Orofacial Infections
Authors: Anthony W. Chow, MD, FRCPC, FACP
The clinical spectrum of orofacial infections affecting the skin or mucous membranes of the face and oral cavity is quite diverse. Such infections may be localized and indolent, or invasive and life-threatening. These infections may be conveniently categorized as odontogenic and non-odontogenic. Odontogenic infections include dental caries, periodontal disease, and suppurative deep space infections. Non-odontogenic infections include pyogenic infections of the face and neck, infections of the oral mucosa, oropharyngeal candidiasis, sialadenitis and parotitis. In this chapter, the more common soft tissue infections of the face and oral mucosa from odontogenic as well as non-odontogenic sources will be briefly discussed.
ANATOMICAL CONSIDERATIONS
Structure of the Tooth
Humans have 32 permanent teeth consisting of 2 incisors, 1 canine, 2 premolars, and 3 molars in each half-jaw. Each tooth has a visible crown that projects above the gingiva (gum), with one or more roots that extend into the alveolar bone of the maxilla or mandible. The tooth forms a peg and socket joint with the alveolar bone and is held in place by the periodontal membrane that allows slight movement of the tooth. The hard tissues of the tooth are dentin, enamel, and cementum, while the soft tissues are the pulp, the periodontal membrane, and the gingiva. Odontogenic infections originate in either the dentoalveolar structures, the periodontium, or the pericoronal tissues (Figure 1). Dental caries and pulpal infection are by far the most common.
The Oral Mucosa
The oral mucosa consists of layers of epithelial cells composed mainly of keratinocytes resting on a basal membrane. Junctions between these epithelial cells are tightly regulated for the passage of molecules into the underlying connective tissue, lymphatics, or regional microcirculation. The oral epithelium constantly undergoes cellular renewal and turnover. Microorganisms seeking to colonize mucosal surfaces must develop a strategy to counteract the constant turnover of the epithelial cell layer. Thus, the oral mucosa has three types of antimicrobial defenses: physical barrier of the epithelial layer; non-specific (innate) immunity derived from salivary constituents, neutrophils and epithelial antimicrobial peptides; and adaptive immunity associated with mucosa-associated lymphatic tissues (MALT) (Figure 2).
Diseases of the oral mucosa often result from breakdown or abnormality of the epithelium. Typical epithelial lesions include hyperkeratosis (increased thickness of the keratin layer), acanthosis (increased thickness of the prickle cell layer with or without associated hyperkeratosis), atrophy (thinning of the epithelium, often associated with incomplete keratinization), or acantholysis (loss of the intercellular attachments in the prickle cell layer leading to separation of the cells). An atrophic epithelium may readily become ulcerated following minor trauma, while acantholytic lesions may lead to intraepithelial blisters due to collection of edema fluid in or between the prickle cells. Bullous lesions may develop with collection of edema fluid between the epithelium and the lamina propria in the region of the basal complex.
The Maxillofacial Skin
The skin is a major organ, complete with epithelial, vascular, lymphoid and neuronal tissues as well as specialized appendages. It consists of two layers, the epidermis derived from the ectoderm and the dermis derived from the mesoderm. The epidermis comprises 4 types of cell layers: stratum corneum, stratum granulosum, stratum spinosum, and the basal layer. It is devoid of blood vessels, nerves, and lymphatics and is, therefore, relatively removed from cellular and humoral host defenses, especially in the more superficial region. The dermis consist primarily of collagen fibers and specialized appendages, including hair follicles, sweat glands, and sebaceous glands, which may descend into the superficial fascia. The arterial supply to the skin arises from a flat plexus of vessels located in the superficial fascia near its junction with the dermis. Smooth muscle cells in the precapillary arterioles function to control blood perfusion of the skin, thereby helping to regulate skin temperature. Venules and lymphatics accompany the arteries. Lymphatics in the dermis contain no valves, whereas those in the superficial fascia contain a few valves. The intact skin is extraordinarily resistant to invasion by microbes unless its vascularity is impaired. Thus, the major factors which predispose to orofacial skin and soft tissue infections are related to minor traumatic or ischemic injury which causes a breakdown in the integrity of the epidermis. Even when these conditions are met, a relatively high bacterial inoculum is required to initiate a soft tissue infection. The presence of foreign bodies, such as dirt, grease, or suture material, dramatically lowers the inoculum size necessary to induce infection.
MICROBIAL ETIOLOGY
The microbial etiology of orofacial infections may be anticipated from the normal resident flora of the contiguous mucosal surfaces from which the infection originated. Due to the close anatomic relationship, the resident flora of the oral cavity, upper respiratory tract, and certain parts of the ears and eyes share many common organisms. Anaerobes generally outnumber aerobes at all sites by a factor of 10:1. Important differences in bacterial compositions have been noted from various sites within the oral cavity (Table 1). Although less is known about the pathogenic potential of individual species, it is clear that as a group these endogenous organisms are structural opportunists and may invade deep tissues when normal mucosal barriers are disrupted. Invasiveness is often influenced by synergistic interactions of multiple species, both aerobic and anaerobic. Moreover, certain species or combinations may be more invasive or more resistant to therapy than others. While anaerobes are likely the predominant pathogens in most orofacial infections (Table 2), other pathogens such asStaphylococcus aureus and facultative gram-negative rods including Pseudomonas aeruginosa may be present in a small but significant proportion of cases, particularly in immunocompromised patients. In contrast to odontogenic infections, suppurative infections arising from the pharynx contain both oral anaerobes and facultative streptococci, particularly Streptococcus pyogenes, whereas rhinogenic or otogenic infections may harbor Streptococcus pneumoniae,Haemophilus influenzae, facultative gram-negative rods as well as anaerobes.
CLINICAL SYNDROMES
Odontogenic Infections
Dental Caries and Pulpitis
Dental caries remain prevalent in North America today, even though there has been a decline in carious tooth surfaces in the 24-44 years age-group within the past decade. In the adolescent population, 25% of children in the 5-17 years age-group accounted for 80% of all caries involving permanent dentition. Due to gingival recession and subsequent exposure of dentin, the elderly population has a higher incidence of root caries, while enamel-related caries tend to occur at a constant rate throughout life. Changes in dental practice have significantly impacted the geriatric population in that whereas 70% of adults over 75 years of age had no natural teeth in 1957, fewer than 35% of this age group lack all teeth by 1995.
There is growing awareness of the infectious nature of dental caries, and that only certain microorganisms residing within dental plaques are cariogenic (the specific plaque hypothesis of dental caries and periodontal disease). Dental caries are primarily caused by microorganisms within the supragingival plaque composed mainly of gram-positive facultative and microaerophilic cocci and rods. The microbial aetiology of dental caries has been well characterized. There is clear evidence that the mutans streptococci group - notably S. mutans and S. sobrinus, are the primary organisms associated with dental caries, both in animal models and in human studies. Root caries are associated with Actinomyces species including A. naeslundii and A. viscosusin addition to mutans streptococci. Investigations of decayed fissures have identified high levels of these mutans streptococci, as have studies comparing the microflora of carious and non-carious teeth. Longitudinal studies also support these conclusions. These streptococci are transmissible between humans, and infants are often infected by salivary contact with their mothers.
Caries originate from dental plaque – the biofilm found on the tooth surface. Initially, a clean tooth surface is coated by the adhesion of bacteria-free salivary protein polymers, known as the acquired pellicle. This acquired pellicle is subsequently colonized by an oral microflora which adhere to the pellicle, form coaggregates with other attached cells, and deposit an extracellular matrix to form a biofilm that has an open architecture. Plaque tends to build within fissures and grooves of the posterior teeth on surfaces that are not easily exposed to cleaning forces such as salivary flow, mastication and the tongue. Plaque bacteria ferments sugar leading to acid production which penetrates the tooth surface and causes demineralisation by lowering the pH and driving out calcium and phosphate. This process can be reversed by plaque removal, pH neutralization and re-mineralization of the tooth, provided that the tooth superstructure is intact. Demineralized lesions appear as white spots, while re-mineralized, inactive caries have a darker color.
Progressive demineralization undermines the tooth surface leading to collapse and cavitation. Untreated, cavitation will progress until the enamel surface is destroyed and the dental pulp is exposed and become infected, either directly via the apical foramen or by contiguous spread from a neighboring source of infection. Once ongoing inflammation has been established within the pulp chamber, pressure builds rapidly, leading to vascular occlusion at the apical foramen, ischemia and necrosis of the pulp tissue. The inflammatory contents of the pulp may drain into the oral cavity via the cavitary lesion in the enamel, but more often extends through the necrotic apical root canal to invade the periapical soft tissue and alveolar bone, resulting in a localized periodontitis or dento-alveolar abscess (Figure 1).
Clinical Manifestations and Therapy
Dental caries are initially asymptomatic. Once the tooth structure is disrupted, odontoblastic processes are exposed to the oral environment, and these expand and contract in response to heat, cold and other environmental shifts, transmitting pain via sensory nerves in the ondontoblast layer. Pulpitis and the associated pressure manifests as severe toothache, often exacerbated by hot drinks (a reaction thought to be secondary to the expansion of gases produced by bacteria within the tooth chamber). There may be accompanying symptoms of low grade fever, facial swelling, or radiation of pain along branches of the fifth cranial nerve.
Diagnosis of dental caries is primarily by visual inspection and radiographic detection. Clinical inspection may detect white spots or darker inactive lesions. Radiographic examination (bitewing radiography or orthopantomogram) is essential for diagnosing interproximal carious lesions, but current radiographic techniques lack sensitivity in recognizing non-cavitating lesions or root surface caries. Newer techniques such as quantitative light-induced fluorescence and fiber-optic transillumination are currently under development and may improve caries detection.
Current therapeutic interventions focus primarily on caries prevention and control strategies. Dietary changes by decreasing sugar consumption are recommended for longterm reduction of caries. Fluoride therapy, which enhances remineralization of hydroxyapatite, has long been advocated for caries prevention (Table 2). Brushing two to three times daily with a fluoridated dentifrice (1,000 ppm of fluoride, usually as 1.1% sodium fluoride) will effectively deliver fluoride to the tooth-plaque surface. In high-risk individuals, additional fluoride therapy in the form of fluoride varnishes (22,600 ppm fluoride as 5% sodium fluoride), professionally applied three or four times a year, has been efficacious in caries prevention. Other interventions include antimicrobial agents such as chorhexidine rinses which suppress oral mutans streptococci, and the use of xylitol gum, a nonfermentable five carbon sugar as a sugar substitute between meals. Dental restoration of cavitary lesions and removal of teeth with compromised pulp is usually necessary. Regular follow-up is essential in at-risk individuals to monitor compliance with preventative strategies, dietary restrictions of sugars, and to evaluate the development of new cavitary lesions.
Periodontal Diseases
Periodontal disease affects the connective tissues supporting the tooth, including the gingiva, periodontal ligament and the alveolar bone. Infection may be confined to the soft tissues around the tooth (gingivitis), or involve deeper structures (periodontitis) with loss of alveolar support for the tooth and eventually tooth loss. Gingivitis and periodontitis are prevalent in the general population. Earlier national surveys revealed that over 90% of the study population 13 years or older had evidence of gingival loss of attachment, and a third had periodontitis. Approximately 3-4% of patients will develop aggressive disease with onset between 14 and 35 years of age. In addition, certain factors such as advancing age, smoking and diabetes mellitus are associated with an increased risk of periodontal disease.
Gingivitis and periodontal disease is mainly caused by microorganisms within the subgingival dental plaque, which penetrate the gingival epithelium and elicit an inflammatory host response. The microflora associated with gingivitis is predominated by Actinomyces speciessuch as A. viscosus and A. naeslundii, and Porphyromonas gingivalis. Other species such as S. sanguis and S. anginosus are also found. As periodontal disease advances, Porphyromonas gingivalis, Prevotella intermedia, Bacteroides forsythus, Campylobacter species and Treponema denticola become predominant. Two specific clinical entities, Localized Juvenile Periodontitis and Acute Necrotizing Ulcerative Gingivitis are associated with specific pathogens, namelyActinobacillus actinomycetemcomitans in the former, and Fusobacterium nucleatum in addition to Treponema denticola in the latter. A unifying hypothesis postulating the microbial shift from a plaque-free tooth surface and progression to supragingival and subgingival plaque organisms and various odontogenic infections is shown in Figure 3.
In addition to plaque-induced inflammation, other conditions that can trigger gingival inflammation include hormonal changes such as during pregnancy, and medications such as dilantin or cyclosporin. The inflammatory host response ultimately results in destruction of the periodontium surrounding the root of the tooth. It is this loss of connective tissue and bony support resulting in deepening of the gingival sulcus which defines active periodontitis.
Clinical Manifestations and Therapy
Gingivitis is characterized by inflammation, swelling and discolouration, and easy bleeding on brushing. Acute Necrotizing Ulcerative Gingivitis, also known as Vincent’s angina or trench mouth, tends to occur in younger individuals. The patient usually presents with a sudden onset of pain and necrosis of the interdental papilla and gingival tissues, accompanied by systemic manifestations including fever, malaise or adenopathy. An association with conditions of stress has been noted. Periodontitis is classified according to patient age and the extent of disease. Current classification includes Chronic Periodontitis, Aggressive Periodontitis, periodontitis as a manifestation of systemic disease (hematologic or genetic disorders), and Necrotizing Periodontal Disease. Clinical entities may be localized or generalized. As periodontitis advances, the loss of supportive tissue leads to formation of a deep gingival sulcus or periodontal pocket. The depths of these pockets indicate the extent of disease progression, with a significant loss being >4mm, and severe disease being >6mm. This process is usually painless, with symptoms being limited to fetor or altered taste. Aggressive Periodontitis includes conditions previously described as Early-Onset Periodontitis (occurring in individuals younger than 35 years of age), and Localized Juvenile Periodontitis. The latter occurs in adolescents and classically manifests as rapid vertical bone loss confined to the first molars and incisors. In contrast to Adult Periodontitis, there is little evidence of plaque or calculus. In all instances, progressive periodontitis results in eventual tooth loss.
Early studies had indicated an association between HIV infection and severe periodontal disease. These included linear gingival erythema, necrotizing ulcerative gingivitis and necrotizing ulcerative periodontitis. The prevalence of these conditions is now felt to have been overestimated, and no major differences in the microflora or complications from dental procedures are found between HIV-positive and negative individuals. HIV positive individuals should have regular dental care as part of their ongoing health evaluation.
Treatment for periodontal disease is similar to that of dental caries, and requires regular dental care including mechanical removal of plaques at the gumline. For severe recession, pocket reduction surgery may be necessary. Acute conditions require local tissue debridement. With the elucidation of the role of specific oral microbial pathogens, antimicrobials have gained prominence in the treatment of certain periodontal conditions (Table 2). Acute Necrotizing Ulcerative Gingivitis should be treated with systemic antimicrobials such as metronidazole or penicillin. Certain types of severe periodontitis are amenable to systemic antimicrobials in conjunction with mechanical debridement (scaling and root planing).
Suppurative Odontogenic and Deep Fascial Space Infections
Suppurative odontogenic infections may lead to mandibular osteomyelitis, or deep fascial space infections of the oral cavity, head and neck. Soft tissue infections of odontogenic origin tend to spread along planes of least resistance from the supporting structures of the affected tooth to various potential spaces in the vicinity. Accumulated pus, therefore, must perforate bone generally at the site where it is thinnest and weakest, before extending into the periapical areas or deeper fascial spaces. In the mandible, the bone is weakest and perforation tends to occur on the lingual aspect in the region of the molar teeth, and on the buccal aspect when more anterior teeth are involved. In the maxilla, the bone is weakest on the buccal aspect throughout and relatively thicker on the palatal aspect. If pus perforates through either the maxillary or mandibular buccal plate, it will present intraorally if inside the attachment of the buccinator muscle to the maxilla or mandible, and extraorally if outside this muscle attachment (Figure 4A). Therefore, infection of the upper and lower molars, lower incisors, and lower canine teeth is often accompanied by extraoral manifestations. When a mandibular infection perforates lingually, it presents in the sublingual space if the apices of the involved teeth lie above the attachment of the mylohyoid muscle (e.g., mandibular incisor, canines, premolars, and first molars), and in the submandibular space if below the attachment of the muscle (e.g., the second and third molars) (Figure 4B).
The clinically important "fascial spaces" around the face and oral cavity and their potential routes of spread are illustrated in Figure 5. A thorough understanding of the "anatomic routes" of infection not only provides valuable information about the nature and extent of infection, but also suggests the optimal surgical approach for drainage.
Culture of pus is the gold standard for microbiologic diagnosis; however, sampling may be contaminated with flora from saliva or fistula tracts. The microorganisms associated with dentoalveolar abscess are generally not the organisms associated with dental caries, but tend to be predominantly anaerobic species from deep within the gingival sulcus. Cultures of pus from periapical abscess show gram negative bacilli such as Prevotella, Porphyromonasand Fusobacterium as well as gram-positive cocci including Peptostreptococcus and Streptococcus species. Cultures from periodontal abscesses show a similar polymicrobial microflora.
Mandibular osteomyelitis arising from odontogenic infections is usually associated with predisposing conditions such as compound fracture, irradiation, diabetes mellitus, or steroid therapy. Following bacterial invasion, the local inflammatory response causes a marked increase in the intramedullary pressure, further compromising blood supply, leading to bone necrosis. Pus travels through the haversian and perforating canals, accumulates beneath the periosteum, and elevates it from the cortex. If pus continues to accumulate, the periosteum is eventually penetrated, and mucosal or cutaneous abscesses and fistulas may develop. As the inflammatory process becomes more chronic, granulation tissue is formed. Spicules of necrotic and nonviable bone may become either totally isolated (sequestrum) or encased in a sheath of new bone (involucrum). Severe mandibular pain is a common symptom, and may be accompanied by anaesthesia or hypoesthesia on the affected side. In protracted cases, mandibular trismus may develop.
Odontogenic space infections include the more superficial masticator, buccal, canine, parotid, submental, and infratemporal spaces. Further extension may involve the deeper fascial spaces of the head and neck, such as the submandibular, sublingual, lateral pharyngeal and retropharyngeal spaces. The salient clinical features of these “space infections” are summarized in Table 3. The third mandibular molars are most commonly implicated in the origin of these infections. The masticator space consists of the interconnected masseteric, pterygoid and temporal spaces, and communicates directly with the submandibular and lateral pharyngeal spaces. Clinically, patients may have minimal swelling as the muscle bulk in this region serves to obscure the inflammatory response. Mandibular pain and trismus may be the only clinical findings. Infections arising from maxillary molar teeth predominantly involve the infratemporal space, which may present with pre-auricular swelling in the early phases and swelling of the cheek or eyelid and possible orbital involvement in the later stages. Buccal space infections arise primarily from the maxillary bicuspid (first premolar) and molar teeth. There is obvious swelling of the cheek, but patients are less sick in comparison to other odontogenic space infections that may require intensive care. Canine space infections arise from maxillary incisors and canines. Clinically, there is marked swelling of the upper lip with extension to the periorbital tissues.
A more serious form of submandibular space infection is Ludwig’s angina. In addition to systemic signs of infection such as fever chills and malaise, the patient presents with mouth pain, stiff neck, drooling, dysphagia, and verbalization with a muffled “hot potato” voice if they are able to speak at all. There is a characteristic lack of trismus as the submandibular space is not in communication with the muscles of mastication. Trismus implies a spread into the lateral pharyngeal space along the styloglossus muscle. There is also typically no lymph node involvement. The distinguishing feature of Ludwig’s angina is a rapidly spreading “woody” inflammation of the submandibular area without overlying cellulitis. On palpation of the submandibular region there is none of the normal tissue compliance. A striking aspect of the physical examination is often the patient’s protruding tongue, which is forced outward due to the internal limitation of swelling by the strong fibers of the deep cervical fascia. Not only will the tongue be displaced, but the whole floor of the oropharnyx will be elevated, erythematous and tender to palpation. The inflammation may spread to involve the epiglottis.
Radiographic studies of dentoalveolar or periodontal abscesses can be normal, but may show periapical lucencies. Diagnosis of deep space infections has dramatically improved with modern imaging techniques such as CT, MRI and indium scans. A plain film of the lateral neck may show tracheal deviation or retropharyngeal soft tissue swelling.
Definitive therapy of suppurative odontogenic and deep space infections of the head and neck require surgical drainage of purulent collections, and removal of the source of infection. Endodontic (root canal) intervention or extractions may be required. Systemic antibiotic therapy is not generally required for dentoalveolar or periodontal abscesses that are superficial and adequately drained surgically. For more serious infections involving deeper tissues, and in immunocompromised hosts, antimicrobial therapy is important in halting the local spread of infection and preventing hematogenous dissemination. Although penicillin monotherapy has been the treatment of choice for odontogenic infections in the past, the emergence of ß-lactamase production among certain oral anaerobes, particularly pigmented Prevotella species and Fusobacterium nucleatum, is increasingly recognized and treatment failure with penicillin alone has been well documented. Thus, penicillin monotherapy is no longer recommended. Metronidazole is active against anaerobic gram-negative bacilli and recommended in combination with penicillin. Stable outpatients may be treated with amoxicillin-clavulinic acid. Penicillin allergic patients should be treated with clindamycin which has excellent activity against the majority of oral pathogens. Gastro-intestinal upset and the association with Clostridium difficile-associated diarrhea are the main adverse effects. In more complicated infections, particularly in immunocompromised hosts, the choice of antibiotic therapy is primarily directed at presumed etiologic agents in the oral microflora, and broad-spectrum coverage with a second or third generation cephalosporin, a carbapenem, or ureidopenicillin may be warranted (Table 2). The role of newer fluoroquinolones with activity against oral streptococci and anaerobes, such asgatifloxacin and moxifloxacin, for the treatment of severe infections in immunocompromised hosts remains to be determined.
Non Odontogenic Infections
Pyogenic Infections of the Face and Neck
These include superficial infections such as impetigo, folliculitis, furunculosis, erysipelas, cellulites and necrotizing fasciitis.
Impetigo
Impetigo is a superficial infection of the skin that is confined to the epidermis, and is characterized by multiple lesions with little or no systemic toxicity. Streptococcus pyogenes accounts for the majority of cases, primarily among pre-school children in warm and humid climates. Poverty, crowding, and poor personal hygiene are other contributing factors. The early lesions are vesiculo-pustular in appearance and associated with localized lymphadenopathy. These lesions progress rapidly to crusting which is characteristically thick and adherent to the underlying skin. A variant of streptococcal pyoderma which extends through the epidermis of the skin and presents as ulcerated lesions is known as ecthyma. A well-recognized complication of streptococcal impetigo is immune complex acute glomerulonephritis. Although S. aureus is often co-isolated with S. pyogenes from impetiginous lesions, it can be the sole cause of impetigo in approximately 10 per cent of cases. Staphylococcal impetigo is clinically distinct from streptococcal impetigo by the presence of large bullous lesions that rupture, leaving thin and nonpurulent crusts overlying the involved skin. Localized lymphadenopathy is not a prominent finding. This form of impetigo is more often seen in newborns and younger children.
Folliculitis and Furunculosis
Folliculitis is an inflammatory process localized to the hair follicles resulting from pore obstruction. S. aureus is the most common bacterial cause. The lesions appear as small pinhead-sized, erythematous papules which may progress to form pustules. A deeper infection may lead to the development of a furuncle or boil, which is a localized abscess of the follicle with some destruction of its walls. A carbuncle forms when a group of furuncles coalesce into a large abscess. Midfacial furuncles have a potential risk of developing cavernous sinus thrombosis owing to extensive communications between the facial veins and the orbital venous plexuses. Recurrent furunculosis is more prone to develop in patients with obesity, diabetes mellitus, corticosteroid therapy, and defects in neutrophil function. A pruritic form of folliculitis associated with exposure to contaminated whirlpools and hot tubs is caused by Pseudomonas aeruginosa. Characteristically, the skin lesions involve the buttocks, trunk and other immersed areas of the body, less commonly the face and neck. Folliculitis is a relatively self-limiting condition and generally does not need specific antimicrobial therapy. Furunculosis and carbuncles should be treated with systemic antibiotics mainly directed at S. aureus. Carbuncles usually require surgical drainage as well.
Erysipelas
Erysipelas is an inflammatory process involving primarily the superficial layers of the skin including the dermis. The lesion has a distinctive fiery-red appearance, and is indurated with a raised and sharply demarcated margin. Typically, there is a bilateral or "butterfly" distribution across the bridge of the nose. Erysipelas is classically caused by S. pyogenes, but can occasionally be caused by other organisms such as S. pneumoniae and H. influenzae. As with other pyogenic streptococcal skin infections, the lesions tend to spread rapidly. The patient may appear quite toxic with fever, chills, and malaise.Lymphadenopathy and lymphangitis is usually present. As the lesions resolve, there is often desquamation of the skin overlying the involved area. Antibiotic treatment directed at S. pyogenes should be continued for 10 to 14 days.
Cellulitis
Cellulitis is an acute infection of the skin extending deep into subcutaneous tissues underlying the dermis. It can result either by inoculation of organisms through an open wound, contiguous spread of infection from a more superficial source, or by hematogenous seeding. The involved tissue is erythematous, warm, edematous, and tender. In contrast to erysipelas, the margins of the lesion in cellulitis are non-elevated and poorly defined. The patient usually demonstrates constitutional signs of illness such as fever, chills, and malaise. In adolescents and adults, Streptococcus pyogenes is the most common etiologic agent of cellulitis in the maxillofacial region which usually results from minor wounds and punctures. Once established, the infection progresses rapidly via the involved lymphatics, causing destruction of the superficial layers of the skin which may progress to streptococcal gangrene. Treatment generally requires surgical debridement of gangrenous skin, incision and drainage of the underlying tissue and fascial planes, as well as appropriate systemic antibiotics.S. aureus can also induce a cellulitis or pyoderma, but in contrast to streptococcal cellulitis, the infection usually arises from a furuncle and tends to be more slowly progressive. Prior to the advent of conjugated vaccines, cellulitis in the maxillofacial region was most commonly caused by Haemophilus influenzae, and Streptococcus pneumoniae. Bacteremia is frequently present, and the child appears toxic. H. influenzae cellulitis was particularly common in children under two years of age, and the infected site may take on a bluish or purplish hue. This condition is less common nowadays. Periorbital cellulitis is also more common in children and is generally preceded by trauma, sinusitis, otitis media, or other upper respiratory tract infections. There is diffuse swelling of the eyelid that may spread across the nasal bridge. Additional manifestations may include conjunctival edema, proptosis, limitation of ocular motility, and decreased vision. Antibiotic treatment should be directed at staphylococci, streptococci, and H. influenzae, the most common pathogens in this entity.
Synergistic Necrotizing Cellulitis and Necrotizing Fasciitis
Synergistic necrotizing cellulitis is a rapidly progressive infection involving the subcutaneous tissues and superficial fascia. In the area of the face and neck, it more commonly results as a complication of an odontogenic "space" infection, but occasionally can occur following minor trauma or a postoperative wound infection. The initial lesion is a reddish-brown bleb which is accompanied by local tenderness. However, the superficial appearance often belies the wide spread destruction of the deeper tissues. There is extensive gangrene of the subcutaneous tissue with gelatinous fat necrosis of the superficial fascia. There is often a copious, brown, watery, and foul discharge ("dish-water pus"). Gram-stain of the exudate reveals mixed organisms, and cultures usually demonstrate a polymicrobial infection involving both aerobic and anaerobic organisms, primarily Peptostreptococcus, Bacteroides, Fusobacterium, and Enterobacteriaceae. Treatment is primarily by surgical debridement of necrotic tissues, incision and drainage of tunnelling dish-water pus, and broad spectrum systemic antibiotics. Necrotizing fasciitis is an aggressive infection characterized by severe gangrene of the skin and of the superficial and deep fascia. The majority of cases involving the neck are either post-traumatic, postsurgical, or odontogenic in nature. Often the patient has underlying ischemic small-vessel disease or diabetes mellitus. Although S. pyogenes and S. aureus can both produce this syndrome with or without a toxic-shock-like presentation, most cases are polymicrobial in etiology, commonly involving anaerobic and/or aerobic gram-positive cocci and gram-negative bacilli. Once clinically apparent, the infection spreads rapidly within hours. Pain is a prominent finding initially, usually at the site of trauma or surgery. The area becomes edematous, warm, and erythematous, and rapidly develops a tense, shiny appearance that later changes to a dark, dusky discoloration. Bullous formation may occur, and the underlying skin becomes necrotic and may slough due to frank gangrene. Subcutaneous emphysema (crepitus) is present in up to 25 per cent of cases. As the necrosis progresses, the pain may actually decrease or abate completely, leaving local anaesthesia due to destruction of the cutaneous nerves in the subcutaneous tissues. Systemic toxicity with sepsis syndrome is a common presentation, and hypotension or shock may develop due to sequestration of large volumes of fluid in the necrotic fascial spaces. Treatment requires aggressive surgical excision, incision and drainage, as well as systemic broad-spectrum antibiotics.
Infections Of the Oral Mucosa
These include acute hepetic gingivostomatitis, aphthous stomatitis, herpangina, gangrenous stomatitis or noma, oropharyngeal candidiasis, and mucositis in the severely immunocompromised host.
Acute Herpetic Gingivostomatitis
This condition is caused by a primary infection with Herpes simplex virus, most commonly in children between two and five years of age and less frequently in adults. The initial symptoms consist of a sore throat, enlarged submandibular lymph nodes, and a burning sensation of the oral mucosa. This is rapidly followed by a vesicular eruption of the oral mucosa which soon becomes ulcerated. The mucosal ulcers may be small at first but often coalesce into large shallow lesions with serpiginous borders, and become covered by a fibrinous, yellowish, firmly adherent membrane. The ulcer is very painful, and the patient is febrile and has considerable difficulty talking, eating and swallowing. The microbiologic diagnosis is confirmed by a positive Tzanck smear (prepared from scrapings of the ulcer base which demonstrate the presence of multinucleated giant cells with intranuclear inclusions), and by immunofluorescence staining or virus isolation. Treatment requires topical analgesics and systemic antiviral agents (acyclovir, vidarabine, or foscarnet) (Table 2).
Aphthous Stomatitis
Aphthous ulcers are among the most common causes of recurrent oral lesions, and must be distinguished from other conditions such asherpes simplex virus or coxsackie virus infections, agranulocytosis, and Behcet's disease. The etiology of aphthous ulcers remains uncertain, although a number of infectious agents including viruses have been implicated. The most prevailing hypothesis suggests that the mechanisms for mucosal ulceration is autoimmune in nature. Three major clinical variants are recognized: 1) minor aphthous ulcers, 2) major aphthous ulcers, and 3) herpetiform aphthous ulcers. Minor aphthous ulcers appear as a number of small ulcers on the buccal and labial mucosa, the floor of the mouth, or the tongue. The palatal soft tissues, pharynx and tonsillar fauces are rarely involved. A prodromal stage is usually present. The ulcers appear gray-yellow, often with a raised and erythematous margin, and are exquisitely painful. Lymph node enlargement is seen only with secondary bacterial infection. The course of ulceration varies from a few days to several weeks, and is followed by spontaneous healing. Major aphthous ulcers are more protracted and last up to several months. All areas of the oral cavity including the soft palate and tonsillar areas may be involved. Prolonged periods of remission may be followed by intervals of intense ulcer activity. Herpetiform aphthous ulcers are small and multiple, and characteristically affect the lateral margins and tips of the tongue. The ulcers are gray with a delineating erythematous border, and are extremely painful which makes eating and speaking difficult. Despite its name, there is little clinical resemblance to an acute herpetic gingivostomatitis. Although intranuclear inclusions have been demonstrated in herpetiform aphthous ulcers, there is no evidence to suggest that these inclusions bear any relationship to presence of viruses. Treatment is primarily symptomatic with antiseptic mouthwashes and local anaesthetic lozenges or gels. Topical or systemic steroids may be beneficial in selected individuals with extensive disease.
Herpangina and Hand, Foot and Mouth Disease
Herpangina is an acute infection of the oropharynx caused by type A Coxsackie viruses, and affects mostly young children. The onset is sudden, with relatively severe systemic reactions including fever, sore throat, dysphagia, vomiting, and abdominal pains. Small greyish papules and vesicles surrounded by red areolae develop on the tonsillar fauces, soft palate, uvula, tongue and oropharynx; these rarely occur on the buccal mucosa or periodontium. The disease is usually self-limiting and lasts three to four days, followed by complete recovery. Treatment consists of symptomatic measures only. Hand, foot and mouth disease is also caused by type A Coxsackie viruses and affects primarily young children. It is characterized by the development of a maculopapular rash on the hands and soles of the feet, which may vesiculate. Maculopapular and vesicular lesions may also develop on the cheeks, hard and soft palate, tongue, oropharynx, tonsillar fauces, and the buccal mucosa. This disease is self-limiting, and lasts only several days, followed by complete recovery.
Gangrenous Stomatitis or Noma
This condition, also known as cancrum oris, is an acute, fulminating and gangrenous infection of the oral and facial tissues. It usually occurs in the presence of severe debilitation and malnutrition, and children are most often affected. The earliest lesion is a small, painful, red spot or vesicle on the attached gingiva in the premolar or molar region of the mandible. A necrotic ulcer rapidly develops and undermines the deeper tissue. Painful cellulitis of the lips and cheeks is observed as the lesion extends outward in a cone-like fashion. Within a short period, sloughing of necrotic soft tissues occurs, exposing the underlying bone, teeth, and deeper tissues. Fusospirochetal organisms such as Borrelia vincentii and Fusobacterium nucleatum are consistently cultured from the lesions. Prevotella melaninogenica may also be present. Specimens obtained by biopsy from the advancing lesion show a mat of predominantly gram-negative threadlike bacteria that cannot be positively identified. Thus, this lesion bears some resemblance to acute necrotizing ulcerative gingivitis but appears to be more focal and destructive, involving deeper tissues beyond the gingiva. Treatment requires high doses of intravenous penicillin. Every effort should be made to correct the severe dehydration and underlying malnutrition and debility. Loose teeth and sequestra may be removed, but saucerization should be avoided. Healing is by secondary intention. Serious mutilation and facial deformity may require subsequent cosmetic surgery.
Oropharyngeal Candidiasis
Colonization of the oral cavity by Candida species increases in the elderly, although the frequency and intensity of carriage appears to be independent of denture use. Clinical disease may be precipitated by the use of broad spectrum antibiotics and inadequately cleaned or ill-fitting dentures. Oral candidiasis is particularly common in the late stages of HIV disease. The most common oral manifestation is pseudomembranous candidiasis (thrush). It is characterized by soft white, slightly raised adherent plaques which can be wiped off leaving an erythematous or bleeding surface. Acute erythemic or atrophic candidiasis is characterized by painful erythematous mucosal lesions and a “bald” (depapillated) appearance of the tongue, with a matching lesion on the opposing surface of the palate. Chronic atrophic candidiasis or denture-induced stomatitis is commonly found in denture wearers and elderly persons with diffuse inflammation of denture-bearing areas due to prolonged irritation. Chronic hyperplastic candidiasis is a leukoplakic or keratoticlesion that cannot be removed by scraping, and is usually located on the anterior buccal mucosa. Candida species can also cause painful fissures at the corners of the mouth, a condition known as angular cheilitis or perliche. Treatment options include topical antifungal agents, such as nystatin oral suspensions orclotrimazole troches, and systemic agents with azoles (fluconazole, itraconazole, voriconazole) or caspofungin (Table 2). The longterm use of azoles for suppressive therapy of chronic mucocutaneous candidiasis in patients with AIDS has led to the recognition of resistant isolates, and current recommendations for therapy include using only topical agents in patients with CD4 counts over 50 while receiving anti-retroviral therapy, systemic anti-fungals for those with CD4 counts less than 50 and no anti-retroviral agents, and avoidance of prolonged use of antifungals.
HIV positive individuals have specific concerns with regards to oral health. In addition of oropharyngeal candidiasis, oral hairy leukoplakia secondary to Epstein-Barr virus is another common manifestation in HIV-positive individuals. Kaposi’s sarcoma (KS), associated with Human Herpes Virus 8, may present orally in up to 60% of patients with the condition. Therapy is still not clearly defined, and in addition to immune restoration with anti-retrovirals, local surgical or chemotherapeutic interventions may be necessary. Reactivation of other viral agents such as herpes simplex, varicella-zoster andcytomegalovirus can all affect the oral mucosa. Culture or histopathologic examination of oral lesions is necessary, as the etiology often cannot be readily predicted clinically.
Mucositis in the Severely Immunocompromised
Much of what is known about the management of oromucosal infections has been ascertained from cancer patients being treated with radiotherapy, chemotherapy or bone marrow transplantation, and from patients with the acquired immunodeficiency syndrome (AIDS). The underlying mechanism of infection in these patients appears to be a breakdown of the mucosal epithelium that leads to mucositis, secondary bacterial or fungal infection, or reactivation of a latent viral infection. Oral candidiasis, herpes simplex, varicella-zoster, and cytomegalovirus infections are the most common manifestations. Mucositis that complicates radiation or chemotherapy most commonly involves the non-keratinized oral epithelium, including the buccal and labial mucosa, soft palate, oropharynx, floor of the mouth, and ventral and lateral surfaces of the tongue. Ulceration and pseudomembrane formation are evident usually between 4 and 7 days after the initiation of chemotherapy, when the rate of destruction of the basal epithelium exceeds that of proliferation of new cells. The clinical manifestations may be quite variable. The lesions are often protracted in duration and may not be associated with an inflammatory reaction, thereby masking the usual signs and symptoms of infection. Pain or tenderness may be the only abnormal finding. Since the etiologic agents of infection cannot be readily predicted on clinical grounds alone in such patients, specific microbiologic diagnosis by culture, histopathology, or antigen detection techniques is critical for appropriate treatment. Topical as well as systemic antimicrobial agents may be indicated, along with antiseptic (e.g. chlorhexidine) and anaesthetic (e.g. benzydamine, viscous lidocaine, etc.) applications. Frequent saline rinses may reduce mucosal irritation, remove thickened secretions or debris, and increase moisture in the mouth. Coating agents such as milk of magnesia or aluminum hydroxide gel (amphogel) have been useful for symptomatic relief of painful oral lesions. Topical or oral cytoprotective agents (e.g., sucralfate) or nonsteroidal antiinflammatory analgesics (e.g., benzydamine, salicylates, etc.) may provide additional benefit. Meticulous oral and dental hygiene, effective management of xerostomia, selective suppression of oropharyngeal microbial colonization, and early control of reactivation by latent viral infections appear to be the critical steps to prevent and reduce the overall morbidity of oromucosal infections in the severely immunocompromised.
Sialadenitis and Parotitis
Sialadenitis, or infection of salivary tissue, most commonly involves the parotid gland, but submandibular and sublingual glands can also be affected. Risk factors for infection include ductal obstruction caused by calculi, dehydration, and chronic medical conditions such as diabetes mellitus, renal failure, sialogogic drugs, and conditions leading to xerostomia. Common pathogens include S. aureus, Enterobacteriaceae, and anaerobic gram negative rods such as Prevotella and Fusobacterium species. Clinically, patients present with a sudden onset of firm, erythematous swelling over the affected gland, and exquisite local pain and tenderness. Systemic findings of high fevers, chills, and marked toxicity are generally present. Treatment requires reversal of salivary stasis, and duct cannulation may be necessary. Antibiotic therapy should be directed at S. aureus and mixed anaerobic pathogens.
Viral parotitis is typified by mumps, the classic syndrome caused by a paramyxovirus. The prodromal period is followed by acute swelling of the involved gland, which can last five to ten days and is often bilateral. Other viral causes of parotitis which can mimic mumps are influenza and coxsackie viruses. HIV can cause parotid inflammation or chronic enlargement. Hepatitis C is also implicated in cases of sialadenitis. Serologic evaluation is helpful in establishing the etiologic diagnosis, and treatment is primarily directed at supportive care and symptomatic relief.
CONCLUSIONS
A systematic approach is necessary to establish the etiologic diagnosis and formulate appropriate therapy. Oropharyngeal infection in immunocompromised patients presents a particular challenge. Effective control of infection and management of oral symptoms by the judicious use of topical and systemic agents are both important. A major focus must be directed towards active promotion of oral hygiene, and more ready accessibility to dental care and restorative prosthodontic treatment programs.
READING LIST
1. Barakate MS, Jensen MJ, Hemli JM, Graham AR. Ludwig's angina: report of a case and review of management issues. Ann Otol Rhinol Laryngol 2001;110: 453-456. [PubMed]
2. Bensinger W, Schubert M, Ang KK, Brizel D, Brown E, Eilers JG et al. NCCN Task Force Report. prevention and management of mucositis in cancer care. J Natl Compr Canc Netw 2008;6 (Suppl 1): S1-S21. [PubMed]
3. Bresco-Salinas M, Costa-Riu N, Berini-Aytes L, Gay-Escoda C. Antibiotic susceptibility of the bacteria causing odontogenic infections. Med Oral Patol Oral Cir Bucal 2006;11: E70-E75. [PubMed]
4. Brook I. Antibiotic resistance of oral anaerobic bacteria and their effect on the management of upper respiratory tract and head and neck infections. Semin Respir Infect 2002;17: 195-203. [PubMed]
5. Brook I. Acute bacterial suppurative parotitis: microbiology and management. J Craniofac Surg 2003;14: 37-40. [PubMed]
6. Brook I. Microbiology and principles of antimicrobial therapy for head and neck infections. Infect Dis Clin North Am 2007;21: 355-391. 2007. [PubMed]
7. Brook I, Gober AE. Recovery of interfering and beta-lactamase-producing bacteria from group A beta-haemolytic streptococci carriers and non-carriers. J Med Microbiol 2006;55:1741-1744. [PubMed]
8. Caufield PW, Griffen AL. Dental caries. An infectious and transmissible disease. Pediatr Clin North Am 2000;47: 1001-1019. 2000. [PubMed]
9. Chow AW. Orofacial and odontogenic infections. Yoshikawa TT, Norman DC, editors. Infectious Diseases in Aging – A Clinical Handbook. 147-162. 2000. Totowa, NY, Humana Press Inc.
10. Chow AW. Infections of the oral cavity, neck and head. Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 7th ed.: 855-871. 2010. Philadelphia, PA, Churchill Livingstone Elsevier.
11. Chow AW, Roser SM, Brady FA. Orofacial odontogenic infections. Ann Intern Med 88: 392-402. 1978. [PubMed]
12. Dahlen G. Microbiology and treatment of dental abscesses and periodontal-endodontic lesions. Periodontol 2000. 2002;28: 206-239. [PubMed]
13. Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol 2005;7:119-133. [PubMed]
14. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J Clin Pediatr Dent 2003;28: 47-52. [PubMed]
15. Hull MW, Chow AW. An Approach to Oral Infections and Their Management. Curr Infect Dis Rep 2005;7:17-27. [PubMed]
16. Hull MW, Chow AW. Indigenous microflora and innate immunity of the head and neck. Infect Dis Clin N Am 2007;21: 265-282. [PubMed]
17. Hurley MC, Heran MK. Imaging studies for head and neck infections. Infect Dis Clin North Am 2007;21: 305-353. 2007. [PubMed]
18. Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007;109: 820-831. [PubMed]
19. Loesche W. Dental caries and periodontitis: contrasting two infections that have medical implications. Infect Dis Clin North Am 2007;21: 471-502. [PubMed]
20. Loesche WJ. The nonsurgical treatment of patients with periodontal disease: results after 6.4 years. Gen Dent 53: 298-306. 2005. [PubMed]
21. Novak MJ, Dawson DR, III, Magnusson I, Karpinia K, Polson A, Polson A et al. Combining host modulation and topical antimicrobial therapy in the management of moderate to severe periodontitis: a randomized multicenter trial. J Periodontol 2008;79: 33-41. [PubMed]
22. Paster BJ, Falkler JW, Jr., Enwonwu CO, Idigbe EO, Savage KO, Levanos VA et al. Prevalent bacterial species and novel phylotypes in advanced noma lesions. J Clin Microbiol 2002;40: 2187-2191. [PubMed]
23. Reichart PA. Oral manifestations in HIV infection: fungal and bacterial infections, Kaposi's sarcoma. Med Microbiol Immunol 2003;192:165-169.[PubMed]
24. Reynolds SC, Chow AW. Life-threatening infections of the peripharyngeal and deep fascial spaces of the head and neck. Infect Dis Clin North Am 2007;21: 557-576. [PubMed]
25. Robinson PG, Adegboye A, Rowland RW, Yeung S, Johnson NW. Periodontal diseases and HIV infection. Oral Dis 2002;8 Suppl 2: 144-150.[PubMed]
26. Roscoe DL, Hoang L. Microbiologic investigations for head and neck infections. Infect Dis Clin North Am 2007;21: 283-304. [PubMed]
27. Scully C, Gorsky M, Lozada-Nur F. The diagnosis and management of recurrent aphthous stomatitis: a consensus approach. J Am Dent Assoc 2003;134: 200-207. [PubMed]
28. Tong AC, Ng IO, Yeung KM. Osteomyelitis with proliferative periostitis: an unusual case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102: e14-e19. 2006. [PubMed]
Tables
Table 1. Predominant Cultivable Bacteria from Various Sites of the Oral Cavity
Type |
Predominant Genus or Family |
Total Viable Count (Mean %) |
|||
|---|---|---|---|---|---|
Gingival Crevice |
Dental Plaque |
Tongue |
Saliva |
||
Facultative Gram-positve cocci
Gram-positive bacilli Gram-negative cocci Gram-negative bacilli |
Streptococcus S. sanguis S. mitis S. salivarious Enterobacteriaceae |
32.8 (0-30) (10-20) (10-30) (0-1) 15.3 0.4 1.2 |
28.2 (0-50) (40-60) (20-40) (0-1) 23.8 0.4 ND |
44.8 (0-1) (10-20) (10-30) (40-60) 13.0 3.4 3.2 |
46.2 (0-1) 10-30) (30-50) (40-60) 11.8 1.2 2.3 |
Anaerobic Gram-positive cocci Gram-positive bacilli
Gram-negative cocci Gram-negative bacilli |
PeptostreptococcusActinomyces, Eubacterium, Leptotrichia Total |
7.4 20.2
10.7 16.1 1.9 4.7 |
12.6 18.4
6.4 10.4 4.1 ND |
4.2 8.2
16.0 8.2 0.7 0.2 |
13.0 4.8
15.9 4.8 0.3 ND |
Modified from Hull and Chow. Endogenous microflora and innate immunity of the head and neck. Infect Dis Clin N Am 21: 265-282. 2007.
Table 2: Microbiology and Antimicrobial Regimens in Selected Odontogenic and Non-odontogenic Orofacial Infections
Clinical Entity |
Unique Microbial Species |
Antimicrobial Regimens |
|---|---|---|
Supragingival dental plaqueand dental caries |
Streptococcus mutans, otherstreptococci, Actinomyces spp |
Fluoride-containing dentifrices or oral rinses (e.g. sodium fluoride 1.1% or stannous fluoride 0.4%) 2 or 3 times daily Fluoride-containing varnishes (e.g. sodium fluoride 5%) applied 3 or 4 times yearly |
Subgingival dental plaqueand simple gingivitis |
Streptococci, Actinomyces spp, spirochetes |
Chlorhexidine 0.12% oral rinses |
Gingivitis, acute necrotizing ulcerative |
Prevotella intermedia,Fusobacterium species, spirochetes |
Penicillin G 2-4 MU IV q4-6h (or penicillin V 500 mg q8h) plus Metronidazole 500 mg PO or IV q8h Ampicillin-sulbactam 1.5-3 g IV q6h (or amoxicillin-clavulanate 500 mg PO q8h) Clindamycin 450 mg PO or 600 mg IV q6-8h |
Periodontitis, juvenile |
Actinobacillus actinomycetemicomitans,Porphyromonas gingivalis, Pr. intermedia, Spirochetes |
Doxycycline 200 mg PO or IV q12h (only in patients >8 years of age) |
Periodontitis, adult |
Spirochetes, Porphyromonas gingivalis, Prevotella melaninogenica |
Doxycycline 200 mg PO or IV q12h Metronidazole 500 mg PO or IV q8h Topical application of minocycline microspheres (aristinâ) Topical application of doxycycline hyclate periodontal extended-release liquid (Atridox®) |
Suppurative orofacial odontogenicInfections including Ludwig’s angina |
Viridans and other streptococci,Peptostreptococcus spp, Bacteroides spp, and other oral anaerobes |
Normal Hosts Penicillin G 2-4 MU IV q4-6h, plus metronidazole 0.5 g IV q6h Ampicillin-sulbactam 2 g IV q4h Clindamycin 600 mg IV q6h Cefoxitin 1-2 g IV q6h Immunocompromised Hosts Cefotaxime 2 g IV q6h or ceftriaxone 1 g IV q12h or cefepime 2 g IV q12h Ticarcillin-clavulanate 3.1 g or piperacillin-tazobactam 3.375 g IV q4h Imipenem 500 mg IV q6h or meropenem 1 g IV q8h Moxifloxacin 400 mg IV q24h Tigecycline 100 mg IV, then 50 mg IV q12h |
Mandibular osteomyelitis |
Same as suppurative odontogenic space infections above |
Clindamycin 450 mg PO or 600 mg IV q6h Moxifloxacin 400 mg PO or IV q24h |
Gangrenous stomatitis (noma) |
Fusobacterium nucleatum, Borrelia vincentii, Prevotella melaninogenica, other oral anaerobes |
Penicillin G 2-4 MU IV q4-6h plus metronidazole 500 mg PO or IV q8h Ampicillin-sulbactam 1.5-3 g IV q6-8h or amoxicillin-clavulanate 500 mg PO q8h Clindamycin 450 mg PO q6-8h or 600 mg IV q6-8h |
HSV gingivostomatitis |
Normal Hosts Acyclovir 200 mg PO 5 times daily or valacyclovir 1 g PO q12h for 7 day Immunocompromised Hosts Acyclovir 250 mg/m2 IV q8h or foscarnet 40 mg/kg IV q8h |
|
Oropharyngeal candidiasis |
Other Candida species |
Nystatin oral suspension (100,000 units/mL), rinse with 5 Clotrimazole troches, 10 mg QID x 7-10 days Itraconazole 200 mg PO daily Fluconzazole 200 mg PO or IV daily or voriconazole 200 mg PO (3 mg/kg IV) q12h Caspofungin 70 mg IV loading, then 50 mg IV q24h |
Severe oral mucositis in immunocompromised hosts |
Viridans and other streptococci, ,Peptostreptococcus spp, Bacteroides appand other oral anaerobes, facultative gram-negative bacilli |
Topical chlorhexidine (0.1%) mouth rinses TID plus one of the following: Cefotaxime 2 g IV q6h Ticarcillin-clavulanate 3.1 g or piperacillin-tazobactam 3.375 g IV q4h Imipenem 500 mg IV q6h or meropenem 1 g IV q8h |
Sialadenitis and suppurative parotitis |
Mixed anaerobes
|
Nafcillin 2 g IV q4h or vancomycin 1 g IV q12h plus one of the following: Clindamycin 600 mg IV q6h Metronidazole 0.5 g IV q6h |
Table 3. Clinical Presentation of Deep Space Infections of the Face and Oral Cavity
|
|
Clinical Features |
||||
|---|---|---|---|---|---|---|
Space Infections |
Usual Site of Origin |
Pain |
Trismus |
Swelling |
Dysphagia |
Dyspnea
|
Masticator, Masseteric, & Pterygoid |
Molars (especially third) Absent |
Present |
Prominent |
May not be evident (deep) |
Absent |
Absent |
Temporal |
Post, maxillary molars |
Present |
None |
Face, orbit (late) |
Absent |
Absent |
Buccal |
Bicuspids, molars |
Minimal |
Minimal |
Cheek (marked) |
Absent |
Absent |
Canine |
Maxillary canines, incisors |
Moderate |
None |
Upper lip, canine fossa |
Absent |
Absent |
Infratemporal |
Posterior maxillary molars |
Present |
None |
Face, orbit (late) |
Occasional |
Occasional |
Submental |
Mandibular incisors |
Moderate |
None |
Chin (firm) |
Absent |
Absent |
Parotid |
Masseteric spaces intense |
None |
Angle of jaw |
Absent |
Absent |
Absent |
Submandibular |
2nd, 3rd mandibular molars |
Present |
Minimal |
Submandibular |
Absent |
Absent |
Sublingual |
Mandibular incisors |
Present |
Minimal |
Floor of mouth (tender) |
Present if bilateral |
Present if bilateral |
Lateral pharyngeal Anterior Posterior |
Masticator space Masticator space |
Intense Minimal |
Prominent Minimal |
Angle of jaw Posterior pharynx |
Present Present |
Occasional Severe |
Retropharyngeal (and danger) |
Lateral pharyngeal space, Distant via lymphatics |
Present |
Minimal |
Posterior pharynx (midline) |
Present |
Present |
Modified from Megran DW, Scheifele DW, Chow AW. Odontogenic infections. Pediatr.Infect Dis 3:257-265. 1984.
Figure 1: Odontogenic Infections.
A=dental caries, pulpal infection, and periapical abscess;
B=periodontal infection;
C=pericoronal infection overlying impacted tooth
(From Chow AW, Roser SM, Brady FA. Orofacial odontogenic infections. Ann InternMed 1978;88:392-402). [PubMed]
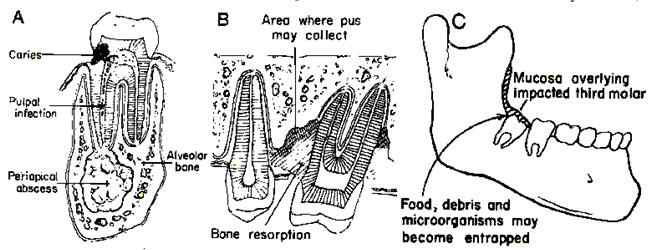
Figure 2: Mucosal Defenses Against Invading Bacteria in the Oral Cavity.
The oral mucosa has three types of antimicrobial defenses: physical barrier of the epithelial layer; non-specific (innate) immunity derived from salivary constituents, neutrophils, and epithelial antimicrobial peptides; and adaptive immunity associated with mucosa-associated lymphatic tissues (MALT) (From Abiko Y, Saitoh M, Nishimura M, et al. Role of beta-defensins in oral epithelial helath and disease. Med Mol Morphol 2007;40:392-402).[PubMed]
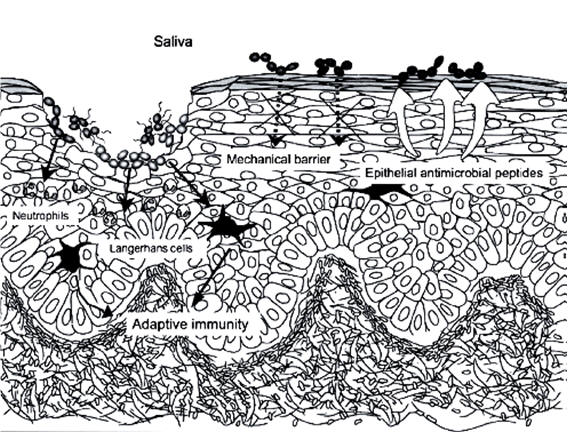
Figure 3: Microbial Specificity in Odontogenic Infections.
A unifying hypothesis demonstrating a microbial shift from a plaque-free tooth surface and progression to supragingival and subgingival plaque organisms
(From Chow AW. Infections of the oral cavity, neck and head. Mandell GL, Bennett JE, Dolin R, editors.Principles and Practice of Infectious Diseases. 7th ed.: 855-871. 2010. Philadelphia, PA, Churchill Livingstone Elsevier).
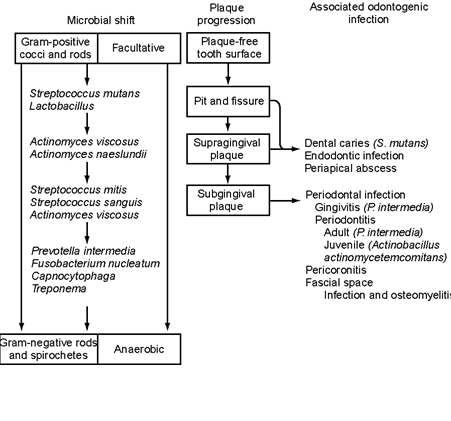
Figure 4A: Routes of Spread of Odontogenic Infections.
a=coronal section at first molar teeth: a, maxillary antrum
b= nasal cavity
c=palatal plate
d=sublingual space (above mylohyoid muscle)
e=submylohyoid space
f=intraoral presentation with infection spreading through the buccal plates inside the attachment of the buccinator muscle
g=extraoral presentation to buccal space with infection spreading through the buccal plates outside the attachment of the buccinator muscle.
Figure 4B: Lingual Aspect of the Mandible
a= tooth apices above the mylohyoid muscle with spread of infection into the sublingual space
b=tooth apices below the mylohyoid muscle with spread of infection into the submylohyoid space.
(From Chow AW: Life-threatening infections of the head, neck, and upper respiratory tract. In: Principles of Critical Care, 2nd ed. Hall JB, Schmidt GA, Wood LH (eds), McGraw-Hill, New York, 1998, pp 890).
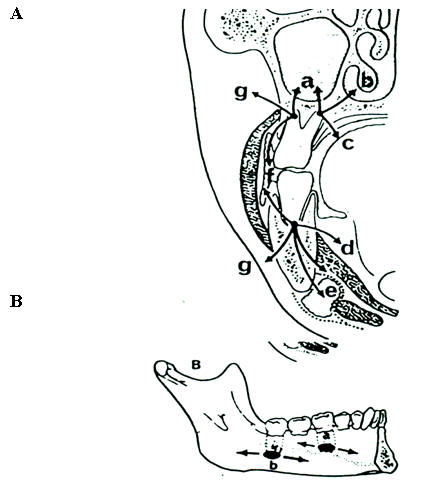
Figure 5: Potential Pathways of Extension in Deep Fascial Space Infections
(From Chow AW. Life-threatening infections of the head and neck. Clin Infect Dis 1992;14:991-1004). [PubMed]

Guided Medline Search For:
Guided Medline Search For Recent Reviews
Guided Medline Search For Historical Aspects
Table of Contents
- Anatomical Considerations
- Microbial Etiology
- Clinical Syndromes
- Odontogenic Infections
- Non Odontogenic Infections
- Pyogenic Infections of the Face and Neck
- Impetigo
- Folliculitis and Furunculosis
- Erysipelas
- Cellulitis
- Synergistic Necrotizing Cellulitis and Necrotizing Fasciitis
- Infections Of the Oral Mucosa
- Acute Herpetic Gingivostomatitis
- Aphthous Stomatitis
- Herpangina and Hand, Foot and Mouth Disease
- Gangrenous Stomatitis or Noma
- Oropharyngeal Candidiasis
- Mucositis in the Severely Immunocompromised
- Sialadenitis and Parotitis
- Conclusions

