Febrile neutropenia
Updated September, 2011
Authors: Dr. M. Aoun
Chemotherapy-induced neutropenia remains the major predisposing factor to infection in cancer patients. The risk of infection correlates with the degree of severity and the duration of neutropenia (3). Because of the decreased inflammatory response during neutropenia, the symptoms and signs of infection are attenuated or even absent and very often, fever is the only early manifestation. This fact justifies the term of febrile neutropenia generally used in this specific situation. Febrile neutropenia is associated with significant morbidity and results in delays in chemotherapy schedules or in reductions of chemotherapy dose-intensity, which may compromise the outcome. The overall mortality rate of febrile neutropenia is approximately 9.5 % in solid tumors and 14 % in lymphoma and leukemia, and the infection-related mortality rates are 2.3 % and 5 % respectively (18).
There is also an increase of costs associated with the management of febrile neutropenia in terms of hospitalization, antibiotic treatment and other supportive measures.
Definitions
Neutropenia refers to an absolute neutrophil count less than 500 cells/µl. Short duration of neutropenia (< 7 days) is usually seen in patients with solid tumor or lymphoma receiving standard chemotherapy and induced intensity conditioning allogeneic HSCT regimen while a long duration of neutropenia (> 7 days) is reported in patients receiving intensive chemotherapy with autologous HSCT rescue (~ 10 days of neutropenia), in patients with allogeneic HSCT and in patients with induction therapy for acute leukemia (> 21 days of neutropenia).
Fever is defined as one peak of axillary temperature above 38.3°C (101°F) or two peaks above 38°C (100°F) within 24 hours and separated by at least 2 hours. Clinically documented infection refers to episodes where a site of infection has been identified either clinically or radiologically.
Microbiologically documented infection refers to episodes where a pathogen has been identified in microbiological samples. Fever of unknown origin indicates episodes of fever where an infection cannot be demonstrated neither clinically nor microbiologically and in whom a non-infectious cause of fever has also been excluded. It is important to stress that the majority of the patients with fever of unknown origin during neutropenia still respond to empiric broad-spectrum antibiotics in the same proportion as patients with documented infections.
Risk Assessment of Chemotherapy-Induced Neutropenia and Its Complications: The occurrence of a febrile neutropenic episode results from the conjunction of several conditions, among which the intensity of the chemotherapy plays a major role in addition to the type and stage of the tumor and to several predisposing factors that are specific to each patient. The risk of febrile neutropenia is highest among patients receiving chemotherapy for acute leukemia or undergoing hematopoietic stem cell transplantation, exceeding 85% of cases. For patients with lymphoma or solid tumor, this risk is much lower although highly variable and more difficult to predict. The type of chemotherapy agents, dosage and number of drugs given in combination markedly influence the rate of febrile neutropenia. For many common regimens of chemotherapy, categorization lists are available, based on mean percentage of febrile neutropenia reported in clinical trials (NCCN, ASCO, EORTC). Paclitaxel, docetaxel, irinotecan, topotecan and high-dose cyclophosphamide, anthracycline, cisplatin, ifosfamide, etoposide, cytarabine have all been identified as significant predictors for febrile neutropenia. The combination of more than 2 myelosuppressive agents and a planned delivery higher than 85 % have been reported in a prospective cohort study, as risk factors for febrile neutropenia and validated subsequently in a randomly selected sample (19). Several patient-specific factors were found to be significant predictors of febrile neutropenia occurrence in multivariate risk-model studies. These include advanced age, poor performance and nutritional status, the presence of co-morbid conditions such as renal, liver or heart insufficiency and diabetes, pre-treatment low blood cell counts and other laboratory abnormalities like serum albumin concentration below 3.5 g/dl or high LDH values. A body temperature higher than 40°C at presentation, hypotension or shock, hematological malignancy, pneumonia and infection at intravenous site, are all significant predictors of bacteremia. Once a febrile neutropenic episode has occurred, the determination of the MASCC (Multinational Association of Supportive Care in Cancer) risk-index score which integrates several clinical parameters that can be readily assessed at the bed-side is a good risk predictor of complications including death (16).
Sites of Involvement and Pathogens: The major sites of infection during neutropenia and the most common offending pathogens are summarized in Table 1. The alimentary tract including oral cavity, esophagus, colon and rectum is the most common source of infection in neutropenic patients. Breaches in mucosal barriers accompanying intense chemotherapeutic regimens, allow members of the indigenous flora to enter the bloodstream. Oral mucositis is the portal of entry of viridans streptococci bacteremia with S. mitis and S. sanguis being the most common isolated species. Potentially lethal complications such as shock and acute respiratory distress syndrome have been associated with viridans streptococcal primary bacteremia in 11 % of cases (9, 10, 24).
Fusobacterium bacteremia, mainly due to F. nucleatumand less frequently to F. necrophorum, is another complication associated with oral mucositis during neutropenia. Despite the well established virulence of this anaerobic gram-negative bacilli, most of the reported cases in neutropenic patients have a favorable outcomes (10). Stomatococcus mucilaginosus, another inhabitant of the oral cavity, has been reported since the 1990’s mainly in primary bacteremias. However, in the pediatric population cases of meningitis have been described. Our experience in 8 neutropenic adult patients with bacteremia due to Stomatococcus mucilaginosus was that of manifestations of sepsis without central nervous system involvement and all patients survived (11). Other commensals of the mouth flora less frequently reported include Leptotrichia buccalis, Capnocytophaga spp., Eikenella corrodens, Rothia dentocariosa, Eubacterium spp. and Prevotella buccae. Necrotizing gingivitis is associated with Bacteroides species and esophagitis is mainly due to Candida spp. or Herpes simplex virus.
Neutropenic enterocolitis (or typhlitis) is an extreme
form of mucosal barrier injury induced by chemotherapy through the generation of
reactive oxygen radicals and causing damage to the cells, tissues and blood
vessels of the intestine. The ileo-cecal region is the most frequently involved
with longitudinal extension to the ascending colon or small bowel. Hemorrhagic
necrosis, ulcerations with bacterial colonization, transmural inflammation and
marked thickening of the bowel wall are the main pathological findings. The
clinical manifestations include fever, abdominal pain, diarrhea, right lower
quadrant pain and abdominal distension. Historically, typhlitis has been
associated with Clostridium septicumbacteremia but recent series,
reported concomitant bacteremia with enterococci, streptococci,
enterobacteriacae, P. aeruginosa and Candida spp. Rare cases due
to Aspergillus spp. or zygomycetes have been discovered on
histopathology examination and deep-seated tissue cultures. Bowel wall
thickening more than 4 mm and over more than 30 mm, demonstrated either by
ultrasound or computed tomography  is suggestive of neutropenic enterocolitis
(13).
is suggestive of neutropenic enterocolitis
(13).
Perirectal abscess is usually of polymicrobial origin involving anaerobes, predominantly Bacteroides fragilis, which are associated with facultatively anaerobic Gram-negative bacteria and Gram-positive cocci. Pain on defecation may be the only manifestation in neutropenic patients.
The respiratory tract, mainly sinuses and lungs, is a common site of infection during neutropenia. During a short period of neutropenia (< 7 days), pneumonia and sinusitis are usually caused by common respiratory pathogens such as Streptococcus pneumoniae, and Haemophilus influenzae and less frequently byEnterobacteriacaeand P. aeruginosa. While for long durations of neutropenia (> 10 days), the risk of having a respiratory infection caused by nosocomial multidrug resistant bacteria or by a filamentous fungi increases substantially.Aspergillusspp. and zygomycetes occur predominantly in acute leukemia, myelodysplastic syndrome and allogeneic hematopoietic stem cell transplant patients. The symptoms and signs of pneumonia may be lacking in neutropenic patients, and the chest CT scan is superior to the standard X-ray in showing the presence, the type and exact topography of a pulmonary infiltrate.
The skin including the catheter-site can be a major source
of severe infections during neutropenia. Catheter-site cellulitis and tunnelitis  are caused by S. aureus, coagulase-negative Staphylococcus (CNS), Bacillus spp., Corynebacterium jeikeium, Candida spp., Fusarium spp.,Hansenula anomala, Rhodotorulaand Malassezia
furfur in case of total parenteral nutrition. Furonculosis
are caused by S. aureus, coagulase-negative Staphylococcus (CNS), Bacillus spp., Corynebacterium jeikeium, Candida spp., Fusarium spp.,Hansenula anomala, Rhodotorulaand Malassezia
furfur in case of total parenteral nutrition. Furonculosis  due toS. aureusand perionyxis
due toS. aureusand perionyxis  due toS. aureusor fungi are
important clues for adequate management. Moisture-laden areas of the
skin such as the scrotum, the inguinal, axillary and perineal regions and the
skin around the nares, are the predilection sites of primary echtyma gangrenosum,
a severe necrotizing cellulitis most often induced by P. aeruginosa. (view images
due toS. aureusor fungi are
important clues for adequate management. Moisture-laden areas of the
skin such as the scrotum, the inguinal, axillary and perineal regions and the
skin around the nares, are the predilection sites of primary echtyma gangrenosum,
a severe necrotizing cellulitis most often induced by P. aeruginosa. (view images  ).
).
Finally, secondary skin lesions may be the only manifestation of many
disseminated infections  .
.
In a prospective trial which addressed extensively the causes of fever in 116 consecutive neutropenic episodes (23) we found that 70% were due to documented infections either clinically only in 24%, or microbiologically only in 22% or both clinically and microbiologically in 24%. Bacteremia represented 32% with primary bacteremia in 18% and secondary bacteremia in 14 %. Fever of unknown origin occurred in 17% of episodes while 13% were of non-infectious origin (Figure 1). Head and neck sites of infection were the most prevalent representing 21% and include oral mucositis grade 3 or 4, pharyngitis and sinusitis. Lower respiratory tract of infections accounted for 15%. Gastrointestinal and urinary tract infections represented 7.5% each, skin and soft tissue infections 6.3% and central nervous system infections were rare representing only 2.5%. Septic shock was present in 6.3% of all episodes.
Diagnosis and Laboratory Testing
The evaluation of patients with febrile neutropenia implicates a collection of medical history including previous infectious episodes, recent antimicrobial administration as prophylaxis or treatment and any allergy to antimicrobial agents. Subtle symptoms accompanying fever should be noted and a thorough physical examination should be undertaken in order to detect the least indice that can help directing further investigations and adequate management. Hidden sites of the body such as scrotum, axillary, inguinal and perianal areas, very often overlooked, should be part of the systematic clinical evaluation.
Blood cultures should be taken through all central line lumens and from peripheral vein. Mid-stream urine culture for bacteria and yeasts is performed whether urinary symptoms are present or not. Additional specimens collection include nasopharyngeal aspiration for rapid antigen detection, PCR and culture for respiratory viruses during epidemics or in patients with upper airway respiratory symptoms, throat swab culture for bacteria and yeasts and mouth rinses for Herpes simplex virus detection in case of oral mucositis. Sputum culture, in case of productive cough, and stool specimens culture and Clostridium difficile toxin detection in case of diarrhea are very helpful. Biopsy of cutaneous or subcutaneous lesions if present for histology, direct examination and cultures for bacteria, mycobacteria and fungi is recommended.
Other more invasive procedures such as lumbar, pleural, ascites and articular
punctures should be evaluated according to the clinical presentation. Chest
X-ray is performed routinely whether the patients have respiratory symptoms or
not. Abdominal ultrasonography is not recommended routinely but should be
performed is case of abdominal pain or diarrhea and whenever an abnormality is
found on physical examination of the abdomen. In patients with persisting fever
despite broad-spectrum antibiotics and having a duration of neutropenia more
than 10 days, a chest and sinus CT scans will be performed. If abnormal findings
are present, this will be completed by sinusoscopy and/or bronchoscopy with bronchoalveolar lavage and ELISA galactomannan test will be done on serum and
BAL fluid.![]()
Treatment
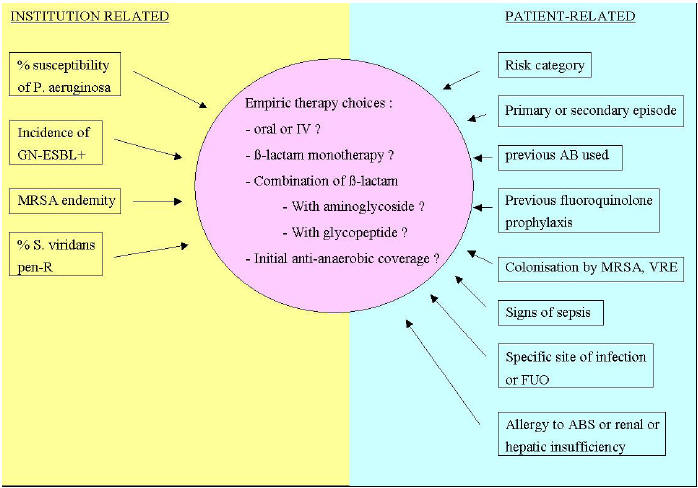
The management of the febrile neutropenia syndrome has evolved progressively over the years. In the 1960s, the initiation of broad-spectrum antibiotics at the very onset of fever in neutropenic patients, decreased dramatically the mortality. This concept of empiric therapy is still one of the major elements of standard care of the febrile neutropenia nowadays. Until the 1980s, a combined therapy of a ß-lactam and an aminoglycoside has prevailed as the regimen of choice for empiric therapy, justified by a predominance of Gram-negative bacteria causing severe infection in neutropenic patients. Moreover, the available ß-lactam antibiotics at this period had a modest intrinsic activity against P. aeruginosa. However, since the mid 1980s, and in parallel to the shift from a predominance of Gram-negative to that of Gram-positive bacteria causing bacteremia in neutropenic patients, several comparative studies were able to show equivalent efficacy between a ß-lactam alone and its combination with an aminoglycoside. In fact, the introduction of new ß-lactam drugs with better intrinsic activity against Gram-negative bacteria in general, and against P. aeruginosa in particular, contributed to decrease the role of combination with aminoglycosides and its potential for nephrotoxicity and ototoxicity. More recently, since the mid 1990s, the attitude consisting in extra safety measures with hospitalization and intravenous antibiotic therapy for all neutropenic patients began to wane in favor of a risk-adapted therapy based on prediction rules of complication rates during the course of a febrile neutropenic episode. Refinement of selection of patients at low-risk of complications was achieved and oral antibiotic therapy with early discharge was applied to this low-risk population. Thus, the risk categorization of the febrile neutropenic episode allows to determine if the patient should be kept in the hospital and treated intravenously, or if he can receive oral antibiotics and be discharged early. Additional key elements are to be addressed for those who receive intravenous empiric antibiotics in order to know which ß-lactam to choose, in monotherapy or in combination with aminoglycosides or other antibiotics (Figure 2).
Treatment of Patients at Low-Risk: Once a febrile neutropenic episode has occurred a risk-assessment of complications is undertaken. For this purpose, the MASCC index scoring system is the most widely used and allows selecting low-risk patients with good sensitivity and specificity. Once the patient is categorized as being at low-risk, the next question then is whether this patient is eligible for oral therapy. In fact, several conditions should be present including the absence of severe oral mucositis, diarrhea and vomiting. Two large, prospective randomized trials compared oral and intravenous antibiotics in patients with febrile neutropenia at low risk of complications, both using amoxicillin-clavulanate plus ciprofloxacin for oral therapy and both showed equivalent efficacy and safety between the oral and the intravenous arms. Based on these results, the association of amoxicillin-clavulanate plus ciprofloxacin has been recommended for oral therapy providing that patients have not received fluoroquinolone prophylaxis. Patients who are allergic to penicillin may benefit from an alternative regimen associating ciprofloxacin and clindamycin although creatinine elevation has been reported with this regimen in neutropenic patients. Two small studies evaluated new-generation fluoroquinolone monotherapy for oral empiric therapy of low risk febrile neutropenic patients. The first one included 54 patients who received oral moxifloxacin 400 mg once daily with a response rate of 91 %. The second one enrolled 43 patients treated with oral gatifloxacin 400 mg once daily and showed a response rate of 95 %. Although promising, these results must be confirmed by larger randomized trials. For patients at low-risk who received intravenous antibiotics for temporary reasons, a step-down strategy with a rapid shift to oral antibiotics may be applied. Outpatient management for patients with febrile neutropenia at low-risk, with oral antibiotic therapy has been evaluated recently in a randomized trial which compared oral antibiotics, followed by early discharge after a 24 h observation period, with in-patient intravenous antibiotics until resolution of the febrile neutropenic episode. The efficacy with both strategies was equivalent and the readmission rate in the outpatient arm was low. Whether some patients at low-risk would benefit from immediate discharge, without a period of observation is not yet established and the safety of such a procedure needs to be carefully assessed before it could be generalized.
Treatment of Patients at High-Risk: The standard care of febrile neutropenia at high-risk of complications includes in-patient management with intravenous broad-spectrum antibiotics. A ß-lactam agent active against Gram-negative bacteria including P. aeruginosa is the corner stone around which the whole management strategy is build up. Although detailed global and updated epidemiological data are lacking in the neutropenic population, it is more likely that local institution epidemiology would be more appropriate for the selection of initial ß-lactam agents for empiric therapy. Among the different ß-lactams, the highest percentage of susceptibility ofP. aeruginosais observed for piperacillin-tazobactam and meropenem although no one single ß-lactam delineates 100 % activity againstP. aeruginosaanymore (12, 21). Another epidemiologic feature to be taken into account, is the rising incidence of extended spectrum ß-lactamase (ESBL)-producing Gram-negative bacilli, especially E. coli and Klebsiella spp., in the neutropenic patients (2). Among the few ß-lactam agents that are suitable for empiric therapy of febrile neutropenia, it is important to make a distinction between molecules such as ceftazidime, cefepime or piperacillin-tazobactam among which the probability of cross-resistance is high, and the carbapenems such as imipenem or meropenem which retain activity in case of emergence of resistance to other ß-lactams. Based on these considerations, it is recommended to use a strategy that differentiates between first-line therapy based on molecules such as cefepime or piperacillin-tazobactam, and second-line therapy constituted of the carbapenems. This allows keeping a valuable alternative in case of emergence of resistance. However, such a strategy can be implemented only if the baseline incidence of ESBL-producing Gram-negative bacilli is low. In fact, carbapenems are the most active drugs against these bacteria, and reduced the mortality in ESBL-producingK. pneumoniaebacteremia. Among the first-line ß-lactam agents, there are some theoretical advantages of piperacillin-tazobactam and cefepime over ceftazidime. These include a better activity against streptococci and methicillin-susceptibleS. aureus with less need for the addition of a glycopeptide. Furthermore, less induction and decreased emergence of ESBLs, are reported with cefepime and piperacillin-tazobactam.
Several randomized comparative trials have assessed the potential of each the ß-lactams, namely ceftazidime, cefepime, piperacillin-tazobactam, imipenem, meropenem and aztreonam, for empiric therapy of primary episodes of febrile neutropenia at high-risk. No significant differences in response rates or mortality were observed in these individual trials. So, the clinical evidence-based medicine assessment of these different ß-lactams will result in a strong recommendation with good evidence to support their use. But, the continuously changing microbial distribution and the emergence of new mechanisms of resistance that occurred after the completion of many of these studies, contribute to obscure the interpretation of results and their relevance to the actual epidemiology. Nevertheless, a recent meta-analysis focusing on response rate in the different comparative trials of empiric therapy of febrile neutropenia, showed superiority of the carbapenems and piperacillin-tazobactam over ceftazidime. In some situations, specific anti-anaerobic coverage is indicated. These include severe mucositis or gingivitis, typhlitis, peri-anal and allogeneic HSCT where up to 17 % of bloodstream infections were anaerobes (17). Piperacillin-tazobactam and carbapenems cover the majority of anaerobes, otherwise, for cephalosporins or aztreonam, metronidazole should be added. Although this is not a current first choice, in case of penicillin-allergy mediated by IgE where the risk of anaphylaxis is important, aztreonam combined with a glycopeptide is an acceptable alternative.
Is There Still a Role for Combination Therapy of a ß-lactam Plus an Aminoglycoside for Empiric Therapy? This question has been addressed in two recent meta-analyses which reviewed the studies that compared monotherapy with combination therapy. The conclusion was that of no advantage of the combination with an excess of toxicity. However, it should be mentioned that mortality is highest in the subgroup of febrile neutropenic patients with sepsis, varying between 18 and 40 % as compared with 2.8 % for non-septic febrile neutropenic patients. If any benefit is to be expected from combination therapy, it is within this subgroup that would occur. But, no single study targeted specifically such patients. Thus, if for the majority of patients with febrile neutropenia, a combination therapy is not justified by the existing data, in the subgroup of patients with sepsis and high mortality, the benefits of combining a ß-lactam with an aminoglycoside may outweigh the risk of toxicity. Another important question has been the addition of a glycopeptide to the initial regimen. In fact, among the Gram-positive pathogens that cause infection or bacteremia during neutropenia, very few cause a fulminant infection course with significant morbidity and mortality. These include viridans streptococci, S. pneumoniae and S. aureus, which need optimal coverage upfront. Again, the local epidemiology and the penicillin or methicillin-resistance of these organisms, which is highly variable between institutions, plays an important role. A recent Cochrane review of 13 randomized trials comparing the addition of an anti-Gram-positive antibiotic to the initial empiric regimen, did not show any benefit in reducing treatment failure, all cause mortality or Gram-positive superinfections. However, there are circumstances in which a glycopeptide or another antibiotic active against resistant Gram-positive bacteria, should be added up-front. These include patients who are already known to be colonized by MRSA, if MRSA is endemic in the institution and in several skin infections that could be caused by resistant staphylococci as folliculitis, furonculosis and periporth cellulitis and also if penicillin-resistant viridans streptococci are prevalent in the institution.
When and How to Modify Initial Empiric Therapy in Persistently Febrile Neutropenic Patients? Few studies have specifically addressed this question. According to the study by Cometta et al (6) conducted by the anti-infection group of the EORTC, the mean time for defervesence on piperacillin-tazobactam monotherapy was 5 days. Therefore, for patients without hypotension or sepsis and in whom no resistant pathogen is isolated, it seems reasonable to wait until day 5 before modification. On the contrary, for those who develop signs of sepsis, hypotension or other early findings of deterioration, at any time, a shift to non-cross resistant antibiotic is recommended. After day 5, those who remain febrile and had no non-infectious cause of fever, may benefit from a shift in ß-lactam coverage i.e. from a first-line treatment with piperacillin-tazobactam or cefepime to a carbapenem. Simultaneously, a thorough investigation including chest and sinus CT scan, galactomannan test and other diagnostic tests for viral or parasitic infections, should be undertaken. Algorithms 1, 2, 3 and 4 provide general lines of management according to specific situations of febrile neutropenia.
Antifungal Empiric Therapy
The rationale for empiric antifungal therapy in persistently febrile neutropenic patients, is based on the fact that early diagnosis of invasive fungal infections is difficult to establish and the mortality is increased by delay in adequate therapy. The concept of empiric antifungal therapy in neutropenic patients with persistent fever despite broad-spectrum antibiotics, has been introduced following two studies with a limited number of patients, comparing amphotericin B with placebo and showing a decrease in fungal infection-related mortality in patients receiving amphotericin-B deoxycholate. These studies were accomplished at a period where antifungal prophylaxis was ineffective and CT-scans and ELISA galactomannan tests on PCR for Aspergillus detection were inexistent. Is this concept still true today? We just don’t know, because all the large studies that have been done thereafter addressing the question of empiric antifungal therapy were not placebo controlled. With this in mind, we see in the latest trials that the true rate of failure of empiric antifungal therapy which is the development of a breakthrough fungal infection and the absence of cure of base line fungal infection is 6.9 % for liposomal amphotericin-B, 3.6 % for voriconazole and 7.7 % for caspofungin. The need for empirical antifungal therapy during neutropenia has decreased secondarily to the implementation of more effective antifungal prophylaxis. In one of the most recent trials using posaconazole prophylaxis in neutropenic patients, the need for empiric therapy was only of 27 %. The next question is whether our new imaging techniques and new laboratory methods are able to detect invasive fungal infections early enough and whether they can serve to build-up a pre-emptive or a diagnostic-driven strategy in order to save unnecessary costs and drug exposure, without increase in mortality. One such a pre-emptive study by Maertens et al (20), based on positive ELISA galactomannan test or suggestive infiltrate on chest CT-scan, showed a reduction of 78 % in the use of empiric antifungal drugs. Another study by Cordonnier et al (5) comparing prospectively the empiric with the pre-emptive strategy in high-risk febrile neutropenic patients showed again a decrease of 20 % in the use of antifungal drugs with the pre-emptive strategy, a similar mortality rate but surprisingly similar median medication costs. Thus, the pre-emptive or diagnostic-driven strategy is able to reduce the rate of antifungal overtreatment but the safety and cost effectiveness should be confirmed by large comparative prospective trials.
Adjunctive Therapy
Th e recovery of neutrophils in patients with severe infection and hematological malignancy plays a major role in resolution and survival. In order to palliate a temporary deficit in neutrophils, a logical approach was the development of transfusion of donor neutrophils as adjunctive therapy to antibiotics and antifungals, in neutropenic patients with refractory infections. Although initial clinical successes were reported between 1970s and beginning 1980s, ganulocyte transfusion adjunctive therapy declined progressively due to several reasons. In fact, adverse effects such as allo-immunization, CMV transmission, GVHD and pulmonary reactions, have been reported. Besides, the availability of more advanced antimicrobials, the time and cost consuming procedure and a marginal effect demonstrated in randomized trials, all together contributed to this decline. Moreover, the lack of standard optimal granulocyte dose required daily (>1010cells) and storage conditions and duration (8-24h) add to the uncertainty of study results. More recently, a revival in granulocyte transfusion use has occurred as donors were stimulated more efficiently by G-CSF at 5 to 10 µg/kg. Higher yields and improved phagocytosis and killing of neutrophils with prolonged intravascular survival are obtained after stimulation. However, the clinical efficacy of this new generation of granulocyte transfusion is still limited to case reports and small non randomized series. A recent meta-analysis of 8 randomized controlled trials involving granulocyte transfusions, given therapeutically to neutropenic patients showed inconclusive evidence to support or refute the generalized use granulocyte transfusion therapy. Thus, in the absence of definitive data, it is reasonable to provide granulocyte transfusions for neutropenic patients with hematological malignancy and documented bacterial or invasive fungal infection, not controlled by adequate antimicrobial therapy.
The role of G-CSF and GM-CSF as adjunct therapy to antimicrobials in febrile neutropenic patients is controversial. Individual small studies have generated conflicting results. A recent meta-analysis on 13 controlled trials comparing CSFs plus antibiotics versus antibiotics alone for the treatment of established febrile neutropenia, showed a benefit in terms of length of hospitalization and time to neutrophil recovery, in favor of CSFs, but no advantage on overall mortality and a trend toward a decrease in infection-related mortality. This latter effect needs further investigation.
Prevention
Basic hygiene measures are reinforced in patients receiving chemotherapy. The prevention of oral mucositis is essential requiring standard dental care, gentle tooth brushing and chlorexidine mouth rinses.Is neutropenic diet prohibiting uncooked meat, raw eggs, fresh vegetables, fruits and juices, necessary and effective in preventing infections? There are no evidence-based studies that addressed this issue and the existing ones are of small sample-size and biased by concomitant antibacterial and antifungal prophylaxis. One recent randomized study including 153 patients showed less bacteremia and fungemia in the group fed with cooked food (9% vs 23%; p=0.03) (8). A recent review on this controversial issue failed to show clear evidence from the literature (14). Until we have more definitive data, it seems prudent to maintain the use of basic principles, prohibiting uncooked meats, raw eggs, seafood, fresh vegetables, fruits except oranges and bananas and aged cheese.Palifermin, a recombinant human keratinocyte growth factor is able to protect several types of epithelial tissues exposed to radiotherapy or chemotherapy. It enhances cell proliferation and increases thickness of the epithelium of the gastro-intestinal tract. Skin rash and disconfort due to mucosal hypertrophy of tongue and gingiva are the main adverse events whereas high cost and risk of growth enhancement in tumors expressing keratinocyte growth factor receptor are additional drawbacks. Another procedure that has demonstrated encouraging results in the prevention of oral mucositis with good tolerance is low level energy laser therapy. However, the two major strategies that have been developed to decrease complications during neutropenia are the administration of myeloid growth factors and antibiotic prophylaxis. A recent meta-analysis based on 17 randomized trials and including 3493 patients showed that myeloid growth factors decrease the risk of febrile neutropenia by 46 %, the infection-related mortality by 45 % and the early mortality by 40 % as compared to controls. Recommendations concerning the use of myeloid growth factors for the prevention of febrile neutropenia have been formulated by the EORTC (1) and an update of the ASCO guidelines released in 2006 along with the NCCN clinical practice guidelines in oncology (22), all adopted a 20 % threshold risk of febrile neutropenia for primary prophylaxis with myeloid growth factors. Moreover, all recognize several special circumstances in which patients at lower risk of FN than 20 % may receive myeloid growth factors prophylaxis because of bone marrow compromise or co-morbidity. On the other hand, the concept of antibiotic prophylaxis takes its origin from the decontamination of the gastro-intestinal tract developed in the 1970s by non absorbable antimicrobials such as neomycin, colistin, polymyxin B or gentamicin, vancomycin and nystatin. Study results with these drugs were discordant mainly because of the gastro-intestinal intolerance and bad tasting. In 1980s, trimethoprim-sulfamethoxazole and nalidixic acid were used for prophylaxis showing also mixed results. Trimethoprim-sulfamethoxazole was associated with high incidence of cutaneous rashes, and increased colonization byP. aeruginosawhile with nalidixic acid, there was a rapid emergence of resistant Gram-negative bacilli.
The fluoroquinolones, introduced in the mid 1980s, had good bactericidal activity against enterobacteriacae and for ciprofloxacin, activity againstP. aeruginosacertainly at the high concentrations achieved in stools from 100 to 2000 µg/g. Fluoroquinolones are well tolerated, have little toxicity and preserve the anaerobic flora. These characteristics made them attractive for prophylaxis in neutropenic patients. Two large randomized double-blind trials comparing levofloxacin with placebo, for prophylaxis in cancer patients receiving chemotherapy, tried to clarify somewhat this controversial issue.
The first one included ambulatory patients with solid tumor or lymphoma with a mean risk for febrile neutropenia of 7.9 % and the second one was conducted in hospitalized patients with a mean risk for febrile neutropenia of 85 %. Both studies showed an advantage for levofloxacin in terms of reduction of febrile episodes, and infection including bacteremia. However, the number needed to treat (NNT) in order to avoid one episode of febrile neutropenia is 70 for the first and 5 for the second study. The emergence of resistance was carefully assessed in the second trial and showed an increase of resistance in levofloxacin arm, in both Gram-negative and Gram-positive bacteria. This implicates strict infection-control measures in order to limit the dissemination of clonal resistance within the unit or the hospital. All the individual studies were not powered enough to show a decrease in mortality with fluoroquinolone prophylaxis but a recent meta-analysis including 95 randomized trials did show a benefit with significant decrease in mortality with antibiotic prophylaxis.
Primary antifungal prophylaxis with the use of azoles mainly, has been extensively evaluated in patients with hematological malignancies. Fluconazole at the dose of 400 mg/d, was associated with a decrease in Candida albicans and tropicalis infections and a decrease in Candida infection-related mortality, in patients with allogeneic bone marrow transplant recipients (15). Itraconazole oral solution, at a dose of 400 mg/d or higher, reduced significantly the incidence of invasive candidiasis and invasive aspergillosis as well as Aspergillus-related mortality in patients with prolonged neutropenia (4). More recently, posaconazole at 200 mg three times daily showed superiority over either fluconazole or itraconazole by decreasing the incidence and mortality of invasive aspergillosis in neutropenic patients with acute leukemia or myelodysplastic syndrome (7). In brief, several strategies for prevention including myeloid growth factors, antibacterial and antifungal prophylaxis have shown their potential with advantages and disadvantages. Consequently, each specific situation of the patient under chemotherapy will be assessed carefully in order to select patients at high-risk who should benefit from prevention and select one strategy rather another or combine these strategies for the patients at highest risk.
Conclusion
The febrile neutropenia syndrome remains a life-threatening complication of cancer therapy. Major progress has been achieved in prediction of occurrence, risk assessment of outcome and prevention. The management based on a risk-adapted strategy with intravenous or oral antibiotics provides security and preserves the quality of life.
REFERENCES
1. Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M, Kearney N, Lyman GH, Tjan-Hejnen VC, Waleswki J, Weber DC, Zielinski C, European Organization for Research and Treatment (EORTC) Granulocyte Colony Stimulating Factor (G-CSF) Guidelines Working Party. EORTC guidelines for the use of granulocyte-colony stimlating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patient with lymphomas and solid tumours Eur J Cancer 2006:42:2433-2453. [PubMed]
2. Akova M. Emerging problem pathogens: a review of resistance patterns over time. Int J Infect Dis 2006;10:S3-S8.[PubMed]
3. Bodey GP, Buckley M, Sathe YS,, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukaemia. Ann Intern Med 1996;64:328-340. [PubMed]
4. Bow EJ, Laverdiere M, Lussier N, Rotstein C, Cheang MS, Ioannou S. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta-analysis of randomized-controlled clinical trials. Cancer. 2002, 94, 3230-3246. [PubMed]
5. Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, Basile M, Isnard F, Ades L, Kuhnoskbi F, Reman O, Chehata S, De Revel T, Lepretre S, Raffoux E, Bretagne S, Schwarzinger M. Empirical versus pre-emptive antifungal therapy in high-risk febrile neutropenic patients: a prospective randomized study. Blood 2006;108:Abstract 2019. [PubMed]
6. Cometta A, Zinner T, De Bock R, Calandra T, Gaya H, Klastersky J, Langenaeken J, Paesmans M, Viscoli C, Glauser MP. Piperacillin-Tazobactam plus vancomycin versus ceftazidime plus amikacin as ermpiric therapy for fever in granulocytopenic patients with cancer. Antimicrob Agents Chemother 1995:339;445-452 [PubMed]
7. Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzales D. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348-359. [PubMed]
8. Deresinski S. Is the neutropenic diet effective and necessary? Clin Infect Dis 2009;49 (1): iii-iv doi:10./598964[PubMed]
9. Elting LS, Bodey Gp, Keefe BH. Septicemia and shock syndrome due to viridans streptocci : a case control study of predisposing factors. Clin Infect Dis 1992;14:1201-1207. [PubMed]
10. Fanourgiakis P, Vekemans M, Georgala A, Daneau D, Vandermies A, Grenier P, Aoun M. Febrile neutropenia and Fusobacterium bacteremia : clinical experience with 13 cases. Support Care in Cancer 2003;11:332-335. [PubMed]
11. Fanourgialis P. Georgala A, Vekemans M, Daneau D, Heymans C, Aoun M. Bacteremia due to Stomatococcus mucilaginosus in neutropenic patients in the setting of a cancer institute. Clin Microbiol Infect 2003;9:1068-1072 [PubMed]
12. Gales AC, Jones RN, Turnidge J, Rennie R, Ramphal R. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis 2001:32;S146-155. [PubMed]
13. Gorschlüter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG, Sauerbruch T, Glasmacher A. Neutropenic enterocolitis is adults : systematic analysis of evidence quality. Eur J Haematol 2005;75:1-13. [PubMed]
14. Jubelirer ST. The benefit of the neutropenic diet : fact or fiction? Oncologist 2011, April 6[PubMed]
15. Kanda Y, Yamamoto R, Chizuka A et al. Prophylactic action of oral fluconazole against fungal infection in neutropenic patients: a meta-analysis of 16 randomized, controlled trials. Cancer. 2000, 89, 1611-1625. [PubMed]
16. Klastersky J, Paesmans M, Rubinstein ER, Boyer M, Elting L, Feld R, Gallagher J, Herrstedt J, Rapoport B, Rolston K, Talcott J. The multinational association for supportive care in cancer risk index : a multinational scoring system for identify low-risk febrile neutropenic cancer patients. J Clin Oncol 2000;18:3038-3051. [PubMed]
17. Lark RL, McNeil SA, van der Hyde K, Noorani Z, Uberti J, Chenoweth C. Risk factors for anaerobic bloodstram infections in bone marrow transplant recipients. Clin Infect Dis 2001;33:338-343. [PubMed]
18. Leibovici L, Paul M, Cullen M, Bucaneve G, Gafter-Gvilli A, Fraser A, Kern WV. Antibiotic prophylaxis in neutropenic patients : new evidence, practical decisions. Cancer 2006;107:1743-1751. [PubMed]
19. Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, D. C. Dale for the ANC Study Group. Prospective validation of a risk model for first cycle neutropenic complications in patients receiving cancer chemotherapy (ASCO Meeting Abstracts). J Clin Oncol 2006 24 (18S): 8561 [PubMed]
2 0. Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, Wilmer A, Verhaegen J, Boogaerts M, Van Eldere J. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection : a prospective feasibility study. Clin Infect Dis 2005;41:1242-1250 [PubMed]
21. Mastertom RG, Kuti JL, Turner PJ, Nicolau DP, The OPTAMA program : utilizing MYSTIC (2002) to predict critical pharmacodynamic target attainment against nosocomial pathogens in Europe. J Antimicrob Chemother 2005;55:71-77. Eratum in : J Antimicrob Chemother 2005;55:990. [PubMed]
22. Smith TJ, Khatcheressian J, Lyman GH et al. 2006 update of recommendations for the use of white blood cell growth factors : an evidence-based clinical practice guideline. J Clin Oncol 2006;24:3187-3205 [PubMed]
23.Toussaint E, Behel-Ball E, Vekemans M, Georgala A, Al-Hakak L, Paesmans M, Aoun M. Causes of fever in cancer patients (prospective study over 477 episodes). Supportive Care in Cancer 2006;10 mars:E-pub. [PubMed]
24.Van der Lelie H, van Ketel RJ, von dem Borne AE, van Oers RH, Thomas BL, Goudsmit R. Incidence and clinical epidemiology of streptococcal septicemia during treatment acute myeloid leukaemia. Scand J Infect Dis 1991;23:163-168. [PubMed]
Algorithm 1: Approach to the Presence of Clinical Signs of Febrile Neutropenia

Always |
ß-lactam active against P. aeruginosa |
Cellulitis around a catheter |
Add a glycopeptide |
Perianal abscess, necrotic gingivitis or typhilitis |
Add anaerobic coverage (piperacillin, tazobactam, imipenem, 3rd of 4th generation cephalosporin plus metronidazole) |
Severe mucositis |
Optimal coverage of streptococci + acyclovir |
Cutaneous cellulitis |
Optimal coverage of S. aureus and Pseudomonas aeruginosa. If important resistance rate to oxacillin in institution : add glycopeptide |
Diarrhea |
Toxin detection of Clostridium difficile and add oral metronidazole |
Algorithm 2: Approach of Empiric Antibiotic Therapy for Febrile Neutropenia by MASCC Score
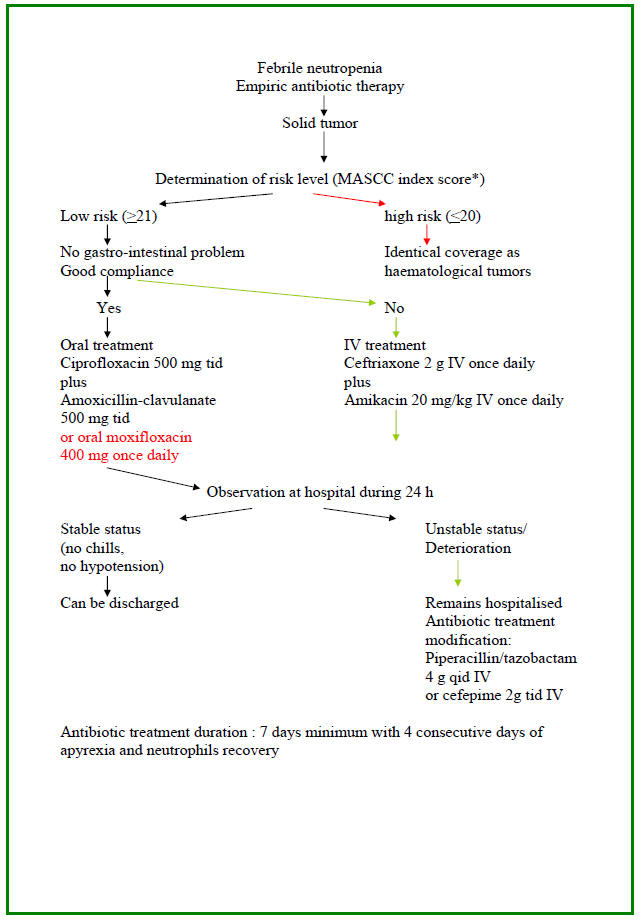
Algorithm 3: Approach to Febrile Neutropenia with Stable Hemodynamic Status
 |
Algorithm 4: Approach to Febrile Neutropenia with Unstable Hemodynamic Status
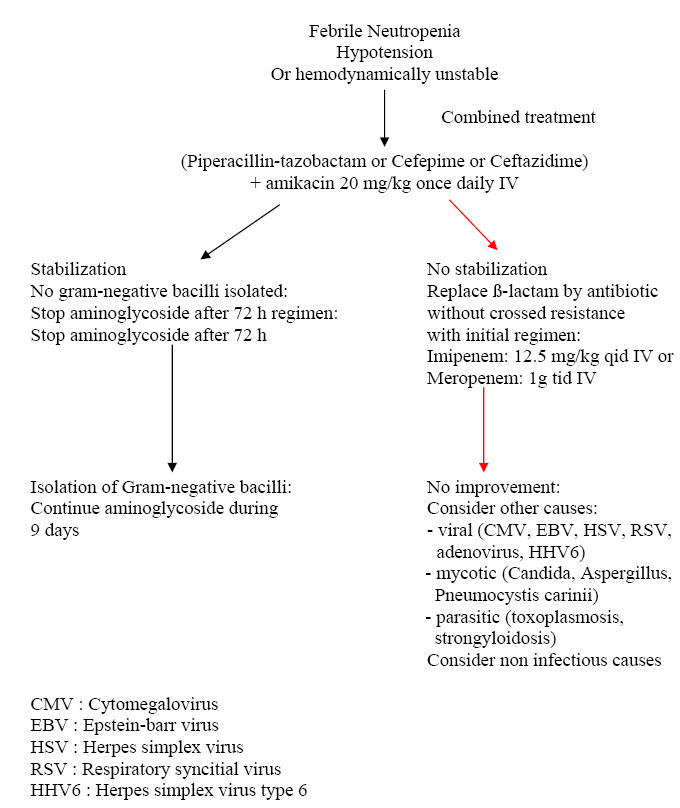 |
Table 1: Sites of Involvement and Common Pathogens
PRIMARY BACTEREMIA viridans Streptococci Enterobacteriacae : E. coli, Klebsiella spp. Pseudomonas aeruginosa Candida spp. PNEUMONIA OR SINUSITIS Conventional bacteria (frequent concomitant bacteremia) Aspergillus spp. Zygomycetes spp. Fusarium spp. SKIN AND SOFT TISSUE (INCLUDING CATHETER SITE) Coagulase negative staphylococci Staphylococcus aureus Bacillus spp. Corynebacterium jeikeium Pseudomonas aeruginosa (ecthyma gangrenosum) Candida spp., Trichosporon spp., Malassezia furfur ORAL MUCOSITIS HSV Oral bacterial flora (viridans Streptococci, Fusabacterium spp, Prevotella, Stomatococcus spp., Eikenella corrodens, Leptotrichia, …) Bacteroides spp. (necrotizing gingivitis) PERIANAL ABSCESS Polymicrobial (Gram-positive cocci, Gram-negative bacilli and anaerobes) TYPHLITIS Clostridium septicum Other Clostridia Enterobacteriacae Bacteroides fragilis Candida spp. |
Figure 1 Causes of Fever During Neutropenia
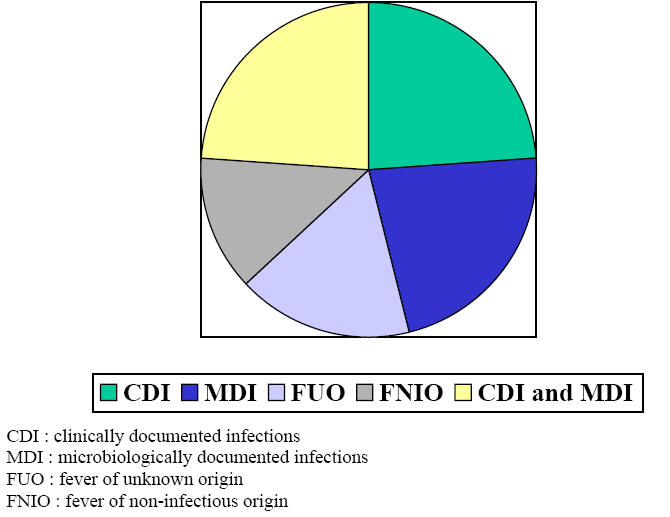
Figure 2: Factors that Influence the Choice of Empiric Therapy
GN-ESBL : Gram-negative bacilli producing extended-spectrum ß-lactamases
MRSA : Methicillin-resistant Staphylococcus aureus
Pen-R : Penicillin-resistant
AB(s) : Antibiotic(s)
VRE : Vancomycin resistant enterococci
FUO: Fever of unknown origin
What's New
Jansen RR, et al. Febrile neutropenia: significance of elaborated screening for respiratory viruses, and the comparison of different sampling methods in neutropenic patients with hematological malignancies. Virol J 2013;10:212.
Vedi A, et al. Oral versus intravenous antibiotics in treatment of paediatric febrile neutropenia. J Paediatr Child Health 2013;49:170-8.
Torres JP, et al. Frequency and clinical outcome of respiratory viral infections and mixed viral-bacterial infections in children with cancer, fever and neutropenia. Pediatr Infect Dis J 2012;31:889-93.
Aguilar-Guisado M, Martín-Peña A, Espigado I, Ruiz Pérez de Pipaon M, Falantes J, de la Cruz F, Cisneros JM. Universal antifungal therapy is not needed in persistent febrile neutropenia: a tailored diagnostic and therapeutic approach. Haematologica. 2012 Mar;97(3):464-71. Epub 2011 Nov 4.
Bochennek K. Liposomal Amphotericin B Twice Weekly as Antifungal Prophylaxis in Pediatric Hematologic Malignancy Patients. Clinical Microbiol Infect. Feb. 2011, Epub ahead of publication.
Cooper MR. Single-agent, broad-spectrum fluoroquinolones for the outpatient treatment of low-risk febrile neutropenia. Ann Pharmacother. 2011 Sep;45(9):1094-102.
Deresinski S. Is the Neutropenic Diet Effective and Necessary? Clin Infect Dis 2009;49: iii-iv.
Manji A, Beyene J, Dupuis LL, Phillips R, Lehrnbecher T, Sung L. Outpatient and oral antibiotic management of low-risk febrile neutropenia are effective in children--a systematic review of prospective trials. Support Care Cancer. 2012 Jun;20(6):1135-45. Epub 2012 Mar 9.
Lingaratnam S, Worth LJ, Slavin MA, Bennett CA, Kirsa SW, Seymour JF, Dalton A, Koczwara B, Prince HM, O'Reilly M, Mileshkin L, Szer J, Thursky KA. A cost analysis of febrile neutropenia management in Australia: ambulatory v. in-hospital treatment. Aust Health Rev. 2011 Nov;35(4):491-500.
Sun HY, Singh N. Mucormycosis: its contemporary face and management strategies. Lancet Infect Dis 2011;11:301-310.
GUIDED MEDLINE SEARCH FOR:
Review Articles
Ramaprasad C, Pursell K. Cytopenias after Solid Organ Transplantation
