferring a cognitive plan to administer antibiotics to the successful infusion into the patient involves many steps that require the medical, nursing, pharmacy and information technology programs to work cohesively. In most hospitals, a substantial number of patients do not receive their antibiotics within 30-60 minutes of an order being written. For septic shock in particular, clinicians must work with nursing and pharmacy to assure that the patient has enough ports for infusion, that infusion times of individual drugs are considered in their selection and prioritization. Clinicians must recognize that blood cultures will be negative > 50% of patients with septic shock. Thus, once a clinical diagnosis of severe sepsis or septic shock has been established, patients should receive a full course of therapy (usually 7-10 days) unless another cause of the syndrome has been conclusively identified.
SPECIFIC ANTIMICROBIAL THERAPY
Once the causative pathogen has been conclusively identified and antibiotic susceptibility results have been obtained, the broad empiric regimen can usually be narrowed. Clinicians must recognize, however, that certain infectious syndromes may be polymicrobial. Finding a single organism in the bloodstream does not invariably indicate that only a single organism is involved. For instance, in a patient with a colonic perforation, finding E. coliin 4 blood cultures is useful information, but the likelihood is that many other aerobic and anaerobic organisms care also pathologically important in causing the septic syndrome. Thus, judgment is required in determining whether to narrow the spectrum of antibiotic therapy. In contrast, if Streptococcus pneumoniaeis isolated from the bloodstream in a patient with sepsis and pneumonia, it is extremely likely this is the sole pathogen, and antibiotic therapy can be significantly narrowed.
There has been considerable debate in the past about the use of bactericidal antibiotics versus bacteristatic drugs. Except for endocarditis, there has been no evidence that bactericidal drugs are more effective, i.e. that their use produces improved patient outcome. Many clinicians prefer bactericidal drugs for neutropenic patients or for severely ill patients. However, there is no clinical evidence support this preference. There has also been debate regarding the desirability of combination therapy versus combination therapy for treatment of a specific pathogen. Combination therapy is a logical strategy in terms of assuring that the breadth of coverage include all likely pathogens when patients are severely ill, and there is no margin for error. However, for treating specific pathogens, there are few examples in which combination therapy is superior to monotherapy when modern, potent agents are used (70).
The optimal duration of antibiotic therapy has not been studied for most types of sepsis, severe sepsis, and septic shock. The duration of therapy will be determined by the rapidity of host improvement, host immunologic status, presence of undrained purulence or unremoved foreign bodies. While 7-10 days in non neutropenic patients with no undrained focus is often recommended, there is little data on which to base this recommendation on. Moreover, there are situations where shorter courses are clearly effective (75). The optimal drugs for specific microorganisms isolated in septic patients can be found in other chapters in this book.
ADJUNCTIVE THERAPIES FOR SEPSIS AND SEPTIC SHOCK
The four essential components of early therapy for patients with sepsis or septic shock are: source control, prompt administration of empiric antibiotics that take into account the most likely pathogen and anti-microbial sensitivity, fluid and vasopressor administration to maintain organ perfusion and source control (i.e., drainage of abscess, removal of infected hardware etc) (32, 49). Several adjunctive therapies are also commonly used although the evidence supporting their efficacy is less convincing.
Fluid Resuscitation and Vasopressors
Severe sepsis or septic shock requires fluid resuscitation in order to maintain perfusion of vital organs (49). The usual treatment approach for severe sepsis or septic shock in adults is to initiate fluid support using either boluses crystalloid (1000 ml of normal saline or Ringer’s lactate) or colloid (500 ml of 5% albumin) given over 15 to 30 minutes with a goal to maintain a mean arterial pressure of ≥ 65 mmHg or a systolic blood pressure of ≥ 90 mmHg. Hydroxyethyl starch is not recommended since it is associated with higher rates of renal failure (9). Fluid resuscitation should be guided by hemodynamic monitoring (preferably with an arterial line and a central venous catheter), and evidence of end organ perfusion (e.g., urine output >0.5ml·kg-1·hr-1, lactate<4mmol/L and maintenance of mental status). If resuscitation goals are not being promptly achieved after several liters of fluid or if clinical evidence of pulmonary edema develops then vasopressor therapy should be administered via a central line. Norepinephrine has emerged as the standard vasopressor agent for septic shock although other vasopressors are used including dopamine less commonly epinephrine (38). There is a theoretical advantage to treating septic shock patients with low dose vasopressin; however, in a large prospective trial the use of vasopressin in conjunction with norepinephrine did not confer a survival benefit (33, 68). Nonetheless, adjunctive vasopressin therapy should be considered as a rescue maneuver in patients with septic shock who are not responding to either norepinephrine or dopamine alone (59).
De Backer D, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779-789.
Maitland K. et al. Mortality after Fluid Bolus in African Children with Severe Infection . New Eng J Med. 2011;364(26):2483-95.
Glucocorticoids
The use of glucocorticoids in sepsis is controversial. Meta-analysis has shown that low doses (hydrocortisone 200 to 300mg daily for 5 to 7 days) confer a mortality benefit in severely septic patients (46). A recent trial of septic shock patients with a relatively low mortality rate (control group mortality 36%) showed that steroid therapy reduced the duration of hypotension, but had no mortality benefit (77). In light of these data, a reasonable approach is to consider low dose glucocorticoid therapy in severely ill septic shock patients who are not improving (i.e., vasopressor refractory shock) with standard treatment. Once steroid therapy is initiated, there should be a low threshold for suspecting occult secondary infections as bacteremic patients on steroids will not consistently mount a fever response (40).
Intensive Insulin Therapy
Intensive insulin therapy (serum glucose maintained between 80-110 mg/dl) has been proposed as an adjunctive therapy for sepsis based upon one trial--performed in critically ill postoperative surgical patients—that showed a survival benefit (84). In two subsequent trials of intensive insulin therapy, including one study which specifically enrolled severe septic patients, there was no mortality benefit due to intensive insulin therapy. Importantly, episodic severe hypoglycemia (< 40mg/dl) occurred at an unacceptably high rate (9, 83). In light of the absence of reproducible benefit and the potential harm, intensive insulin therapy is not recommended for septic patients.
Review Article: No longer recommended? Steroids and insulin for septic shock. N Engl J Med 2008.
Early Goal Directed Therapy
Early goal directed therapy (EGDT) in sepsis is defined as prompt fluid resuscitation targeting optimal central venous pressure, mean arterial pressure and central venous oxygenation saturation (ScvO2) (51). While the benefit of treating septic patients in a timely fashion is self-evident, the evidence that measuring ScvO2 is beneficial is less than compelling. In the only prospective trial of EGDT patients in the treatment arm differed from the controls in one aspect: a catheter capable of measuring ScvO2 was used to guide red blood cells transfusions or dobutamine administration. This one positive trial contrasted with the only other prospective trial evaluating titrating therapy to ScvO2 (albeit in critically ill patients) which showed no benefit. There have been no further trials testing EGDT (specifically ScvO2-guided therapy) as a stand-alone treatment (19, 30, 31, 45, 52, 72, 82). Analysis of these trials and other data cast doubt as to the contribution of ScvO2 monitoring to patient outcome (85). Thus, at this time such ScvO2 catheters should not be considered necessary for achieving optimal outcome despite their inclusion in well publicized guidelines and bundles (12).
Drotrecogin alfa
Activated protein C (drotrecogin alfa) is a component of the coagulation cascade and functions as an inhibitor of factors Va and VIIIa thereby promoting fibrinolysis. In addition, drotrecogin alfa has anti-inflammatory properties and is capable of inhibiting proinflammatory cytokine production. Low plasma levels of protein C occur in septic patients (66). Based upon the results of a single randomized controlled trial, this adjunctive therapy was approved for patients in septic shock who had a severity of illness score (APACHE II) greater than 25 within 24 hours of the onset of shock (3). Drotrecogin alfa is administered as a 96 hour infusion. Two subsequent randomized controlled trials, one pediatric study and another in adults septic patients with a low likelihood of death (APACHE II < 25), were both stopped early on the grounds of futility(1, 15). An increased risk of serious bleeding including intracerebral hemorrhage, however, has been observed in all trials of drotrecogin alfa (14). Pending the results of an ongoing confirmatory trial of (PROWESS-SHOCK), drotrecogin alfa has the potential to cause serious bleeding and should be viewed as an optional therapy in patients with sepsis and a high likelihood of death.
CONCLUSION
Sepsis is a major cause of morbidity and mortality in the United States. Recognition of sepsis, severe sepsis or impending septic shock in the emergency department and in hospitalized patients remains a challenge that requires clinical judgement. Management principles for patients with distributive shock focus on an expeditious response involving source control, antimicrobial therapy, fluids, vasopressors and adjunctive therapies. Health care providers and medical facilities have an obligation to develop a systems based approach to assure that therapy is prompt, coordinated, and comprehensive in order to provide optimal patient outcome.
(Printable Version of Management of Sepsis)
REFERENCES [PubMed]
1 . Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, Francois B, Guy JS, Bruckmann M, Rea-Neto A, Rossaint R, Perrotin D, Sablotzki A, Arkins N, Utterback BG, Macias WL. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med 353 (13): 1332-41, 2005. [PubMed]
2 . Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med 35 (10): 2408-16, 2007. [PubMed]
3 . Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ, Jr. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344 (10): 699-709, 2001. [PubMed]
4 . Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229 (4716): 869-71, 1985. [PubMed]
5 . Bleck TP, Smith MC, Pierre-Louis SJ, Jares JJ, Murray J, Hansen CA. Neurologic complications of critical medical illnesses. Crit Care Med 21 (1): 98-103, 1993. [PubMed]
6 . Bolton CF, Young GB, Zochodne DW. The neurological complications of sepsis. Ann Neurol 33 (1): 94-100, 1993. [PubMed]
7 . Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101 (6): 1644-55, 1992. [PubMed]
8 . Brandtzaeg P, Osnes L, Ovstebo R, Joo GB, Westvik AB, Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med 184 (1): 51-60, 1996. [PubMed]
9 . Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 358 (2): 125-39, 2008. [PubMed]
10 . Charles PE, Ladoire S, Aho S, Quenot JP, Doise JM, Prin S, Olsson NO, Blettery B. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect Dis 8: 38, 2008. [PubMed]
11 . Cunnion RE, Schaer GL, Parker MM, Natanson C, Parrillo JE. The coronary circulation in human septic shock. Circulation 73 (4): 637-44, 1986. [PubMed]
12 . Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36 (1): 296-327, 2008. [PubMed]
13 . Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med 158 (2): 444-51, 1998. [PubMed]
14 . Eichacker PQ, Natanson C. Increasing evidence that the risks of rhAPC may outweigh its benefits. Intensive Care Med 33 (3): 396-9, 2007. [PubMed]
15 . Eisenberg P. Discontinuation of study F1K-MC-_EVBP, investigation of the efficacy and safety of Drotrecogin alfa (activated) in pediatric severe sepsis (letter), 2005.
16 . Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg FW. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 85 (5): 1341-7, 1995. [PubMed]
17 . Fisher CJ, Jr., Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. Jama 271 (23): 1836-43, 1994. [PubMed]
18 . Frutos-Vivar F, Nin N, Esteban A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Curr Opin Crit Care 10 (1): 1-6, 2004. [PubMed]
19 . Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 333 (16): 1025-32, 1995. [PubMed]
20 . Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181 (1): 176-80, 2000. [PubMed]
21 . Grover R, Zaccardelli D, Colice G, Guntupalli K, Watson D, Vincent JL. An open-label dose escalation study of the nitric oxide synthase inhibitor, N(G)-methyl-L-arginine hydrochloride (546C88), in patients with septic shock. Glaxo Wellcome International Septic Shock Study Group. Crit Care Med 27 (5): 913-22, 1999. [PubMed]
22 . Herbertson MJ, Werner HA, Russell JA, Iversen K, Walley KR. Myocardial oxygen extraction ratio is decreased during endotoxemia in pigs. J Appl Physiol 79 (2): 479-86, 1995. [PubMed]
23 . Heron M. Deaths: leading causes for 2004. Natl Vital Stat Rep 56 (5): 1-95, 2007. [PubMed]
24 . Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med 25 (8): 1298-307, 1997. [PubMed]
25 . Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27 (7): 1230-51, 1999. [PubMed]
26 . Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 151 (2 Pt 1): 293-301, 1995. [PubMed]
27 . Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118 (1): 146-55, 2000. [PubMed]
28 . Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 3 (5): 371-82, 2003. [PubMed]
29 . Janeway C, Jr. Immunobiology: The Immune System in Health and Disease. Third ed. New York: Garland Publishing, p. 9:1-27, 1997.
30 . Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest 132 (2): 425-32, 2007. [PubMed]
31 . Kortgen A, Niederprum P, Bauer M. Implementation of an evidence-based "standard operating procedure" and outcome in septic shock. Crit Care Med 34 (4): 943-9, 2006. [PubMed]
32 . Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34 (6): 1589-96, 2006. [PubMed]
33 . Landry DW, Levin HR, Gallant EM, Ashton RC, Jr., Seo S, D'Alessandro D, Oz MC, Oliver JA. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 95 (5): 1122-5, 1997. [PubMed]
34 . Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 345 (8): 588-95, 2001. [PubMed]
35 . Leaver SK, Finney SJ, Burke-Gaffney A, Evans TW. Sepsis since the discovery of Toll-like receptors: disease concepts and therapeutic opportunities. Crit Care Med 35 (5): 1404-10, 2007. [PubMed]
36 . Liu KD, Matthay MA. Protein C as a surrogate end-point for clinical trials of sepsis. Crit Care 12 (2): 139, 2008. [PubMed]
37 . Mariscalco MM. Innate immunity in critical care. Semin Pediatr Infect Dis 17 (1): 25-35, 2006. [PubMed]
37 a. Marshall JC. SIRS and MODS: What is their relevance to the science and practice of intensive care? Shock 2000;14:586-589. [PubMed]
38 . Martin C, Viviand X, Leone M, Thirion X. Effect of norepinephrine on the outcome of septic shock. Crit Care Med 28 (8): 2758-65, 2000. [PubMed]
39 . Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348 (16): 1546-54, 2003. [PubMed]
40 . Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 131 (4): 954-63, 2007. [PubMed]
41 . Medzhitov R, Janeway C, Jr. Innate immunity. N Engl J Med 343 (5): 338-44, 2000. [PubMed]
42 . Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest 113 (10): 1473-81, 2004. [PubMed]
43 . Mermel LA, Maki DG. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann Intern Med 119 (4): 270-2, 1993. [PubMed]
44 . Merx MW, Weber C. Sepsis and the heart. Circulation 116 (7): 793-802, 2007. [PubMed]
45 . Micek ST, Roubinian N, Heuring T, Bode M, Williams J, Harrison C, Murphy T, Prentice D, Ruoff BE, Kollef MH. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 34 (11): 2707-13, 2006. [PubMed]
46 . Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med 141 (1): 47-56, 2004. [PubMed]
47 . Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49 (9): 3640-5, 2005. [PubMed]
48 . Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med 163 (2): 316-21, 2001. [PubMed]
49 . Natanson C, Danner RL, Reilly JM, Doerfler ML, Hoffman WD, Akin GL, Hosseini JM, Banks SM, Elin RJ, MacVittie TJ, et al. Antibiotics versus cardiovascular support in a canine model of human septic shock. Am J Physiol 259 (5 Pt 2): H1440-7, 1990. [PubMed]
50 . Natanson C, Eichenholz PW, Danner RL, Eichacker PQ, Hoffman WD, Kuo GC, Banks SM, MacVittie TJ, Parrillo JE. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med 169 (3): 823-32, 1989. [PubMed]
51 . Nguyen HB, Corbett SW, Menes K, Cho T, Daugharthy J, Klein W, Wittlake WA. Early goal-directed therapy, corticosteroid, and recombinant human activated protein C for the treatment of severe sepsis and septic shock in the emergency department. Acad Emerg Med 13 (1): 109-13, 2006. [PubMed]
52 . Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, Edwards J, Cho TW, Wittlake WA. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 35 (4): 1105-12, 2007. [PubMed]
53 . O'Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, Masur H. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med 36 (4): 1330-49, 2008. [PubMed]
54 . Opal SM, Fisher CJ, Jr., Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 25 (7): 1115-24, 1997. [PubMed]
55 . Opal SM, Huber CE. Bench-to-bedside review: Toll-like receptors and their role in septic shock. Crit Care 6 (2): 125-36, 2002. [PubMed]
56 . Papadopoulos MC, Davies DC, Moss RF, Tighe D, Bennett ED. Pathophysiology of septic encephalopathy: a review. Crit Care Med 28 (8): 3019-24, 2000. [PubMed]
57 . Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 100 (4): 483-90, 1984. [PubMed]
58 . Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 328 (20): 1471-7, 1993. [PubMed]
59 . Parrillo JE. Septic shock--vasopressin, norepinephrine, and urgency. N Engl J Med 358 (9): 954-6, 2008. [PubMed]
60 . Parrillo JE, Burch C, Shelhamer JH, Parker MM, Natanson C, Schuette W. A circulating myocardial depressant substance in humans with septic shock. Septic shock patients with a reduced ejection fraction have a circulating factor that depresses in vitro myocardial cell performance. J Clin Invest 76 (4): 1539-53, 1985. [PubMed]
61 . Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 27 (2): 304-12, 1999. [PubMed]
62 . Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. Jama 277 (13): 1058-63, 1997. [PubMed]
63 . Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med 140 (1): 18-25, 2004. [PubMed]
64 . Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. Jama 273 (2): 117-23, 1995. [PubMed]
65 . Remick DG, Kunkel RG, Larrick JW, Kunkel SL. Acute in vivo effects of human recombinant tumor necrosis factor. Lab Invest 56 (6): 583-90, 1987. [PubMed]
66 . Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest 112 (4): 460-7, 2003. [PubMed]
67 . Rigato O, Salomao R. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock 19 (2): 113-6, 2003. [PubMed]
68 . Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358 (9): 877-87, 2008. [PubMed]
69 . Safdar N, Fine JP, Maki DG. Meta-analysis: methods for diagnosing intravascular device-related bloodstream infection. Ann Intern Med 142 (6): 451-66, 2005. [PubMed]
70 . Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis 4 (8): 519-27, 2004. [PubMed]
71 . Shahidi H, Kilbourn RG. The role of nitric oxide in interleukin-2 therapy induced hypotension. Cancer Metastasis Rev 17 (1): 119-26, 1998. [PubMed]
72 . Shapiro NI, Howell MD, Talmor D, Lahey D, Ngo L, Buras J, Wolfe RE, Weiss JW, Lisbon A. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 34 (4): 1025-32, 2006. [PubMed]
73 . Shorr AF, Lazarus DR, Sherner JH, Jackson WL, Morrel M, Fraser VJ, Kollef MH. Do clinical features allow for accurate prediction of fungal pathogenesis in bloodstream infections? Potential implications of the increasing prevalence of non-albicans candidemia. Crit Care Med 35 (4): 1077-83, 2007. [PubMed]
74 . Shorr AF, Nelson DR, Wyncoll DL, Reinhart K, Brunkhorst F, Vail GM, Janes J. Protein C: a potential biomarker in severe sepsis and a possible tool for monitoring treatment with drotrecogin alfa (activated). Crit Care 12 (2): R45, 2008. [PubMed]
75 . Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 162 (2 Pt 1): 505-11, 2000. [PubMed]
76 . Sirard JC, Vignal C, Dessein R, Chamaillard M. Nod-like receptors: cytosolic watchdogs for immunity against pathogens. PLoS Pathog 3 (12): e152, 2007. [PubMed]
77 . Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J. Hydrocortisone therapy for patients with septic shock. N Engl J Med 358 (2): 111-24, 2008. [PubMed]
78 . Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, Hinshaw LB. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med 18 (8): 801-6, 1990.
79 . Svistunenko DA, Davies N, Brealey D, Singer M, Cooper CE. Mitochondrial dysfunction in patients with severe sepsis: an EPR interrogation of individual respiratory chain components. Biochim Biophys Acta 1757 (4): 262-72, 2006. [PubMed]
80 . Thomas L. Germs. N Engl J Med 287 (11): 553-5, 1972. [PubMed]
81 . Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330 (6149): 662-4, 1987. [PubMed]
82 . Trzeciak S, Dellinger RP, Abate NL, Cowan RM, Stauss M, Kilgannon JH, Zanotti S, Parrillo JE. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest 129 (2): 225-32, 2006. [PubMed]
83 . Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med 354 (5): 449-61, 2006. [PubMed]
84 . van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med 345 (19): 1359-67, 2001. [PubMed]
84 a. Vincent J. Dear SIRS, I'm sorry to say that I don't like you. . . Crit Care Med 1997;25:372-374. [PubMed]
85 . Vitberg D EP, Natanson C. American Thoracic Society. Toronto, Canada.
86 . Waage A, Halstensen A, Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet 1 (8529): 355-7, 1987. [PubMed]
87 . Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 167 (5): 695-701, 2003. [PubMed]
88 . Wenzel RP. Treating sepsis. N Engl J Med 347 (13): 966-7, 2002. [PubMed]
89 . Zanotti Cavazzoni SL, Dellinger RP. Hemodynamic optimization of sepsis-induced tissue hypoperfusion. Crit Care 10 Suppl 3: S2, 2006. [PubMed]
90 . Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 25 (7): 1095-100, 1997. [PubMed]
Table 1. Definitions of Sepsis and Organ Failurea
|
Infection |
Pathologic process caused by the invasion of normally sterile tissue or fluid or body cavity by pathogenic microorganisms |
Crude mortalityb |
|---|---|---|
|
Response to infection or other severe clinical insult manifested by two or more of the following: Temperature: > 38° C or <36° C Heart rate: > 90 beats/min Respiratory rate: > 20 breaths/min or PaCO2 < 32 mm Hg WBC>12,000 cells/mm3, <4,000 cells/mm3 or >10% bands |
7% |
|
|
Sepsis |
Clinical syndrome defined by the presence of both infection and a systemic inflammatory response |
15% |
|
Severe Sepsis |
Sepsis with organ dysfunction as evidenced by hypotension or hypoperfusion(i.e., hypoxemia, renal failure, change in mental status) |
20% |
|
Septic Shock |
Severe sepsis with hypoperfusion despite adequate fluid resuscitation |
45% |
|
Multiple Organ Dysfunction Syndrome |
Presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without intervention |
|
|
aAdapted from Crit Care Med 1992;20:864-874;b based on Jama 273:117-123 and N Engl J Med 347:966-967 |
||
Table 2: Differential Diagnosis of Shock
|
Hypovolemic Shock |
Distributive Shock |
Cardiogenic Shock |
Extracardiac Obstructive Shock |
|---|---|---|---|
|
1. Hemorrhagic
|
1. Septic Shock |
1. Myocardial
|
1. Increased Intrathoracic Pressure
|
|
2. Nonhemorrhagic |
2. Anaphylactic |
2. Mechanical
|
2. Intrinsic Vascular Flow Obstruction
|
|
3. Neurogenic |
3. Arrhythmias |
Table 3: Hemodynamic Profiles of Shock States
|
Hypovolemic Shock |
Distributive Shock |
Cardiogenic Shock |
Extracardiac Obstructive Shock |
|
|---|---|---|---|---|
|
CO |
Decreased |
Increased |
Decreased |
Decreased |
|
SVR |
Increased |
Decreased |
Increased |
Increased |
|
PCOP |
Decreased |
Normal/Decreased |
Increased |
Increased/Decreased |
|
CVP |
Decreased |
Normal/Decreased |
Normal/Increased |
Increased/Decreased |
|
SVO2 |
Decreased |
Decreased |
Decreased |
Decreased |
|
CO; cardiac output, SVR; systemic vascular resistance, PCOP; pulmonary capillary occlusion pressure, CVP; central venous pressure, SVO2; mixed venous oxygen saturation |
||||
Zanotti Cavazzoni SL, Dellinger RP. Hemodynamic optimization of sepsis-induced tissue hypo perfusion. Crit Care 2006;10 Suppl 3:S2.
Table 4: Examples of Some Empiric Regimens for Several Presentations of Sepsis (Note that all examples should be interpreted in the context of individual patient factors and pathogen susceptibility patterns at different hospitals).
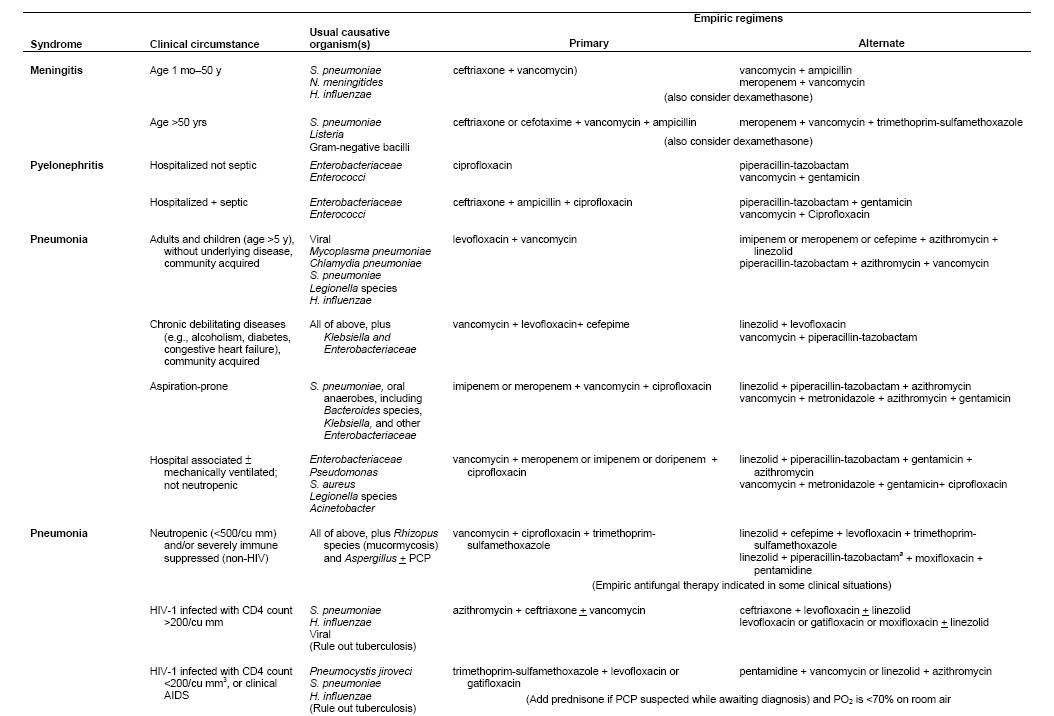
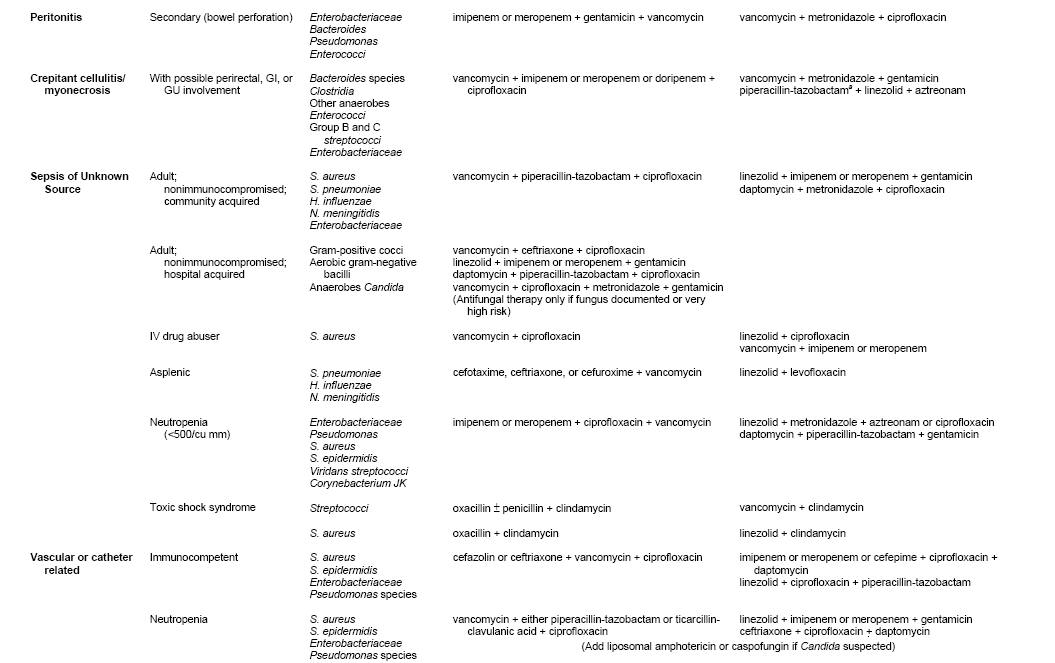
The authors are grateful to Kelly Byrne for technical assistance with Table 4.
Figure 1: Pathogenesis and therapy of sepsis and septic shock. Outline of the sequence of events that occur during the pathogenesis of sepsis and septic shock including examples of mediators and standard therapy.
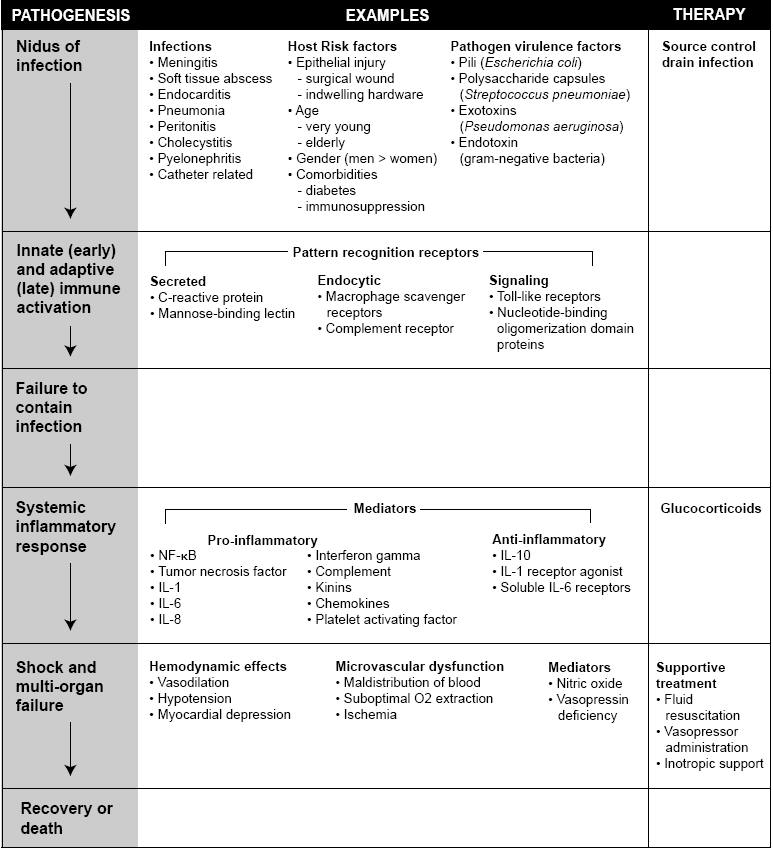
The authors are grateful to Thomas F. Sweeney, Jr. for technical assistance with Figure 1.
What's New
None
Reviews
None
History
None
