Infections in Patients Undergoing Chronic Dialysis
Authors: Steven Berman, M.D.
INTRODUCTION
Patients with renal failure are susceptible to infection. In the predialysis era, 60% of patients with chronic renal failure requiring hospitalization were infected and 39% died from infectious causes. It was assumed that the debility caused by the uremic state increased the risk of infection, and the reversal of uremia would reduce the risk of infection.
Unfortunately, the prescription of chronic hemodialysis to reduce the uremic state did not reduce the problem of infection; it only changed the paradigm. Dialysis superimposes new problems onto patients already suffering relentless deterioration from underlying multi-system disease and poor wound healing. Diabetes mellitus is the responsible cause for one-half of end stage renal disease (ESRD) cases, followed by hypertension, and chronic glomerulonephritis. Heart disease is present in 40% of the patients and 15% suffer from peripheral vascular disease. In addition to infection risk associated with frailty and disability are problems associated with the intravascular connection, white blood cell and complement dysfunction from contact with dialysis membranes, and exposure to bacteria and pyrogens from contaminated dialysis solutions or inadequately cleaned dialysis machines.
To better understand the issue of infection in dialysis patients, we reviewed a 10-year experience of inpatient and outpatient medical records for 433 patients with ESRD requiring chronic hemodialysis. Twenty-four hundred (2,400) infections were identified, requiring 5,111 courses of antibiotics, averaging 10% of total days in chronic dialysis therapy. The most common infection involved hemodialysis vascular access (HVAD). However, 80% of the infectious episodes were not related to dialysis. Infections associated with diabetes mellitus and/or frailty, including infections in the setting of peripheral vascular disease or neuropathy, pneumonia and other skin and soft tissue accounted for 42% of the total. The onset of 80% of the infection episodes occurred in the community setting, of which 44% of the episodes resulted in hospitalization (Figure 1).
Microbiology
Staphylococcus aureus, coagulase negative staphylococci (CONS), P. aeruginosa, E. coli, Klebsiella, and Enterobacter were the most frequent isolates (Table 1). From infections of the HVAD, S. aureus and CONS were the most commonly isolated bacteria. Unexpectedly, gram-negative bacteria were commonly isolated from the initial sputum cultures of patients with community-onset pneumonia. Fifty-five percent (55 %) of 113 patient episodes with positive sputum cultures grew gram-negative bacteria, including 23 isolates of P. aeruginosa. Although outpatient hemodialysis facilities are free-standing and separate from the hospital, the cohorting of patients into large room(s) with multiple dialysis stations, the pervasive and widespread use of antibiotics, and frequency with which patients are in/out of the hospital all contribute to a resident microbiological flora historically associated with nosocomial infections. Infections in these patients are more accurately classified as Health Care Associated (HCA) rather than community acquired.
CLINICAL SYNDROMES
Hemodialysis Vascular Access Device (HVAD) Infections
The vascular access connection is the Achilles heel of dialysis. Complications of the vascular access are frequent and costly to the patient’s health and to society. Thrombosis and infection are the most frequent causes of vascular access failure. The first vascular access devices were silastic tubes (Teflon), which cannulated the artery and vein and provided access for dialysis through exposed external tubing. These Scribner shunts were susceptible to frequent infection, averaging 28 episodes per 1000 days of use. Internalization of the angioaccess through the creation of a subcutaneous fistula dramatically reduced the incidence of infection. This simple arteriovenous fistula (AVF) continues to be the preferred access device for chronic dialysis. It is durable; over 70% are functional after 3 years and the infection rate is only 2-3% over the life of the AVF. The limitations of native AVFs include exhaustion of arteries and veins to create a suitable anastomosis, they may become dysfunctional because of the development of venous aneurysms, and poor venous flow from stenosis. Repair is difficult and the AVF may be unusable as a result of these complications. The alternative to AVF is an expanded polytetrafluoroethylene synthetic graft (Gortex), (AVG). These grafts are not as durable. The 3-year survival rate is 50%, with failure commonly from thrombosis or infection. Unlike the AVF, a thrombectomy procedure or revising and bypassing the affected portion can repair the AVG.
Cuffed central line catheters, permanent catheters, are used for chronic dialysis when there is no suitable limb option. Insertion in an operating suite is necessary as they are tunneled under the skin and feature a protective cuff. Despite these safeguards, they are more prone to infection than the AVF or AVG, with a rate of bacteremia twice the AVG. Central venous catheters inserted at bedside into the right internal jugular or subclavian vein are intended to be a temporary dialysis bridge, up to 30 days, until a permanent graft or fistula matures. A significant number remain in place for much longer, and about one-half are ultimately removed because of suspected or documented infection.
The HVAD is the most common cause of infection in the dialysis population. Signs of infection include erythema, skin breakdown, purulent drainage and, occasionally, bleeding from a pseudoaneurysm. Fever and other signs of sepsis may be present. The clinical presentation, bacteriology, treatment, and complications of VAD infections differ according to the type and location of the access device. Local inflammation is common across the spectrum of shunt, AVF and AVG infections. In our review, erythema and tenderness of the soft tissues overlying AVF or AVG were the most common findings (cellulitis). More ominous signs of progressive infection as fever, drainage, or abscess were present in less than half of the cases. Nephrologists, acutely aware of the complications of infections of the HVAD, have a low bar to start antibiotic treatment for signs of inflammation, which in another setting could reasonably be expected to be self-limited. Because of the intensity of antibiotic use in dialysis patients and potential impact on microbiological flora in these patients, oral antibiotics are a reasonable choice to treat cellulitis overlying the AVF or VAD. As S. aureus is the pathogen isolated from cultures in almost all infections of AVF and unmodified AVG, our preferences include dicloxacillin or doxycycline if MRSA is a concern, as neither agent requires dosage adjustment for renal failure. Trimethoprim/sulfamethoxazole (TMP/SMX) has activity for most community acquired MRSA which is not approved for use in dialysis patients. Mupirocin is applied to the inflamed site and to the nares and patients are instructed to bathe with chlorhexidine to minimize the passage of S. aureus to family members, other patients, or dialysis staff. Blood cultures are mandatory in any patient with fever or an infection with drainage, pus, hematoma, or signs of a pseudoaneurysm as results may be positive in 10% of these more complicated VAD infections. Empiric treatment with parenteral antibiotics for complicated VAD infections is indicated pending the culture results and subsequent clinical events.
An AVF infected with S. aureus should be ligated, as the chances of curing an endovascular infection is poor. Ligation and removal of the AVF prevents future infection but also eliminates one of a limited number of useful sites for further angioaccess. Synthetic grafts can potentially be salvaged though procedures as a thrombectomy and graft revision (bypass). A graft that has been surgically manipulated has an increased risk of infection. Gram-negative bacteria may be isolated from up to 20% of cultures of infected grafts subjected to additional surgery, especially when the AVG is exposed because of skin breakdown.
Central catheter infections (permanent catheters and central venous catheters) usually present with fever and minimal local inflammatory reaction. Less than 10% of the cases feature inflammation at the exit site even though the offending bacteria may be cultured from the catheter tip or surrounding skin. Central catheters are associated with a higher incidence of bacteremia than peripheral vascular access infections. Catheter removal is encouraged in the presence of S. aureus, persistent sepsis, or persistent bacteremia.
Because of the cost and morbidity of removing and changing infected central catheters, instilling and leaving high concentrations of antibiotics +/- heparin in the catheter between hemodialysis sessions has been used in an attempt to salvage central catheters (lock therapy). The results of lock therapy studies have been mixed. There seems to be little improvement in salvage when S aureus is the pathogen, and our experience is catheter-associated bacteremia from coagulase negative staphylococci (CONS) and gram-negative bacteria often respond to antibiotics alone and can be successfully treated without removal of the catheter if the blood can be sterilized in 1 to 2 days and the patient is no longer septic.
Bacteremia
The HVAD is responsible for half of the infections with bacteremia. Thirty percent of the patients with positive blood cultures have another identifiable site of infection as the lungs, abdomen, skin/soft tissue, or urinary tract. In 20% of patients with bacteremia, a source is never found.
Infections from sources other than a HVAD are managed in a similar fashion to those in patients who do not have ESRD. However, the duration of therapy may be longer and clinical response slower because of poor wound healing associated with the uremic state and patient debility such as an inability to effectively clear inspissated secretions.
Gram-positive bacteremia in dialysis patients has serious risk for sequelae. S. aureus bacteremia deserves special attention as the most common organism isolated from blood cultures and the highest predilection of complications including metastic soft tissue, bone infection and endocarditis. Vegetations are demonstrated by trans-esophageal echocardiogram (TEE) on the heart valves of some 40% of patients with S. aureus bacteremia from a central catheter. When gram-positive cocci are isolated from the blood cultures, we advocate treatment with paranteral antibiotics for 4 weeks. The concern for seeding an endovascular device and the possibility of infective endocarditis are the rationale for treating gram-positive bacteremias for 4 weeks with parenteral antibiotics. Without complications as persistent bacteremia, prolonged fever, heart failure, embolization, or conduction defects, a TEE is unnecessary, as it does not impact therapy.
If the organism is methicillin sensitive (MSSA), the treatment is straightforward. Cefazolin is our drug of choice because of a high rate of clinical cure, low toxicity, and the convenience of dosing only after dialysis (1-2 grams) so that treatment can be completed as an outpatient. Nafcillin has somewhat more potent in vitro activity but has not been shown to be more effective in clinical studies.
Vancomycin has been used and overused in hemodialysis patients for more than 35 years. Less than 15% of vancomycin is metabolized/removed each day by non-renal mechanisms. Prior to 10 years ago, vancomycin was dosed once a week because the dialysis membrane did not allow significant passage of large molecules like vancomycin. ‘High-flux’ membranes have become the standard in most dialysis centers in the United States and enough vancomycin is dialyzed that a supplemental dose should be administered after each dialysis treatment. Patients with MSSA bacteremia treated with vancomycin have poorer clinical outcomes and prolonged bacteremia compared to those patients treated with semisynthethic penicillins or first generation cephalosporin antibiotics. Vancomycin should be reserved for empiric treatment of suspected MRSA infections pending the results of cultures and to treat infections with documented methicillin resistant gram-positive organisms.
Fortunately, the vast majority of S. aureus infections have a good outcome. Persistent bacteremia is uncommon; in our series, only 3% of gram-positive cocci bacteremia >3 days. The source may be a persistent nidus as abscess, infected heart valve or the VAD. Unfortunately there is no reliable imaging study to confirm the VAD as the source of fever or bacteremia. Removal of temporary catheters should be the first step, followed by a TEE if the bacteremia persists. Determining when to sacrifice an innocent-appearing AVF or AVG in the presence of persistent bacteremia or sepsis remains a dilemma. Clinical or microbiological failure can also be due to MRSA reported as ‘sensitive’ to vancomycin by a laboratory using a cut point of the MIC < 4 mcg/ml. An upward drift in the MIC (>1 mcg/ml but <4 mcg/ml) of S. aureus to vancomycin has been documented, and these organisms are associated with more unsuccessful outcomes. To combat the higher MIC of some MRSA, it is advised to keep the pre-dialysis vancomycin level above 15. Since nephrotoxicity is not an issue and ototoxicity is unusual when the peak level is less than 40, our goal is a minimum peak vancomycin level of 30.
The workup to define antibiotic failure includes a tube dilution MIC/MBC ratio; the higher the ratio, the greater limitation of antibiotics. A serum bacteriocidal level (Schlicter test) is also useful. If the patient’s serum drawn 2 hours after a dose of antibiotics can kill the organism at a dilution > ¼, the effectiveness of antibiotic therapy has been optimized.
Changing or adding additional antibiotics is an option. Alternative therapies to cover MRSA include Daptomycin and linezolid, the combination of rifampin plus low dose Gentamicin is also commonly used.
Fever During Hemodialysis And Antibiotic Administration
Fever that occurs only during dialysis should be aggressively evaluated. The first manifestation of an indolent vascular access infection may be fever and bacteremia during hemodialysis. Blood cultures should be drawn from any patient with fever during hemodialysis. During the initial period of hemodialysis, a profound neutropenia and the sudden high flow through a colonized vascular access device are two events that occur and may play a role in this presentation. Hemodialysis machines may also be the source of fever and bacteremia. Contamination of the blood by waterborne organisms may occur in several ways: a leak in the system, contamination of the water source, rapid growth of bacteria in dialysate, or by colonization of the patient through contact contamination. If cultures are positive for Burkholderia cepacia, Stenotrophomonas maltophilia, Pseudomonas stutzeri, Pseudomonas aeruginosa or Aeromonas sp., consider a break in sterility. Fever may be non-infectious, caused by endotoxin absorption, activation of interleukin, or leukocyte pyrogen from the dialysis coil.
Empiric antibiotic therapy should be initiated for hemodialysis-related fever. Whenever possible, we prescribe antibiotics at the end of a dialysis session, which lasts until the next session. This post-dialysis approach is simple, ensures compliance, promotes outpatient treatment, and is cheap. Cefazolin, in the absence of methicillin resistance, has been our standard therapy for gram-positive bacterial infections. Cefoxitin, ceftazidime and aminoglycosides can also be dosed in this manner for gram-negative bacterial infections (Table 2). Unless the patient is septic or has had a previously documented MRSA infection, we do not use vancomycin so as to minimize the spread of vancomycin resistant gram-positive bacteria. Instead, we administer 1.5 grams of cefazolin and 120 mg of gentamicin until the results of the cultures are available.
Continous replacement renal therapy as CVVH (Continuous Venovenous Hemofiltration) or CVVHD (Continuous Venovenous Hemodialysis) is often utilized in critically ill patients. Because the clearance of most molecules including creatinine more resembles a functioning kidney, dosing of antibiotics is not the same as in intermittent hemodialysis. Recommendations for antibiotic dosing are included in Table 2. Another approach is to use the serum concentrations of antibiotic, creatinine and published nomograms dosing in renal insufficiency as a guide.
Infections Associated With Peritoneal Dialysis
Continuous ambulatory peritoneal dialysis (CAPD) is the most frequently utilized technique. The advantages of peritoneal dialyses include more independence as they can be done at home or work and do not require incapacitation of 3 to 6 hours, three times a week and, the unpleasant experience of hemodynamic instability during hemodialysis is avoided. Despite these relative attractions, less than 10% of patients with end stage renal disease (ESRD) dialyze with this procedure. Peritoneal dialysis is labor intensive and infection is a common complication averaging 1 episode/ 10 patient months. A major technical problem is inability of the peritoneal catheter to form a perfect seal with skin and bacteria may colonize the dialysis sheath. The bacteria, which cause peritonitis, may come from the skin, water, or the gastrointestinal tract. S. epidermidis suggests problem with hygiene of the skin, waterborne organisms as Stenotrophomonas maltophilia and pseudomonas species are associated with contamination of the exit site with tap water during body wash, and mixed flora often implicates the gastrointestinal tract as the source of infecting bacteria. Gram-positive organisms, especially S. epidermidis, are responsible for 70% of cases of peritonitis in chronic dialysis patients, gram-negative bacteria for 25%. Atypical mycobacteria and yeast account for 5% of cases.
Peritonitis is signaled by the sudden development of cloudy fluid. Other signs of inflammation as abdominal pain and fever may be absent in the early stages. Infection of the catheter exit site features an exudate and purulent drainage. The infection is often indolent and may not lead to peritonitis. Extension along the catheter sheath is a tunnel infection. The diagnosis of a tunnel infection may be difficult if there is no drainage at exit site. Imaging studies as ultrasound and Indium–111 white blood cell scan may be helpful. Peritonitis is the most serious complication of a tunnel infection.
The preferred approach to treatment of peritonitis is to mix and instill antibiotics with peritoneal dialysate fluid directly into the peritoneal cavity as part of a routine exchange. Systemic absorption occurs and in the presence of inflamed peritoneum, serum levels may approximate the concentration of antibiotics in the dialysate. However, with normal peritoneal surfaces, absorption is not predictable and tissue levels may be marginal to treat infections other than peritonitis. This is especially true for vancomycin. Exit site and tunnel infections are best treated with intravenous or oral antibiotics. Until the results of cultures are known, short term empiric therapy with a combination of vancomycin and gentimicin will cover almost all of the pathogens encountered in an infection from a peritoneal catheter.
Pneumonia
Pneumonia is a common infection in patients with end stage renal disease. The X-ray manifestations may be protean, mimicking pulmonary edema, metastasis, or fungal disease. The predisposing conditions include underlying cardiac disease, pulmonary fluid overload, low serum albumin, and a shortened duration of protection from influenza and pneumococcal vaccines. With advanced age, co-morbid disease, and physiological dysfunction, almost all patients with fever and an abnormal X-ray require hospitalization. The causative bacterial pathogens of community-acquired pneumonia (CAP) in the dialysis population include pneumococcus and Haemophilus influenzae, but also S. aureus, and gram-negative rods. A gram stain may be most helpful in choosing the initial regimen, but in the absence of a good specimen, antibiotic coverage should include gram-negative bacteria. Almost one-half of pneumonias in patients with end stage renal disease may be due to gram-negative bacteria. Fluoroquinolones are one of the drugs of choice in the general population for the treatment of CAP. The administration of fluoroquinolones beyond 2 days should be for specific indications such as atypical bacteria.
Urinary Tract Infection
Asymptomatic pyuria and bacturia are present in about 30% of patients with end stage renal disease and, in the absence of symptoms, is not a significant clinical problem and does not require treatment. Yet, the urine may be the origin of the majority of cases of gram-negative bacteremia. A urine culture is advisable in the presence of gram-negative bacteremia if the source of infection is not obvious. When the isolate from the urine is the same as the blood culture, a genital urinary workup should be perused, especially to rule out obstruction. Pyocystitis is an accumulation of gross pus in the bladder and can lead to bacteremia. Treatment includes adequate drainage, systemic antibiotics, and germicidal bladder irrigation.
Autosomal dominant polycystic kidney disease is responsible for about 5% of end stage renal disease. Infection of cyst can occur from bacteremia or from the urine. Diagnosis of infection may be difficult in the absence of a symptomatic urinary tract infection. Computerized tomography or ultrasound is not diagnostic. An Indium-111 white blood cell scan may be helpful. The infections may be notoriously slow to respond, perinephric abscesses occur, and peracutaneous or invasive surgical drainage is often necessary.
Host Dysfunction And Infection
Other infectious problems of chronic renal failure and uremia persist despite maintenance dialysis. Reactivation of tuberculosis and leprosy is a danger. Delayed hypersensitivity and cell mediated immunity is impaired. Yersinia and wisteria infections have been associated with elevated serum ferritin levels from transfusion overload. Serum from uremic patients, even after dialysis, slows the acute local inflammatory response and impairs chemotaxis, phagocytosis and oxidative metabolism of neutrophils.
Prevention Of Infection
Prevention of infection is one of the few avenues available to reduce hospitalizations, control costs, and improve quality of life for these patients. Common pyogenic bacteria from the patient’s endogenous flora are responsible for most infections in patients with end stage renal disease. The carriage rate of S. aureus in patients with end stage renal disease may approach 70%. Vascular access is the risk factor in more than 50% of the infections and S. aureus on the skin the most common pathogen. A previous episode of bacteremia is the most predictive risk factor for subsequent bacteremia, suggesting that the same patients have repeated infections and may be chronic carriers of staphylococcus. Mupirocin applied to the nares significantly reduces the carriage rate as well as subsequent rate of bacteremia. Unfortunately, clinical experience demonstrates that universal use will ultimately lead to mupirocin resistance. Other strategies may have better results including limiting mupirocin prophylaxis to S. aureus carriers only.
The chances of infection of the vascular access are increased in patients with poor personal hygiene, malnutrition, and inadequate dialysis. The type of vascular access is a major risk factor for infection, with VC being at highest risk. Their use is to be discouraged without a plan for a safer VAD.
Twenty-five percent of the patients account for 50% of the total costs of caring for dialysis patients and more than 40% of deaths. Using vascular access data, past infection history, co-morbidity indexes, and physical activity scales we have developed an index to stratify patient risk for future infection. In an attempt to change the paradigm, we are focusing our resources to provide preventative home services in this frail and debilitated group of patients.
SELECTED READING
Beddhu S, Bruns FJ, Saul M, et al. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. American Journal Medicine 2000;108(8):609-13. (Hospitalization of dialysis patients is expensive and high-risk patients can be identified in advance.) [PubMed]
Berman SJ, Johnson EW, Nakatsu C, et al. Burden of infection in patients with end-stage renal disease requiring long term dialysis. Clinical Infectious Diseases 2004;39:1747-53. (A retrospective review of infections in hemodialysis patients which documents the incidence, prevalence, scope of the problem.) [PubMed]
Bloom BS, Fendrick AM, Chernew ME, Patel P. Clinical and economic effects of mupirocin calcium on preventing Staphylococcus aureus infection in hemodialysis patients: a decision analysis. (A pharmaco-economic analysis shows that it is most effective to decrease nasal carriage in hemodialysis patients) (Intermittent nasal mupirocin was effective without minimal emergence of resistance). [PubMed]
Boelaert JR. Staphylococcus aureus infection in haemodialysis patients. Mupirocin as a topical strategy against nasal carriage : a review. J Chemother 1994;6(Suppl 2):19-24. [PubMed]
Bruns FJ, Seddon P, Saul M, Zeidel ML. The cost of caring for end-stage kidney disease patients: an analysis based on hospital financial transaction records. Journal of the American Society of Nephrology 1998;9:884-90. (Efforts to control costs without sacrificing quality of care must center on reducing inpatient costs.) [PubMed]
Chang F-Y, MacDonald BB, Peacock JE Jr., et al. A prospective multicenter study of Staphylococcus aureus bacteremia. Medicine 2003;82(5):322-32. (A comprehensive look at complications/outcomes of S. aureus.) [PubMed]
Fowler VG Jr., Li J, Corey GR, et al: Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. Journal American College Cardiology 1997;30:1072-78. (Valvular vegetations are common with S. aureus bacteremia if looked for using TEE.) [PubMed]
Laupland KB, Conly JM. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin Infect Dis 2003;37(7):933-8. (A review of 16 tirals showed mupirocin was effective in decreasing nasal carriage of S. aureus, but evidence was equivocal for decrease S. aureus infection). [PubMed]
Pien FD, Berman SJ (eds): Infections in Patients with Chronic Renal Failure. Infectious Disease Clinics of North America 2001;15: 709-1008. (A comprehensive review of all aspects of infection in dialysis/transplant patients.)
Sherris JC, Scribner BH: Transaction American Society Artificial Internal Organs 1961;7:37. (Modest publication describing a practical vascular access method for chronic repeat hemodialysis.)
Strippoll GF, Tong A, Johnson D, Schena FP, Craig JC. Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients. Cochrane Database Syst Rev 2004;18(4):CD004679. (A review of 19 trials showed mupirocin was effective in decreasing exit-site/tunnel infection, but not peritonitis). [PubMed]
Stryjewski ME, Szczech, LA, Benjamin DK Jr., et al: Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clinical Infectious Diseases 2007;44:190-6. (In the absence of patient specific circumstances, Vancomycin should not be continued beyond empirical therapy for hemodialysis-dependent patients with MSSA bacteremia.) [PubMed]
Tenover FC, Moellering RC Jr.: The rationale for revising the clinical and laboratory standards institute vancomycin minimal inhibitory concentration interpretative criteria for Staphylococcus aureus. Clinical Infectious Diseases 2007;44:1208-15. (Describes the issue of Vancomycin resistance.) [PubMed]
Tables
Table 1. Microbiology of Infections in Dialysis Patients. 1440 Bacterial Isolated from 1134 Episodes in 433 Patients
| ALL SITES | PNEUMONIA | HVAD | BKI | |||||
|---|---|---|---|---|---|---|---|---|
| TOTAL ISOLATES | 1440 | 186 | 237 | 307 | ||||
| NOS | HCA | NOS | HCA | NOS | HCA | NOS | HCA | |
| TOTAL | 370 | 1070 | 73 | 113 | 36 | 201 | 32 | 275 |
| Gram-Positive Cocci | 158(43) | 539(50) | 16(22) | 29(26) | 30(83) | 149(74) | 23(72) | 147(54) |
| CONS | 53(14) | 153(14) | 0 | 1(1)* | 16(44) | 46(23) | 7(22) | 42(15) |
| MSSA | 19(5) | 194(18) | 0 | 12(11) | 7(19) | 70(35) | 3(9) | 49(18) |
| MRSA | 45(6) | 70(7) | 16(22) | 13(12) | 4(11) | 20(10) | 4(13) | 13(5) |
| Enterococcus | 23(5) | 70(7) | 0 | 1(1)* | 2(6) | 9(5) | 6(19) | 27(10) |
| VRE | 12(8) | 1(0) | 0 | 0 | 0 | 0 | 2(6) | 0 |
| Other Gram- Positive Cocci | 59(16) | 6(1) | 0 | 0 | 17(47) | 1(1) | 1(3) | 16(6) |
| Aerobic Gram- Negative Rods | 126(34) | 384(36) | 47(64) | 62(55) | 5(14) | 37(18) | 9(28) | 92(34) |
| P. aeruginosa | 31(8) | 117(11) | 16(2) | 24(21) | 0 | 9(5) | 5(16) | 25(9) |
| E. coli | 25(3) | 77(7) | 2(3) | 4(4) | 1(3) | 5(3) | 2(6) | 20(7) |
| Klebsiella | 20(3) | 57(5) | 10(14) | 12(11) | 0 | 7(4) | 0 | 9(3) |
| Enterobacter | 22(3) | 42(4) | 9(12) | 5(4) | 0 | 10(5) | 0 | 0 |
| Other aerobic gram negative rods | 28(3) | 91(9) | 10(14) | 17(15) | 3(9) | 6(4) | 2(6) | 38(14) |
| Respiratory Pathogens | 11(3) | 32(4) | 8(12) | 20(20) | 0 | 0 | 0 | 0 |
| Moraxella | 4(1) | 5(1) | 4(6) | 2(2) | 0 | 0 | 0 | 0 |
| H. influenzae | 5(1) | 18(2) | 2(3) | 11(12) | 0 | 0 | 0 | 0 |
| Pneumococcus | 2(1) | 9(1) | 2(3) | 7(6) | 0 | 0 | 0 | 0 |
| Miscellaneous | 75(20) | 115(11) | 2(3) | 2(2) | 1(3) | 15(8) | 0 | 36(13) |
* isolated from an empyema
() = percent of total isolates, nosocomial or community acquired, with the same diagnosis
NOS = nosocomial
HCA = infections acquired living while living at home (community acquired) but cohorting with other chronic hemodialysis patients in an out patient dialysis facility 3x week
HVAD = hemodialysis vascular access device infection
BKI = infection below the knee
CONS = coagulase negative Staphylococcu
MSSA = methicillin sensitive Staphylococcus aureus
MRSA = methicillin resistant Staphylococcus aureus
Table 2. Common Antibiotics Used In Patients With ESRD
| DRUG | HALF LIFE (Hr)NORMAL/ ESRD | LOADING DOSE | DOSAGE BETWEEN DIALYSIS | DOSAGE AFTER HEMO- DIALYSIS (%LOADING DOSE) | DOSAGE CVVH/ CVVHD |
|---|---|---|---|---|---|
| Aminoglycoside Antibiotics | |||||
| Amikacin | 1.4-2.3/17-150 | 5mg/kg | None | 67% | --- |
| Gentamicin | 1.8/20-60 | 1.5mg/kg | None | 67% | --- |
| Tobramycin | 2.5/27-60 | 1.5mg/kg | None | 67% | --- |
| Cephalosporin Antibiotics | |||||
| Cefazolin | 2/40-70 | 1-2g | None | 100% | 1-2g q12h / 2g q12h |
| Cefepime | 2.2/18 | 1-2g | 1g q24h* | 100% | 1-2g q12h / 2g q12h |
| Cefoxitin | 1/13-23 | 1-2g | None | 100% | --- |
| Ceftazidime | 1.2/13-25 | 1-2g | None | 100% | 1-2g q12h / 2g q12h |
| Ceftriaxone | 7-9/12-24 | 1-2g | 1-2g q24h | 0% | 2g q12-24h / 2g q12-24h |
| Miscellaneous Antibacterials | |||||
| Daptomycin | 8/27 | 4-6mg/kg | None | 100% | 4 or 6mg/kg q48h / 4 or 6mg/kg q48h |
| Doxycycline | 24/24 | .1g po/iv | q12-24 h | 0% | --- |
| Imipenem | 1/4 | 5-1g | 25% dose, q6h | 50% | 250mg q6h / 250mg q6h or 500mg q6h |
| Meropenem | 1.1/6-8 | 5-1g | 25% dose, q6h | 50% | 1g q12h / 1g q12h |
| Metronidazole | 6-14/7-21 | 5g | 75% dose, q12h | 75% | --- |
| Vancomycin | 6-8/200-250 | 5-1.5g | Weekly (only if low flux dialysis) | 50% Hi-flux (only) | 1g q48h / 1g q24h |
| Penicillins | |||||
| Penicillin G | 0.5/6-20 | 1-4 million units | q12-24h* | 100% | --- |
| Ampicillin | 1/7-20 | 1-2g | q12-24h* | 100% | --- |
| Dicloxacillin | 0.7/1-2 | .25 | q6h | 0% | --- |
| Nafcillin | 0.5/1.2 | 1-2g | q6h | 0% | 2g q4-6h / 2g q4-6h |
| Quinolones | |||||
| Ciprofloxacin | 4-8 | .4 g iv/.5-.75 g po | 50% q 12h | 0% | 200mg q12h / 200-400mg q12h |
| Levofloxacin | 6-10 | .5g po/iv | 50% q48h | 0% | 250mg q24h / 250mg q24h |
| Moxifloxacin | 12-16 | .4g po/iv | 100% q24h | 0% | 400mg q24h / 400mg q24h |
* Literature documentation scanty
CVVH = continuous venovenous hemofiltration
CVVHD = continuous venovenous hemodialysis
Figure 1: Diagnosis of Infection
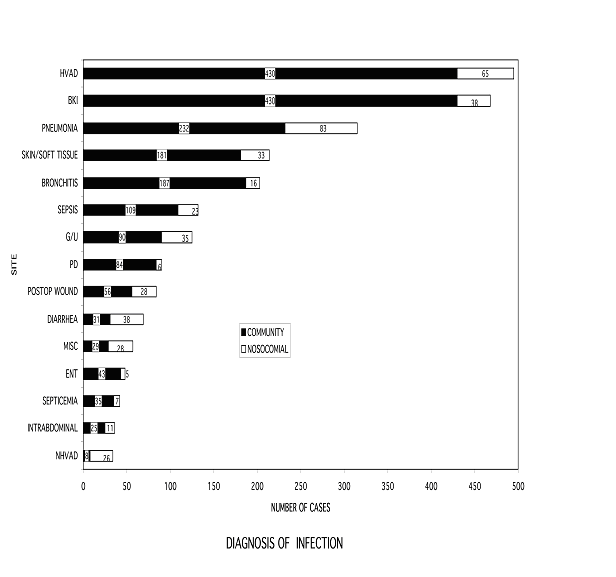
HVAD = hemodialysis vascular access device
BKI = below knee infection
G/U = genital urinary tract
PD = peritoneal dialysis related
Postop Wound = postoperative wound
Diarrhea = enteric bacterial infection
Misc. = miscellaneous
ENT = ears, nose, throat
NHVAD = non-hemodialysis vascular access device

