Urinary Tract Infections
Authors: FME Wagenlehner, MD, PhD, KG Naber, MD, PhD
INTRODUCTION
Epidemiology of Urinary Tract Infections
Urinary tract infections (UTIs) are among the most prevalent infectious diseases with a substantial financial burden on society. In the USA, UTIs are responsible for over 7 million physician visits annually, including more than 2 million visits for cystitis. Approximately 15% of all community-prescribed antibiotics in the USA are dispensed for UTI, at an estimated annual cost of over US $1 billion. Furthermore, the direct and indirect costs associated with community-acquired UTIs in the USA alone exceed an estimated US $1.6 billion. Urinary tract infections account for more than 100,000 hospital admissions annually, most often for pyelonephritis. They also account for at least 40% of all hospital-acquired infections and are in the majority of cases catheter-associated. Nosocomial bacteriuria develops in up to 25% of patients requiring a urinary catheter for > 7 days, with a daily risk of 5%. It has been estimated that an episode of nosocomial bacteriuria adds US $500-1,000 to the direct cost of acute-care hospitalization. In addition, the pathogens are fully exposed to the nosocomial environment, including selective pressure by antibiotic or antiseptic substances. Nosocomial UTIs therefore comprise perhaps the largest institutional reservoir of nosocomial antibiotic-resistant pathogens.
Classification of Urinary Tract Infections
Infections can be classified according to their location within the urinary tract, e.g. pyelonephritis, cystitis. The different parts of the urinary tract, however, communicate with each other to some degree. As a result, bacteria in one area are probably also present elsewhere. For practical clinical reasons, UTIs are classified according to the predominant clinical symptoms:
• uncomplicated lower UTI (cystitis)
• uncomplicated pyelonephritis
• complicated UTI with or without pyelonephritis
• urosepsis
The clinical presentation and management of different UTI categories may vary during life and may depend on the patient’s condition. Therefore, special patient groups (the elderly, those with underlying diseases and the immunocompromised) have also to be considered.
Pathogenesis of Urinary Tract Infections
Micro-organisms can reach the urinary tract by haematogenous or lymphatic spread, but there is abundant clinical and experimental evidence to show that the ascent of micro-organisms from the urethra is the most common pathway leading to a UTI, especially organisms of enteric origin (i.e. Escherichia coliand other Enterobacteriaceae). This provides a logical explanation for the greater frequency of UTIs in women than in men and for the increased risk of infection following bladder catheterization or instrumentation. A single insertion of a catheter into the urinary bladder in ambulatory patients results in urinary infection in 1-2% of cases. Indwelling catheters with open-drainage systems result in bacteriuria in almost 100% of cases within 3-4 days. The use of a closed-drainage system, including a valve preventing retrograde flow, delays the onset of infection, but ultimately does not prevent it. It is thought that bacteria migrate within the mucopurulent space between the urethra and catheter, and that this leads to the development of bacteriuria in almost all patients within about 4 weeks.
Haematogenous infection of the urinary tract is restricted to a few relatively uncommon microbes, such as Staphylococcus aureus, Candidaspp., Salmonellaspp. and Mycobacterium tuberculosis, which cause primary infections elsewhere in the body. Candida albicansreadily causes a clinical UTI via the haematogenous route, but is also an infrequent cause of an ascending infection if an indwelling catheter is present or following antibiotic therapy. The concept of bacterial virulence or pathogenicity in the urinary tract infers that not all bacterial species are equally capable of inducing infection. The more compromised the natural defence mechanisms (e.g. obstruction, bladder catheterization), the fewer the virulence requirements of any bacterial strain to induce infection. This is supported by the well-documented in-vitro observation that bacteria isolated from patients with a complicated UTI frequently fail to express virulence factors. The virulence concept also suggests that certain bacterial strains within a species are uniquely equipped with specialized virulence factors, e.g. different types of pili, which facilitate the ascent of bacteria from the faecal flora, introitus vaginae or periurethral area up the urethra into the bladder, or, less frequently, allow the organisms to reach the kidneys to induce systemic inflammation.
Review Article: Tabibian JH, et al. Uropathogens and Host Characteristics. J Clin Microbiol 2008;46: 3980-3986.
Microbiological and Other Laboratory Findings(Table 8)
The number of bacteria is considered relevant for the diagnosis of a UTI. In 1960, Kass developed the concept of ’significant‘ bacteriuria (≥ 105cfu) in the context of pyelonephritis in pregnancy. Although this concept introduced quantitative microbiology into the diagnostics of infectious diseases and is therefore still of general importance, it has recently become clear that there is no fixed number of significant bacteriuria, which can be applied to all kinds of UTIs and in all circumstances. Sterile pyuria is defined as the presence of leukocytes without bacteria. The following bacterial counts are clinically relevant:
• ≥ 103colony-forming units (cfu) of uropathogen/mL of a mid-stream sample of urine in acute uncomplicated cystitis in a woman
• ≥ 104cfu uropathogen/mL of mid-stream sample of urine in acute uncomplicated pyelonephritis in a woman
• ≥ 105cfu uropathogen/mL of mid-stream sample of urine in a woman, or ≥ 104cfu uropathogen/mL of mid-stream sample of urine in a man or in straight catheter urine in women in a complicated UTI.
In a suprapubic bladder puncture specimen, any count of bacteria is relevant. Asymptomatic bacteriuria is diagnosed if two cultures of the same bacterial strain (in most cases the species only is available) taken ≥ 24 hours apart show bacteriuria of ≥ 105cfu uropathogen/mL.
In clinical routine assessment, a number of basic criteria must be looked at before a diagnosis can be established, including:
• clinical symptoms
• results of selected laboratory tests (blood, urine)
• evidence of the presence of microbes by culturing or other specific tests.
Suggested Reading
1. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002;113(Suppl 1A): 5S-13S. [PubMed]
2. Gales AC, Jones RN, Gordon KA, Sader HS, Wilke WW, Beach ML, Pfaller MA, Doern GV. Activity and spectrum of 22 antimicrobial agents tested against urinary tract infection pathogens in hospitalized patients in Latin America: report from the second year of the SENTRY antimicrobial surveillance program (1998). J Antimicrob Chemother 2000;45: 295-303. [PubMed]
3. Kass EH. Bacteriuria and pyelonephritis of pregnancy. Arch Intern Med 1960;105: 194-198. [PubMed]
4. Maki DG, Tambyah PA. Engineering out the risk of infection with urinary catheters. Emerg Infect Dis 2001;7: 342-347. [PubMed]
5. Mazzulli T. Resistance trends in urinary tract pathogens and impact on management. J Urol 2002;168: 1720-1722. [PubMed]
6. Naber KG, Bergman B, Bjerklund-Johansen TE, Botto H, Lobel B, Jiminez Cruz F, Selvaggi FP; Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur Urol 2001;40: 576-588. [PubMed]
7. Naber KG, Bishop MC, Bjerklund-Johansen TE, Botto H, Cek M, Grabe M, Lobel B, Palou J, Tenke P. EAU guidelines on the management of urinary and male genital tract infections. EAU Working Group on Urinary and Male Genital Tract Infections. European Association of Urology (EAU) Guidelines Office. 2006 edition. 1-126. [PubMed]
8. Patton JP, Nash DB, Abrutyn E. Urinary tract infection: economic considerations. Med Clin North Am 1991;75: 495-513. [PubMed]
9. Ruden H, Gastmeier P, Daschner FD, Schumacher M. Nosocomial and community-acquired infections in Germany. Summary of the results of the First National Prevalence Study (NIDEP). Infection 1997;25: 199-202. [PubMed]
UNCOMPLICATED URINARY TRACT INFECTIONS IN ADULTS
Background
Acute, uncomplicated UTIs in adults include episodes of acute cystitis and acute pyelonephritis occurring in otherwise healthy individuals. These UTIs are seen mostly in women who have no risk factors, i.e. no structural or functional abnormalities within the urinary tract and the kidneys and no underlying disease known to increase the risks of acquiring infection or of failing therapy. Uncomplicated UTIs are extremely common infections. Approximately 25-35% of women between the ages of 20 and 40 years have experienced an episode described by their physician as an uncomplicated UTI.
Definition
The distinction between an uncomplicated and a complicated UTI is important because of implications with regard to pre- and post-treatment evaluation, the type and duration of antimicrobial regimens, and the extent of the evaluation of the urinary tract. In contrast to an uncomplicated UTI, a complicated UTI is an infection associated with a condition that increases the risks of acquiring an infection or of failing therapy. At the time of presentation with an acute onset of urinary tract symptoms, it is usually not possible to classify definitively patients as having a complicated or an uncomplicated UTI. Several factors have been identified, however, that are markers for a potential complicated UTI:
• Male sex
• Elderly
• Hospital-acquired infection
• Pregnancy
• Indwelling urinary catheter
• Recent urinary tract intervention
• Functional or anatomical abnormality of the urinary tract
• Recent antimicrobial use
• Symptoms for > 7 days at presentation
• Diabetes mellitus
• Immunosuppression
These factors only provide guidance to the clinician who must decide, based on limited clinical information, whether to embark on a more extensive evaluation and treatment course. It is generally safe to assume that a pre-menopausal, non-pregnant woman with acute onset of dysuria, frequency or urgency, who has not recently been instrumented or treated with antimicrobials and who has no history of a genitourinary tract abnormality, has an uncomplicated lower (cystitis) or upper (pyelonephritis) UTI. Recurrent UTIs are common among pre-menopausal, sexually active, healthy women, even though they generally have anatomically and physiologically normal urinary tracts. Whether a UTI in pregnancy by itself is to be classified as an uncomplicated or a complicated UTI remains debatable. Although data on UTIs in healthy post-menopausal women without genitourinary abnormalities are limited, it is likely that most UTIs in such women are also uncomplicated. Data on UTIs in healthy adult men are sparse and much less is known about the optimal diagnostic and therapeutic approaches to UTIs in men.
Aetiological Spectrum
The spectrum of aetiological agents is similar in uncomplicated upper and lower UTIs, with E. colibeing the causative pathogen in approximately 70-95% of cases and Staphylococcus saprophyticusin about 5-19% of cases, whereasS. saprophyticusis less frequently found in pyelonephritis than in cystitis. Occasionally, other Enterobacteriaceae, such as P. mirabilisand Klebsiellaspp., or enterococci (mostly in mixed cultures indicating contamination), are isolated from such patients. In as many as 10-15% of symptomatic patients, bacteriuria cannot be detected using routine methods.
Acute Uncomplicated Cystitis in Pre-menopausal, Non-pregnant Women
At this stage in life, the incidence of acute uncomplicated cystitis is high and this infection is associated with considerable morbidity. Therefore, even small improvements in diagnostics, therapy or prophylaxis have a high impact on public health.
Incidence, Risk Factors, Morbidity
A prospective study at a university health centre or a health maintenance organization (HMO) revealed an incidence of 0.7 per person-year in the university cohort and 0.5 per person-year in the HMO cohort. Cohort and case control studies in young women showed that the risk is strongly and independently associated with recent sexual intercourse, recent use of diaphragm with spermicide, preceding asymptomatic bacteriuria, a history of recurrent UTI, the age of first UTI and history of UTI in the mother. On average, each episode of this type of UTI in pre-menopausal women was shown to be associated with 6.1 days of symptoms, 2.4 days of restricted activity, 1.2 days in which they were not able to attend classes or work and 0.4 days in bed.
Diagnosis
A non-pregnant pre-menopausal woman presenting with acute dysuria usually has one of three types of infection:
• acute cystitis
• acute urethritis, caused by Chlamydia trachomatis, Neisseria gonorrhoeae, or herpes simplex virus
• vaginitis caused by Candidaspp. or Trichomonas vaginalis.
A distinction between these three entities can usually be made with a high degree of certainty from the history and physical examination.
Acute cystitis is more likely if the woman complains of urgency and suprapubic pain; has suprapubic tenderness; is a diaphragm-spermicide user; has symptoms that mimic those of previously confirmed cystitis; or has recently undergone urethral instrumentation. Although approximately 40% of women with cystitis have haematuria, this is not a predictor of a complicated infection. Urethritis caused by N. gonorrhoeaeor C. trachomatisis relatively more likely if a women has had a new sexual partner in the past few weeks or if her sexual partner has urethral symptoms; there is a past history of a sexually transmitted disease (STD); symptoms were of gradual onset over several weeks and there are accompanying vaginal symptoms such as vaginal discharge or odour. Vaginitis is suggested by the presence of vaginal discharge or odour, pruritus, dyspareunia, external dysuria and no increased frequency or urgency.
Urinalysis (e.g. using a dipstick method) to look for pyuria, haematuria and nitrites is indicated if a UTI is suspected. Pyuria is present in almost all women with an acutely symptomatic UTI and in most women with urethritis caused by N. gonorrhoeaeor C. trachomatis; its absence strongly suggests an alternative diagnosis. The definitive diagnosis of a UTI is made in the presence of significant bacteriuria, the definition of which remains somewhat controversial. The traditional standard for significant bacteriuria is ≥ 105cfu uropathogen/mL in voided mid-stream sample of urine, based on studies of women with acute pyelonephritis and asymptomatic bacteriuria that were carried out four decades ago. Several more recent studies have shown that this is an insensitive standard when applied to acutely symptomatic women and that approximately one-third to one-half of cases of acute cystitis have bacteriuria < 105cfu/mL. For practical purposes, colony counts ≥ 103cfu/mL should be used for the diagnosis of acute uncomplicated cystitis (Table 8). Sterile pyuria is defined as the presence of leukocytes without bacteria. The determination of a urine culture is generally not necessary in women with uncomplicated cystitis because the causative organisms and their antimicrobial susceptibility profiles are predictable. Also, culture results become available only after the patient’s symptoms have resolved or are considerably improved. Voided mid-stream sample of urine or straight catheter (by trained urological personnel) urine cultures should probably be performed if the patient’s symptoms are not characteristic of a UTI. The laboratory must be instructed to look for ‘low count’ bacteriuria if such UTIs are to be detected. A pelvic examination is indicated if any of the factors suggesting urethritis or vaginitis listed above are present or if there is doubt as to the diagnosis. A pelvic examination should include a careful evaluation for evidence of vaginitis, urethral discharge, or herpetic ulcerations; a cervical examination for evidence of cervicitis and cervical and urethral cultures forN. gonorrhoeaeandC. trachomatis(or other sensitive and specific tests in first-voided urine in the morning, such as polymerase chain reaction tests).
Koeijers JJ, Kessels AG , Nys S , Bartelds A , Donker G , Stobberingh EE , Verbon A . Evaluation of the nitrite and leukocyte esterase activity tests for the diagnosis of acute symptomatic urinary tract infection in men. Clin Infect Dis 2007;45: 894-896.
(Printable version of Incidence/Diagnosis in Diagnosis of Uncomplicated UTI in Women)
Treatment
Guideline: Guidelines for antimicrobial therapy of urinary tract infections in Taiwan. J Microbiol Immunol Infect. 2000 Dec;33(4): 271-2.
There seems to be no long-term adverse effects with respect to renal function or increased mortality associated with acute uncomplicated cystitis, even in women who experience frequent recurrences, and in the non-pregnant population. Untreated cystitis rarely progresses to symptomatic upper tract infection. Thus, the significance of lower tract infection in non-pregnant women seems to be limited to the morbidity of symptoms caused by the infection, which can lead to substantial disruption of the lives of affected individuals. In fact, most lower UTIs (50-70%) clear spontaneously if untreated, although symptoms may persist for several months. Knowledge of the antimicrobial susceptibility profile of uropathogens causing uncomplicated UTIs in the community should guide therapeutic decisions. The resistance pattern ofE. colistrains causing an uncomplicated UTI, however, may vary considerably between regions and countries. Short courses of antimicrobials are highly effective in the treatment of acute uncomplicated cystitis in pre-menopausal women (Table 1). Short-course regimens are desirable because of the improved compliance that they promote, their lower cost, and lower frequency of adverse reactions. However, in assessing the potential cost advantages of short-course regimens, it is necessary to consider the potential added expense associated with treatment failures or recurrences arising from short-course therapy. It is also important to consider the potential psychological aspects of single-dose therapy; as symptoms may not subside for 2 or 3 days, the patient may have misgivings during this time about the ‘insufficient’ treatment provided to her. Such a scenario may result in unnecessary visits or calls to the physician.
The following antimicrobial agents can be used for treatment of uncomplicated cystitis: trimethoprim (TMP), trimethoprim-sulfamethoxazole (TMP-SMX), fluoroquinolones (ciprofloxacin, enoxacin, fleroxacin, gatifloxacin, levofloxacin, lomefloxacin, norfloxacin, ofloxacin, pefloxacin, rufloxacin), ß-lactams (amoxicillin, ampicillin-like compounds, cefadroxil, cefuroxime axetil, cefpodoxime proxetil, ceftibuten, pivmecillinam, ritipenem axetil), fosfomycin trometamol, and nitrofurantoin (Table 1).
Hsueh PR.
et al. Consensus review of the epidemiology and appropriate antimicrobial therapy of
complicated urinary tract infections in Asia-Pacific region.
Usta TA. et al. Comparison of single-dose and multiple-dose antibiotics for lower urinary tract infection in pregnancy. Int J Gynaecol Obstet. 2011 Sep;114(3): 229-33.
The following conclusions about antimicrobial therapy can be made:
Treatment Duration: In otherwise healthy, adult, non-pregnant women with acute uncomplicated cystitis, single-dose therapy (with some exceptions) is significantly less effective in eradicating initial bacteriuria than are longer durations of treatment with antimicrobials tested in this manner, such as TMP-SMX, TMP, norfloxacin, ciprofloxacin, fleroxacin, and as a group ß-lactams. However, TMP-SMX, TMP, norfloxacin, ciprofloxacin, and fleroxacin given for 3 days are as effective as the same antimicrobials used over longer durations. Longer treatment usually shows a higher rate of adverse events.
Trimethoprim, Co-trimoxazole: A 3-day regimen with TMP-SMX can be considered to be the standard therapy. TMP alone was equivalent to TMP-SMX with regard to eradication and adverse effects. Considering possible rare, but serious, adverse effects caused by sulphonamides, TMP alone may be considered the preferred drug over TMP-SMX. TMP or TMP-SMX can be recommended as first-line drugs for empirical therapy, but only in communities with rates of uropathogen resistance to TMP < 10-20% because there is a close correlation between susceptibility and the eradication of E. colion the one hand and resistance and persistence of the uropathogen on the other. The risk of emerging resistant uropathogens in the case of recurrence was also much higher when using TMP as a first-line drug than when using pivmecillinam or ciprofloxacin, which had the lowest risk of the drugs investigated.
Fluoroquinolones: The fluoroquinolones (ciprofloxacin, fleroxacin, norfloxacin and ofloxacin) are equivalent to TMP-SMX when given as a 3-day regimen. Pefloxacin and rufloxacin, each as single-day therapies, are interesting options and may be equivalent to TMP-SMX in the eradication of bacteriuria and its recurrence. Questions remain as to the possibility of a higher incidence of adverse effects with these agents than with other recommended therapies. A 3-day regimen with levofloxacin, 250 mg once daily, was similarly effective to a 3-day regimen of ofloxacin 200 mg twice daily, but with levofloxacin there was a trend to lesser adverse events. A 3-day course with CiproXR (500 mg) once daily was equivalent inregard to efficacy and safety as a course of conventional ciprofloxacin (250 mg twice daily).
Fluoroquinolones are more expensive than TMP and TMP-SMX, and are thus not recommended as first-line drugs for empirical therapy except in communities with rates of uropathogen resistance to TMP > 10-20%. In some countries, however, the resistance ofE. colito fluoroquinolones has already increased to more than 10%. In this situation, alternative oral drugs should be considered for empirical therapy. Treatment with any of these agents should result in more than 90% eradication of the bacteriuria.
Talan DA, et al. Prevalence and Risk Factor Analysis of Trimethoprim-Sulfamethoxazole and Fluoroquinolone -ResistantEscherichia coliInfection among Emergency Department Patients with Pyelonephritis . Clinical Infectious Diseases2008;47: 1150–1158.
ß-lactam Antibiotics: In general, ß-lactams as a group are less effective than the aforementioned drugs. First- and second-generation oral cephalosporines are not recommended as first-line antimicrobials for a 3-day treatment of uncomplicated UTI. However, among third-generation oral cephalosporins, a 3-day course with cefpodoxime-proxetil (200 mg twice daily) was as safe and effective as that of TMP-SMX. One exception might be pivmecillinam. 7 days of pivmecillinam, 200 mg twice daily, was equivalent to 3 days of norfloxacin, 400 mg twice daily. With pivmecillinam, however, the rate of vaginal candidiasis was significantly lower than with norfloxacin. Pivmecillinam also shows low resistance rates forE. coliand other Enterobacteriaceae, without cross-resistance to other antimicrobials used for the treatment of UTI.
Fosfomycin: Single dose fosfomycin trometamol (3 g) therapy has shown good results regarding bacteriological eradication. Considering that fosfomycin trometamol has been extensively used in several European countries for single-dose therapy of uncomplicated UTI since 1988, the resistance rate forE. coliremained very low without cross-resistance to other antimicrobials used for the treatment of UTI.
Falagas ME, Kastoris AC, et al. Fosfomycin for the Treatment of Multidrug-Resistant, Including Extended-Spectrum beta-Lactamase Producing, Enterobacteriaceae Infections: A Systematic Review. Lancet Infect Dis. 2010 Jan;10: 43-50.
Nitrofurantoin: Nitrofurantoin (50-100 mg four times daily, or sustained release formulation 100 mg twice daily) cannot be considered a suitable drug for short-term therapy (up to 3 days) of acute uncomplicated cystitis. A course of 5-7 days is recommended if nitrofurantoin is used for this indication. Despite the clinical use of nitrofurantoin for many years, the resistance rate forE. coliandS. saprophyticusis still low throughout Europe, although in some areas a two-fold increase in nitrofurantoin resistance has already been observed forE. coliwithin the last 10 years. Nitrofurantoin is, however, not active against P. mirabilisand Klebsiellaspp., the second and third most frequently isolated Gram-negative uropathogens. There is also some concern about the safety of nitrofurantoin, especially the acute and chronic pulmonary syndromes, which are common in the elderly. These severe adverse events, however, were not observed when nitrofurantoin was used for long-term and low-dose prophylaxis for recurrent UTIs in girls and women.
Other Treatment Modalities: Urinary analgesics, such as phenazopyridine, 200 mg three times daily, can be administered to patients who have experienced severe dysuria for 1 or 2 days. Women with cystitis, including those with severe dysuria and urgency, usually show resolution or marked improvement of symptoms within 2-3 days of initiating therapy. This should be explained to the patient. Thus, the need for, and duration of, analgesic therapy in women with UTIs must be individualized. Although it is generally recommended that patients with UTIs increase their fluid intake to promote micturition and the elimination of uropathogens, it remains unclear as to whether this is beneficial or detrimental to patients with UTI.
Gupta K, Hooton TM, Naber KG, Wult B, Colgan R International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of American and the European Society for Microbiology and Infectious Diseases
Post-treatment Follow-up
Urinalysis (e.g using a dipstick method) is sufficient for routine follow-up. Routine post-treatment cultures in asymptomatic patients may not be indicated because the benefit of detecting and treating asymptomatic bacteriuria in healthy women has been demonstrated only in pregnancy and prior to urological instrumentation or surgery. In women whose symptoms do not resolve by the end of treatment and in those whose symptoms resolve but recur within 2 weeks, urine culture and antimicrobial susceptibility testing should be performed. For therapy in this situation, one should assume that the infecting organism is not susceptible to the agent originally used and retreatment with a 7-day regimen using another agent should be considered.
(Printable Version of Treatment and Follow-up Sections for Cystitis in Treatment for UTIs in Women)
Acute Uncomplicated Pyelonephritis in Pre-menopausal, Non-pregnant Women Diagnosis
Acute pyelonephritis is suggested by flank pain, nausea and vomiting, fever (> 38°C), or costovertebral angle tenderness, and may occur with or without cystitis symptoms. The presentation of an acute uncomplicated pyelonephritis usually varies from a mild to a moderate illness. A life-threatening condition with multi-organ system dysfunction, including sepsis syndrome with or without shock and renal failure, must be considered a complicated case. Urinalysis is indicated to look for pyuria and haematuria. In contrast to cystitis, 80-95% of episodes of pyelonephritis are associated with > 105cfu uropathogen/mL. For routine diagnosis, a breakpoint of 104cfu/mL can be recommended (Table 8). An evaluation of the upper urinary tract with ultrasound should be performed to rule out urinary obstruction. Additional investigations, such as an unenhanced helical computed tomography (to rule out urolithiasis), an excretory urogram or DMSA scan, according to the clinical situation should be considered if the patient remains febrile after 72 hours of treatment to rule out further complicating factors, e.g. urolithiasis, renal or perinephric abscesses. Routine performance of an excretory urogram in patients with acute uncomplicated pyelonephritis has little value because most adults with uncomplicated acute pyelonephritis have a normal upper urinary tract.
(Printable version of this Diagnosis Section with Microbiological and Other Laboratory Finding)
Treatment
The following aspects should be considered for treatment (Table 2):
1. TMP-SMX is preferred over ampicillin.
2. Two weeks of therapy with TMP-SMX for acute uncomplicated pyelonephritis appears to be adequate for the majority of women.
3. In communities in which the resistance rate ofE. coli to TMP is > 10%, a fluoroquinolone should be recommended as the drug of choice for empirical therapy. It was demonstrated that a 7-day regimen of ciprofloxacin, 500 mg twice daily, showed a significantly higher rate of bacterial eradication and a lower rate of adverse effects when compared with a 14-day therapy using TMP-SMX, 960 mg twice daily. The following fluoroquinolones are also comparable to conventional ciprofloxacin 500 mg twice daily: ciprofloxacin extended release formulation (1000 mg once daily), gatifloxacin (400 mg once daily), levofloxacin (250 mg twice daily), and lomefloxacin (400 mg once daily).
4. A 10-day therapy with cefpodoxime proxetil 200 mg twice daily is probably equivalent with ciprofloxacin 500 mg twice daily.
5. In areas with a rate ofE. coliresistance to fluoroquinolones > 10% and in situations in which fluoroquinolones are contraindicated (e.g. pregnancy, lactating women, adolescence), an aminopenicillin plus a beta-lactamase inhibitor, or a group three oral cephalosporin is recommended, either for initial use, or if a patient has to be switched to an oral regimen.
Therefore in mild and moderate cases an oral fluoroquinolone could be used for 7 days as first-line therapy. In situations where a fluoroquinolone is not indicated, a group three oral cephalosporin, e.g. cefpodoxime proxetil, may be an alternative for empirical therapy. More severe cases of acute uncomplicated pyelonephritis should be admitted to hospital and, if the patient cannot take oral medication, treated parenterally with a fluoroquinolone, an aminopenicillin plus a beta-lactamase inhibitor, a group three cephalosporin, or an aminoglycoside. With improvement, the patient can be switched to an oral regimen using one of the above-mentioned antibacterials (if active against the infecting organism) to complete the 1-2 weeks’ course of therapy.
Although approximately 12% of patients hospitalized with acute uncomplicated pyelonephritis have bacteraemia, it is common practice to obtain blood cultures only if the patient appears ill enough to warrant hospitalization. There is no evidence that bacteraemia has prognostic significance or warrants longer therapy in an otherwise healthy individual with pyelonephritis.
Gupta K, Hooton TM, Naber KG, Wult B, Colgan R International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of American and the European Society for Microbiology and Infectious Diseases
Post-treatment Follow-up
Routine post-treatment cultures in an asymptomatic patient may not be indicated; routine urinalysis using a dipstick method is sufficient. In women whose pyelonephritis symptoms do not improve within 3 days, or that resolve and then recur within 2 weeks, a repeat urine culture, antimicrobial susceptibility testing and an appropriate investigation, such as renal ultrasound or scan, should be performed. In the patient with no urological abnormality, it should be assumed that the infecting organism is not susceptible to the agent originally used and retreatment with a 2-week regimen using another agent should be considered. For those patients who relapse with the same pathogen as the initially infecting strain, a 6-week regimen is usually curative. An overview of the clinical management of acute pyelonephritis is shown in Figure 1.
Recurrent (uncomplicated) UTIs in Women
Background
Recurrent urinary tract infection is defined in the literature by three episodes of UTI in the last 12 months or two episodes in the last 6 months. Risk factors for recurrent urinary tract infection are genetic and behavioural. Some studies estimate that 20-30% of women who have a UTI will have a recurrent urinary tract infection. Women who are non-secretors of blood group substances have an increased occurrence of recurrent urinary tract infection. A secretor is defined as a person who secretes their blood type antigens into body fluids and secretions, such as saliva, etc. A non-secretor on the other hand puts little to none of their blood type antigens into these fluids. In the USA about 20% of the population are non-secretors. Women with recurrent urinary tract infection have an increased frequency of urinary infection in first-degree female relatives. In addition, E. coli,the most common uropathogen, adheres more readily to epithelial cells in women who experience recurrent urinary tract infection. Behavioural factors associated with recurrent urinary tract infection include sexual activity, with a particularly high risk in those who use spermicides as a birth control method. According to cohort and case control studies, risk factors associated with recurrent urinary tract infection in sexually active premenopausal women are frequency of sexual intercourse, spermicide use, age of first UTI (less than 15 years of age indicates a greater risk of recurrent urinary tract infection) and history of UTI in the mother, suggesting that genetic factors and/or long-term environmental exposures might predispose to this condition. Following the menopause, risk factors strongly associated with recurrent urinary tract infection are vesical prolapse, incontinence and post-voiding residual urine. Other risk factors such as blood group substance non-secretor status and a history of UTI before the menopause need to be confirmed by further research.
Recurrent UTIs result in significant discomfort for women and have a high impact on ambulatory health care costs as a result of outpatient visits, diagnostic tests and prescriptions. Different approaches have been proposed for the prevention of recurrent urinary tract infection, including non-pharmacological therapies, such as voiding after sexual intercourse or the ingestion of cranberry juice, and the use of antibiotics as preventive therapy given regularly or postcoital prophylaxis in sexually active women.
With respect to antibiotic prophylaxis, it is not known which antibiotic schedule is best or the optimal duration of prophylaxis, the incidence of adverse events, or the recurrence of infections after stopped prophylaxis or treatment compliance.
Prophylactic Antimicrobial Regimens
One effective approach for the management of recurrent uncomplicated UTI is the prevention of infection through the use of long-term, prophylactic antimicrobials taken on a regular basis at bedtime or postcoital. Generally, the number of patients with microbiological recurrent UTIs decreased by eightfold as compared to the period of time before prophylaxis and compared to placebo by fivefold. The UTI episodes per patient-year is reduced in general by 95% during antimicrobial prophylaxis as compared to the period of time before prophylaxis. The duration of prophylactic therapy usually extends between 6 months or 1 year. Prophylaxis does not appear to modify the natural history of a recurrent UTI. When discontinued, even after extended periods, approximately 60% of women will become re-infected within 3-4 months.
The recommendations for antimicrobial regimens for the prevention (prophylaxis) of recurrent uncomplicated UTI in pre-menopausal women are listed in Table 3. Trimethoprim, co-trimoxazole or nitrofurantoin can still be considered as the standard regimen. Fosfomycin trometamol (FT), 3g every 10 days for 6 months can be considered as an alternative. An alternative prophylactic approach is post-intercourse prophylaxis for women in whom episodes of infection are associated with sexual intercourse. Generally, for this approach, the same antimicrobials can be used in the same doses as though recommended for continuous prophylaxis. A patient-initiated treatment may also be suitable for management in well-informed, young women, in whom the rate of recurrent episodes is not too common. This is, however, strictly speaking, not prophylaxis but early treatment.
( Printable Version of Prophylactic Antimicrobial Regimens Section for Recurrent UTI in Treatment for UTIs in Women)
UTIs in Pregnancy
Urinary tract infections are common during pregnancy. There is some debate about whether these infections can be classified as uncomplicated, even in cases where no further risk factors besides pregnancy can be found. Bearing this in mind, the three entities, asymptomatic bacteriuria, acute cystitis and acute pyelonephritis, will be discussed in this section with regard only to pregnancy and not to other risk factors.
The factors that predispose a woman to UTI in pregnancy appear to be related to the anatomical and physiological changes in the kidney and urinary tract that occur during pregnancy. The ureters become dilated above the pelvic brim and the bladder is displaced anteriorly and superiorly by the enlarging uterus. Renal blood flow and the glomerular filtration rate increase by about 30-40% during pregnancy and the kidneys become slightly enlarged and hyperaemic. Urine flow may be sluggish and the bladder may not empty completely.
Epidemiology
The prevalence of asymptomatic bacteriuria in American, European and Australian studies varies between 4% and 7%. Incidence relates to sexual activity and increases with increasing age and gravidity. It is also higher among patients from lower socio-economic groups. Symptomatic infection occurs in about 1-2% of pregnant women.
Most women acquire bacteriuria before pregnancy. At the first examination, the rates of bacteriuria in pregnant women are similar to those in non-pregnant women with similar risk factors. About 37-57% of bacteriuric schoolgirls develop UTIs during pregnancy. An additional 1% of infections occur during pregnancy. Bacteriuria during pregnancy is associated with a significant increase in the number of low-birth-weight infants (< 2500 g), low gestational age (< 37 weeks), and neonatal mortality. Women with persistent infection despite treatment or with evidence of ‘tissue invasion’ are at a higher risk of delivering premature infants. It should, however, be mentioned that bacterial vaginosis is also an important independent risk factor for premature birth; hence, treatment is recommended.
Asymptomatic Bacteriuria
Early studies by Kass and others demonstrated that 20-40% of women with asymptomatic bacteriuria develop pyelonephritis during pregnancy. Treatment of the bacteriuria lowers this risk. It is therefore generally recommended that pregnant women should be screened for bacteriuria by urine culture at least once in early pregnancy, and they should be treated if results are positive. To avoid unnecessary treatment, asymptomatic bacteriuria is defined as two consecutive positive cultures of the same species. The false-positive rate of a single mid-stream sample of urine may be as high as 40%. Therefore, women with a positive urine culture should be asked to return within 1-2 weeks, at which time, after stressing the importance of a careful cleansing of the vulva before micturition, a second mid-stream sample of urine or straight catheter urine specimen is obtained for culture. Treatment should be based on antibiotic sensitivity testing and usually involves a 5- to 7-day course of antibiotics. Follow-up cultures should be obtained 1-4 weeks after treatment and at least once more before delivery.
Acute Cystitis During Pregnancy
Most symptomatic UTIs in pregnant women present as acute cystitis, as occurs in non-pregnant women. Usually a 7-day treatment course is recommended, e.g. with pivmecillinam. Short-term therapy is not as established in pregnant women as it is in non-pregnant women, but it is recommended by smaller studies and expert opinion. Fosfomycin trometamol (3 g single dose) or second- and third generation oral cephalosporins (e.g. ceftibuten 400 mg once daily) could be considered candidates for effective short-term therapy. Otherwise conventional therapy with amoxicillin, cephalexin or nitrofurantoin is recommended.
Follow-up urine cultures should be obtained after therapy to demonstrate eradication of the bacteriuria. As in non-pregnant women, there is no advantage to be gained by using long-term prophylaxis except for recurrent infections. Low-dose cephalexin (125-250 mg) or nitrofurantoin (50 mg) at night are recommended for prophylaxis against re-infection if indicated, lasting up to and including the puerperium. Postcoital prophylaxis may be an alternative approach.
Acute Pyelonephritis in Pregnancy
Acute pyelonephritis tends to occur during the later stages of pregnancy, usually in the last trimester. The incidence of acute pyelonephritis in obstetric patients is 2%. The incidence is increased in the puerperium. Characteristically, the patient is acutely ill with high fever, leucocytosis and costovertebral angle pain. Bacteraemia is common, but mortality and complications are low when the patient is treated with effective therapy. The major causes of concern are the presence of underlying urological abnormalities and associated risks to the mother and fetus, such as toxaemia, hypertension, prematurity and perinatal mortality. Currently, antimicrobial therapy is so effective that, even with bacteraemia, almost all patients with uncomplicated pyelonephritis do well and become afebrile within a few days. Recommended antibiotics include second- or third-generation cephalosporins, an aminopenicillin plus a BLI, or an aminoglycoside. During pregnancy, quinolones, tetracyclines and TMP should not be used during the first trimester, while sulphonamides should not be used in the last trimester. In cases of delayed defeverescence and upper tract dilatation, a ureteral stent may be indicated and antimicrobial prophylaxis until delivery and including the puerperium should be considered.
Usta TA. et al. Comparison of single-dose and multiple-dose antibiotics for lower urinary tract infection in pregnancy. Int J Gynaecol Obstet. 2011 Sep;114(3): 229-33.
(Printable Version of UTI in Pregnancy)
UTIs in Post-menopausal Women
The normal vagina contains only low numbers of Gram-negative enteric bacteria because of competition from the resident microbial flora. Lactobacilli account for the low vaginal pH. They tend to be less abundant in postmenopausal women and after antimicrobial therapy. Oestrogens are presumed to exert a protective force against recurrent UTIs in post-menopausal women because they enhance the growth of lactobacilli and decrease vaginal pH. Gram-negative enteric bacteria do not ordinarily colonize the vagina in postmenopausal women unless these women are prone to recurrent UTIs. In post-menopausal women with recurrent UTIs, therapy with oral or intravaginal oestriol reduced significantly the rate of recurrence. For other patients, an antimicrobial prophylactic regimen should be recommended in addition to hormonal treatment. In the case of an acute UTI, the antimicrobial treatment policy is similar to that in pre-menopausal women. Short-term therapy in post-menopausal women is not, however, as well documented as in younger women.
(Printable this UTIs in Post menopausal Women Section with others for UTIs in Women)
Acute Uncomplicated UTIs in Young Men
Pathogenesis and Risk Factors
It has been conventional to consider all UTIs in men as complicated because most UTIs occurring in the newborn, infant or elderly male are associated with urological abnormalities, bladder outlet obstruction or instrumentation. A UTI in an otherwise healthy adult man between the ages of 15 and 50 years is very uncommon. The large difference in the prevalence of UTIs between men and women is thought to be caused by a variety of factors, including the greater distance between the usual source of uropathogens (the anus and the urethral meatus); the drier environment surrounding the male urethra; the greater length of the male urethra; and the antibacterial activity of the prostatic fluid. It has become clear, however, that a small number of men aged 15-50 years suffer acute uncomplicated UTIs. The exact reasons for such infections are not clear, but risk factors associated with such infections include intercourse with an infected partner, anal intercourse and lack of circumcision; however, these factors are not always present. More than 90% of men with febrile UTI (fever > 38.0°C), with or without clinical symptoms of pyelonephritis, have a concomitant infection of the prostate, as measured by transient increases in serum PSA and prostate volume, irrespective of prostatic tenderness.
Diagnosis(Table 8)
The symptoms of uncomplicated UTIs in men are similar to those in women. Urethritis must be ruled out in sexually active men using a urethral Gram stain or a first-voided urine specimen wet mount to look for urethral leucocytosis. A urethral gram stain demonstrating leucocytes and predominant Gram-negative rods suggests E. coliurethritis, which may precede or accompany a UTI. Dysuria is common to both UTI and urethritis. The aetiological agents that cause uncomplicated UTIs in men are also similar to those in women.
Treatment(Table 9)
Empirical use of the agents discussed previously for uncomplicated cystitis or pyelonephritis in women are recommended. Nitrofurantoin should not be used in men with a UTI, since it does not achieve reliable tissue concentrations. For acute uncomplicated pyelonephritis, the use of a fluoroquinolone as initial empirical treatment is recommended in areas where the rate of E. coliresistance to fluoroquinolones is low (< 10%). Otherwise, alternative drugs have to be considered. Since in most men with febrile UTI or pyelonephritis, prostatic involvement also has to be considered, the goal of treatment is not only to sterilize the urine, but also to eradicate the prostatic infection. Thus, antimicrobials with good prostatic tissue and fluid penetration are preferable, e.g. fluoroquinolones.
A minimum of 7 days of therapy is recommended, because of the relatively greater likelihood of an occult complicating factor in men compared with women. Urological evaluation should be carried out routinely in adolescents and in men with febrile UTI, pyelonephritis and recurrent infections, or whenever a complicating factor is present.
Asymptomatic Bacteriuria
Asymptomatic bacteriuria is common. Populations with structural or functional abnormalities of the genitourinary tract may have an exceedingly high prevalence of bacteriuria, but even healthy individuals frequently have positive urine cultures. Asymptomatic bacteriuria is seldom associated with adverse outcomes.
Pregnant women and individuals undergoing traumatic genitourinary interventions are at risk for complications of bacteriuria and show benefit from screening and treatment programmes. Screening for or treatment of asymptomatic bacteriuria is not recommended for the following persons:
• pre-menopausal, non-pregnant women
• diabetic women
• older persons living in community
• elderly institutionalized subjects
• persons with spinal cord injury
• catheterized patients while the catheter remains in situ.
In fact, treatment of bacteriuria may be associated with harmful outcomes, such as increased shortterm frequency of symptomatic infection, adverse drug effects, and re-infection with organisms of increased antimicrobial resistance. Screening for asymptomatic bacteriuria and treatment is recommended only for selected groups where benefit has been shown:
• pregnant women
• before transurethral resection of the prostate and other traumatic urological interventions.
Antimicrobial therapy should be initiated before the procedure. Short-term antimicrobial treatment of asymptomatic women with catheter-acquired bacteriuria that persists 48 hours after removal of the indwelling catheter may be considered.
(Printable Version of UITs in Young Men)
(Printable Version of Gram Stain)
Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis 2005;40: 643-654.
Suggested Reading
1. Brumfitt W, Hamilton-Miller JM. Efficacy and safety profile of long-term nitrofurantoin in urinary tract infections: 18 years’ experience. J Antimicrob Chemother 1998;42: 363-371. [PubMed]
2. Hamm M, Wawroschek F, Weckermann D, Knopfle E, Hackel T, Hauser H, Krawczak G, Harzmann R. Unenhanced helical computed tomography in the evaluation of acute flank pain. Eur Urol 2001;39: 460-465. [PubMed]
3. Hooton TM. Fluoroquinolones and resistance in the treatment ofuncomplicated urinary tract infection. Int J Antimicr Agents 2003;22(Suppl 2): 65-72. [PubMed]
4. Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton AE, Stergachis A, Stamm WE. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med 1996;335: 468-474. [PubMed]
5. Hooton TM, Scholes D, Stapleton AE, Roberts PL, Winter C, Gupta K, Samadpour M, Stamm WE. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000;343: 992-997. [PubMed]
6. Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 1997;11: 551-581. [PubMed]
7. Hooton TM, Winter C, Tiu F, Stamm WE. Randomized comparative trial and cost analysis of 3-day antimicrobial regimens for treatment of acute cystitis in women. JAMA 1995;273: 41-45. [PubMed]
8. Kahlmeter G; ECO.SENS. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother 2003; 51: 69-76. [PubMed]
9. Lecomte F, Allaert FA. Single-dose treatment of cystitis with fosfomycin trometamol (Monuril): analysis of 15 comparative trials on 2.048 patients. Giorn It Ost Gin 1997;19: 399-404. [PubMed]
10. Naber KG. Short-term therapy of acute uncomplicated cystitis. Curr Opin Urol 1999;9: 57-64. [PubMed]
11. Naber KG, Allin DM, Clarysse L, Haworth DA, James IG, Raini C, Schneider H, Wall A, Weitz P, Hopkins G, Ankel-Fuchs D. Gatifloxacin 400 mg as a single shot or 200 mg once daily for 3 days is as effective as ciprofloxacin 250 mg twice daily for the treatment of patients with uncomplicated urinary tract infections. Int J Antimicrob Agents. 2004;23: 596-605. [PubMed]
12. Naber KG, Bartnicki A, Bischoff W, Hanus M, Milutinovic S, van Belle F, Schonwald S, Weitz P, Ankel- Fuchs D. Gatifloxacin 200 mg or 400 mg once daily is as effective as ciprofloxacin 500 mg twice daily for the treatment of patients with acute pyelonephritis or complicated urinary tract infections. Int J Antimicrob Agents 2004;23(Suppl 1): 41-53. [PubMed]
13. Naber KG, Bishop MC, Bjerklund-Johansen TE, Botto H, Cek M, Grabe M, Lobel B, Palou J, Tenke P. EAU guidelines on the management of urinary and male genital tract infections. EAU Working Group on Urinary and Male Genital Tract Infections. European Association of Urology (EAU) Guidelines Office. 2006 edition. 1-126. [PubMed]
14. Nicolle LE. Asymptomatic bacteriuria: when to screen and when to treat. Infect Dis Clin North Am 2003;17: 367-394. [PubMed]
15. Nicolle LE. Pivmecillinam in the treatment of urinary tract infections. J Antimicrob Chemother 2000;46(Suppl 1): 35-39. [PubMed]
16. Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40: 643-654. [PubMed]
17. Nicolle LE, Harding GK, Preiksaitis J, Ronald AR. The association of urinary tract infection with sexual intercourse. J Infect Dis 1982;146: 579-583. [PubMed]
18. Pfau A. Recurrent UTI in pregnancy. Infection 1994;22(Suppl 1): 49. [PubMed]
19. Raz R, Gennesin Y, Wasser J, Stoler Z, Rosenfeld S, Rottensterich E, Stamm WE. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30: 152-156. [PubMed]
20. Schaeffer AJ, Jones J, Dunn JK. Association of vitro Escherichia coli adherence to vaginal and buccal epithelial cells with susceptibility of women to recurrent urinary-tract infections. N Engl J Med 1981;304: 1062-1066. [PubMed]
21. Talan DA, Klimberg IW, Nicolle LE, Song J, Kowalsky SF, Church DA. Once daily, extended release ciprofloxacin for complicated urinary tract infections and acute uncomplicated pyelonephritis. J Urol 2004;171: 734-739. [PubMed]
22. Talan DA, Stamm WE, Hooton TM, Moran GJ, Burke T, Iravani A, Reuning-Scherer J, Church DA. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA 2000;283: 1583-1590. [PubMed]
23. Vosti KL. Recurrent urinary tract infections. Prevention by prophylactic antibiotics after sexual intercourse. JAMA 1975;231: 934-940. [PubMed]
24. Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 1999;29: 745-758. [PubMed]
25. Weidner W, Ludwig M, Weimar B, Rau W. Rational diagnostic steps in acute pyelonephritis with special reference to ultrasonography and computed tomography scan. Int J Antimicrob Agents 1999;11: 257-259. [PubMed]
URINARY TRACT INFECTIONS IN CHILDREN
Background
The urinary tract is a common source of infection in children and infants. It represents the most common bacterial infection in children less than 2 years of age. The outcome of a UTI is usually benign, but in early infancy it can progress to renal scarring, especially when associated with congenital anomalies of the urinary tract. Delayed sequelae related to renal scarring include hypertension, proteinuria, renal damage and even chronic renal failure, requiring dialysis treatment in a significant number of adults.
The risk of a UTI during the first decade of life is 1% in males and 3% in females. It has been suggested that 5% of schoolgirls and up to 0.5% of schoolboys undergo at least one episode of UTI during their school life. The incidence is different for children under 3 months of age, when it is more common in males. The incidence of asymptomatic bacteriuria is 0.7-3.4% in neonates, 0.7-1.3% in infants under 3 months of age and between 0.2% and 0.8% in preschool boys and girls, respectively. The incidence of symptomatic bacteriuria is 0.14% in neonates, with a further increase to 0.7% in boys and 2.8% in girls aged less than 6 months. The overall recurrence rate for the neonatal period has been reported to be 25%.
Aetiology
The common pathogenic sources are Gram-negative, mainly enteric, organisms. Of these, Escherichia coliis responsible for 90% of episodes of UTIs. Gram-positive organisms (particularly enterococci and staphylocci) represent 5-7% of cases. Hospital-acquired infections show a wider pattern of aggressive organisms, such as Klebsiellaspp., Serratia and Pseudomonas spp. Groups A and B streptococci are relatively common in the newborn. There is an increasing trend towards the isolation of Staphylococcus saprophyticusin UTIs in children, although the role of this organism is still debatable.
Pathogenesis and Risk Factors
Obstruction and dysfunction are among the most common causes of urinary infection. Phimosis predisposes to UTI. Enterobacteria derived from intestinal flora colonize the preputial sac, glandular surface and the distal urethra. Among these organisms are strains of E. coliexpressing P. fimbriae which adhere to the inner layer of the preputial skin and to uroepithelial cells. A wide variety of congenital urinary tract abnormalities can cause UTIs through obstruction, e.g. urethral valves, pelvi-ureteric junction obstruction or non-obstructive urinary stasis (e.g. prune belly syndrome, vesicoureteral reflux). More mundane but significant causes of UTIs include labial adhesion and chronic constipation. Dysfunctional voiding in an otherwise normal child may result in infrequent bladder emptying aided by delaying manoeuvres, e.g. crossing legs, sitting on heels. Neuropathic bladder dysfunction (spina bifida, sphincter dyssynergia, etc) may lead to postvoid residual urine and secondary vesicoureteral reflux.
The link between renal damage and UTIs is controversial. The mechanism in obstructive nephropathy is self-evident, but more subtle changes occur where there is vesicoureteral reflux. Almost certainly the necessary components include vesicoureteral reflux, intrarenal reflux and a UTI. These must all work together in early childhood when the growing kidney is likely to be susceptible to parenchymal infection. Later on in childhood, the presence of bacteriuria seems irrelevant to the progression of existing scars or the very unusual formation of new scars. Another confounding factor is that many so-called scars are dysplastic renal tissue which developed in utero.
Signs and Symptoms
Symptoms are non-specific, and vary with the age of the child and the severity of the disease. Epididymoorchitis is extremely unusual. With scrotal pain and inflammation in a boy, testicular torsion has to be considered.
A UTI in neonates may be non-specific and with no localization. In small children, a UTI may present with gastrointestinal signs, such as vomiting and diarrhea. In the first weeks of life, 13.6% of patients with fever have a UTI. Rarely, septic shock will be the presentation. Signs of a UTI may be vague in small children, but later on, when they are older than 2 years, frequent voiding, dysuria and suprapubic, abdominal or lumbar pain may appear with or without fever.
Shaikh N et al. Does this child have a urinary tract infection? JAMA. 2007 Dec 26;298(24): 2895-904.
Classification
Urinary tract infections may be classified either as a first episode or recurrent, or according to severity (simple or severe). Recurrent UTI may be subclassified into three groups:
• Unresolved infection: subtherapeutic level of antimicrobial, non-compliance with treatment, malabsorption, resistant pathogens.
• Bacterial persistence: may be due to a nidus for persistent infection in the urinary tract. Surgical correction or medical treatment for urinary dysfunction may be needed.
• Reinfection: each episode is a new infection acquired from periurethral, perineal or rectal flora. From the clinical point of view, severe and simple forms of UTIs should be differentiated because to some extent the severity of symptoms dictates the degree of urgency with which investigation and treatment are to be undertaken.
Severe UTI
Severe UTI is related to the presence of fever of > 39ºC, the feeling of being ill, persistent vomiting, and moderate or severe dehydration.
Simple UTI
A child with a simple UTI may have only mild pyrexia, but is able to take fluids and oral medication. The child is only slightly or not dehydrated and has a good expected level of compliance. When a low level of compliance is expected, such a child should be managed as one with a severe UTI.
Diagnosis
Physical Examination
It is mandatory to look for phimosis, labial adhesion, signs of pyelonephritis, epididymo-orchitis, and stigmata of spina bifida, e.g. hairy patch on the sacral skin. The absence of fever does not exclude the presence of an infective process.
Laboratory Tests
The definitive diagnosis of infection in children requires a positive urine culture. Urine must be obtained under bacteriologically reliable conditions when undertaking a urine specimen culture. A positive urine culture is defined as the presence of more than 100,000 cfu/mL of one pathogen. The urine specimen may be difficult to obtain in a child less than 4 years old and different methods such as suprapubic bladder aspiration, bladder catheterization, plastic bag collection are advised since there is a high risk of contamination (Table 4).
Imaging of the Urinary Tract
A ‘gold standard’ imaging technique has to be cost-effective, painless, safe, with minimal or nil radiation, and an ability to detect any significant structural anomaly. Current techniques do not fulfil all such requirements.
Ultrasonography: Ultrasonography (US) has become very useful in children because of its safety, speed and high accuracy in identifying the anatomy and size of the renal parenchyma and collecting system. It is subjective and therefore operator-dependent, and gives no information on renal function. However, scars can be identified, although not as well as with technetium-99m dimercaptosuccinic acid (Tc-99m DMSA) scanning. This technique has been shown to be very sensitive and excretory urography must be reserved only for when images need to be morphologically clarified.
Radionuclide Studies: Tc-99m DMSA is a radiopharmaceutical that is bound to the basement membrane of proximal renal tubular cells; half of the dose remains in the renal cortex after 6 hours. This technique is helpful in determining functional renal mass and ensures an accurate diagnosis of cortical scarring by showing areas of hypoactivity indicating lack of function. A UTI interferes with the uptake of this radiotracer by the proximal renal tubular cells, and may show areas of focal defect in the renal parenchyma. A star-shaped defect in the renal parenchyma may indicate an acute episode of pyelonephritis. A focal defect in the renal cortex usually indicates a chronic lesion or a ‘renal scar’.
A focal scarring or a smooth uniform loss of renal substance as demonstrated by Tc-99m DMSA has generally been regarded as being associated with vesicoureteral reflux (reflux nephropathy). The use of Tc-99m DMSA scans can be helpful in the early diagnosis of acute pyelonephritis. About 50-85% of children will show positive findings in the first week. Minimal parenchymal defects, when characterized by a slight area of hypoactivity, can resolve with antimicrobial therapy. However, defects lasting longer than 5 months are considered to be renal scarring. Tc-99m DMSA scans are considered more sensitive than excretory urography and ultrasonography in the detection of renal scars.
Cystourethrography: 1.Conventional Voiding Cystourethrography: Voiding cystourethrography is the most widely used radiological exploration for the study of the lower urinary tract and especially of vesicoureteral reflux. It is considered mandatory in the evaluation of UTIs in children less than 1 year of age. Its main drawbacks are the risk of infection, the need for retrogrades filling of the bladder and the possible deleterious effect of radiation on children. In recent years, tailored low-dose fluoroscopic voiding cystourethrography has been used for the evaluation of vesicoureteral reflux in girls in order to minimize radiological exposure. Voiding cystourethrography is mandatory in the assessment of febrile childhood UTI, even in the presence of normal ultrasonography. Up to 23% of these patients may reveal vesicoureteral reflux. 2.Radionuclide Cystography (Indirect): This investigation is performed by prolonging the period of scanning after the injection of Tc-99m diethylene triamine pentaacetate (DTPA) or mercaptoacetyltriglycine (MAG-3) as part of a dynamic renography. It represents an attractive alternative to conventional cystography, especially when following patients with reflux, because of its lower dose of radiation. Disadvantages are a poor image resolution and difficulty in detecting lower urinary tract abnormalities.
Cystosonography: Contrast material-enhanced voiding ultrasonography has been introduced for the diagnoses of vesicoureteral reflux without irradiation. Further studies are necessary to determine the role of this new imaging modality in UTI.
Additional Imaging: Excretory urography remains a valuable tool in the evaluation of the urinary tract in children, but its use in UTIs is debatable unless preliminary imaging has demonstrated abnormalities requiring further investigation. The major disadvantages in infants are the risks of side effects from exposure to contrast media and radiation. However, the role of excretory urography is declining with the increasing technical superiority of CT and MRI. However, the indications for their use is still limited in UTI.
Urodynamic Evaluation: When voiding dysfunction is suspected, e.g. incontinence, residual urine, increased bladder wall thickness, urodynamic evaluation with uroflowmetry, (video) cystometry, including pressure flow studies, and electromyography should be considered.
Etoubleau C, Reveret M, et al. Moving from bag to catheter for urine collection in non-toilet-trained children suspected of having urinary tract infection: a paired comparison of urine cultures. J Pediatr. 2009 Jun;154: 803-6.
Schedule of Investigation
Screening of infants for asymptomatic bacteriuria is unlikely to prevent pyelonephritic scar formation, as these usually develop very early in infancy. Only a minority of children with a UTI have an underlying urological disorder, but when present such a disorder can cause considerable morbidity. Thus, after a maximum of two UTI episodes in a girl and one episode in a boy, investigations should be undertaken (Figure 2), but not in the case of asymptomatic bacteriuria. The need for DTPA/MAG-3 scanning is determined by the ultrasound findings, particularly if there is suspicion of an obstructive lesion.
(Printable Version of Background/Diagnosis of UTI in Children)
Treatment
Treatment has four main goals:
1. elimination of symptoms and eradication of bacteriuria in the acute episode
2. prevention of renal scarring
3. prevention of a recurrent UTI
4. correction of associated urological lesions.
Severe UTIs
A severe UTI requires adequate parenteral fluid replacement and appropriate antimicrobial treatment, preferably with cephalosporins (third generation). If a Gram-positive UTI is suspected by Gram stain , it is useful to administer aminoglycosides in combination with ampicillin or amoxicillin/clavulanate. Antimicrobial treatment has to be initiated on an empirical basis, but should be adjusted according to culture results as soon as possible. In patients with an allergy to cephalosporins, aztreonam or gentamicin may be used. When aminoglycosides are necessary, serum levels should be monitored for dose adjustment. Chloramphenicol, sulfonamides, tetracyclines, rifampicin, amphotericin B and quinolones should be avoided. The use of ceftriaxone must also be avoided due to its undesired side effect of jaundice. A wide variety of antimicrobials can be used in older children, with the exception of tetracyclines (because of teeth staining). Fluorinated quinolones may produce cartilage toxicity, but if necessary may be used as second-line therapy in the treatment of serious infections, since musculoskeletal adverse events are of moderate intensity and transient. For a safety period of 24-36 hours, parenteral therapy should be administered. When the child becomes afebrile and is able to take fluids, he/she may be given an oral agent to complete the 10-14 days of treatment, which may be continued on an outpatient basis. This provides some advantages, such as less psychological impact on the child and more comfort for the whole family. It is also less expensive, well tolerated and eventually prevents opportunistic infections. The preferred oral antimicrobials are: trimethoprim (TMP), co-trimoxazole (TMP plus sulphamethoxazole), an oral cephalosporin, or amoxicillin/clavulanate. However, the indication for TMP is declining in areas with increasing resistance. In children less than 3 years of age, who have difficulty taking oral medications, parenteral treatment for 7-10 days seems advisable, with similar results to those with oral treatment. If there are significant abnormalities in the urinary tract (e.g. vesicoureteral reflux, obstruction), appropriate urological intervention should be considered. If renal scarring is detected, the patient will need careful follow-up by a paediatrician in anticipation of sequelae such as hypertension, renal function impairment and recurrent UTI.
An overview of the treatment of febrile UTIs in children is given in Figure 3 and the dosing of antimicrobial agents is outlined in Table 5.
Simple UTIs
A simple UTI is considered to be a low-risk infection in children. Oral empirical treatment with TMP, an oral cephalosporin or amoxicillin/clavulanate is recommended, according to the local resistance pattern. The duration of treatment in uncomplicated UTIs treated orally should be 5-7 days. A single parenteral dose may be used in cases of doubtful compliance and with a normal urinary tract. If the response is poor or complications develop, the child must be admitted to hospital for parenteral treatment.
Prophylaxis
If there is an increased risk of pyelonephritis, e.g. vesicoureteral reflux, and recurrent UTI, low-dose antibiotic prophylaxis is recommended. It may also be used after an acute episode of UTI until the diagnostic work-up is completed. The most effective antimicrobial agents are: Nitrofurantoin, TMP, cephalexin and cefaclor.
Montini G, et al. Prophylaxis After First Febrile Urinary Tract Infection in Children? A Multicenter, Randomized, Controlled, Noninferiority Trial. Pediatrics 2008;122: 1064-1071.
(Printable Version of Treatment for UTIs in Children)
(Printable Version of Gram Stain)
Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis 2005;40: 643-654.
Suggested Reading
1. Bloomfield P, Hodson EM, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database of Syst Rev 2005;(1): CD003772. [PubMed]
2. Britton KE. Renal radionuclide studies. In: Whitfield HN, Hendry WF, Kirby RS, Duckett JW, eds. Textbook of genitourinary surgery. Oxford: Blackwell Science, 1998, pp. 76-103. [PubMed]
3. Haycock GB. A practical approach to evaluating urinary tract infection in children. Pediatr Nephrol 1991;5: 401-402. [PubMed]
4. Hoberman A, Chao HP, Keller DM, Hickey R, Davis HW, Ellis D. Prevalence of urinary tract infection in febrile infants. J Pediatr 1993;123: 17-23. [PubMed]
5. Jacobson SH, Eklof O, Eriksson CG, Lins LE, Tidgren B, Winberg J. Development of uraemia and hypertension after pyelonephritis in childhood: 27 year follow up. BMJ 1989;299: 703-706. [PubMed]
6. Jodal U. The natural history of bacteriuria in childhood. Infect Dis Clin North Am 1987;1: 713-729. [PubMed]
7. Kass EJ, Fink-Bennett D, Cacciarelli AA, Balon H, Pavlock S. The sensitivity of renal scintigraphy and sonography in detecting nonobstructive acute pyelonephritis. J Urol 1992;148: 606-608. [PubMed]
8. Lin DS, Huang SH, Lin CC, Tung YC, Huang TT, Chiu NC, Koa HA, Hung HY, Hsu CH, Hsieh WS, Yang DI, Huang FY. Urinary tract infection in febrile infants younger than eight weeks of Age. Paediatrics 2000;105: E20. [PubMed]
9. Mucci B, Maguire B. Does routine ultrasound have a role in the investigation of children with urinary tract infection? Clin Radiol 1994;49: 324-325. [PubMed]
10. Naber KG, Bishop MC, Bjerklund-Johansen TE, Botto H, Cek M, Grabe M, Lobel B, Palou J, Tenke P. EAU guidelines on the management of urinary and male genital tract infections. EAU Working Group on Urinary and Male Genital Tract Infections. European Association of Urology (EAU) Guidelines Office. 2006 edition. 1-126. [PubMed]
11. Pickworth FE, Carlin JB, Ditchfield MR, de Campo MP, Cook DJ, Nolan T, Powell HR, Sloane R, Grimwood K. Sonographic measurement of renal enlargement in children with acute pyelonephritis and time needed for resolution: implications for renal growth assessment. Am J Roentgenol 1995;165: 405-408. [PubMed]
12. Rosenberg AR, Rossleigh MA, Brydon MP, Bass SJ, Leighton DM, Farnsworth RH. Evaluation of acute urinary tract infection in children by dimercaptosuccinic acid scintigraphy: a prospective study. J Urol 1992;148: 1746-1749. [PubMed]
13. Schulamn SL. Voiding dysfunction in children. Urol Clin North Am 2004;31: 481-490, ix. [PubMed]
14. Stutley JE, Gordon I. Vesico-ureteric reflux in the damaged non-scarred kidney. Pediatr Nephrol 1992;6: 25-29. [PubMed]
15. To T, Agha M, Dick PT, Feldman W. Cohort study on circumcision of newborn boys and subsequent risk of urinary-tract infection. Lancet 1998;352: 1813-1816. [PubMed]
16. Westwood ME, Whiting PF, Cooper J, Watt IS, Kleijnen J. Further investigation of confirmed urinary tract infection (UTI) in children under five years: a systematic review. BMC Pediatr 2005;5: 2. [PubMed]
17. Yeung CK, Godley ML, Dhillon HR, Gordon I, Duffy PG, Ransley PG. The characteristics of primary vesico-ureteric reflux in male and female infants with pre-natal hydronephrosis. Br J Urol 1997;80: 319- 327. [PubMed]
COMPLIC ATED UTIs DUE TO UROLOGICAL DISORDERS
Definitions and Classification
A complicated UTI is an infection associated with a condition, such as structural or functional abnormalities of the genitourinary tract or the presence of an underlying disease, which increases the risks of acquiring an infection or of failing therapy. Two criteria are mandatory to define a complicated UTI: a positive urine culture and one or more of the factors listed in Table 6.
Complicated UTI can arise in a heterogeneous group of patients. But neither patient age nor gender per se are part of the definition of a complicated UTI. With regard to prognosis and clinical studies, it is advisable to stratify complicated UTIs due to urological disorders into at least two groups:
1. Patients in whom the complicating factors could be eliminated by therapy, e.g. stone extraction, removal of an indwelling catheter.
2. Patients in whom the complicating factor could not be or is not removed satisfactorily during therapy, e.g. permanent indwelling catheter, stone residuals after treatment or neurogenic bladder.
Clinical Presentation
A complicated UTI may or may not be associated with clinical symptoms (e.g. dysuria, urgency, frequency, flank pain, costovertebral angle tenderness, suprapubic pain and fever). Clinical presentation may vary from severe obstructive acute pyelonephritis with imminent urosepsis to a catheter-associated post-operative UTI, which might disappear spontaneously as soon as the catheter is removed. It also has to be recognized that symptoms, especially lower urinary tract sympoms (LUTS), are not only caused by UTIs but also by other urological disorders, such as benign prostatic hyperplasia (BPH), TURP, etc.
Apart from urological abnormalities, concomitant medical conditions, such as diabetes mellitus (10%) and renal failure, which can be related to urological abnormalities, are often present in a complicated UTI.
Urine Cultures(Table 8)
Significant bacteriuria in a complicated UTI is defined by counts of ≥ 105cfu/mL and ≥ 104cfu/mL, in the mid-stream sample of urine of women and men, respectively. If a straight catheter urine sample is taken, ≥ 104cfu/mL can be considered relevant. For an asymptomatic patient, two consecutive urine cultures (at least 24 hours apart) yielding ≥ 105cfu/mL of the same micro-organism are required. The requirement for pyuria is ≥ 10 WBC per high-power field (x 400) in the resuspended sediment of a centrifuged aliquot of urine or per mm3in unspun urine. A dipstick method can also be used for routine assessment, including a leukocyte esterase test, haemoglobin and probably a nitrite reaction. Sterile pyuria is commonplace in patients with indwelling bladder catheters.
Microbiology
Spectrum and Antibiotic Resistance
Patients with a complicated UTI, both community and hospital-acquired, tend to show a diversity of microorganisms with a higher prevalence of resistance against antimicrobials, and higher rates of treatment failure if the underlying abnormality cannot be corrected. However, the presence of a resistant strain on its own is not enough to define a complicated UTI. Urinary abnormality (anatomical or functional) or the presence of an underlying disease predisposing to a UTI is also necessary.
A broad range of bacteria can cause a complicated UTI. The spectrum is much larger than with an uncomplicated UTI and the bacteria are more likely to be antibiotic-resistant (especially in a treatment-related complicated UTI) than those isolated in an uncomplicated UTI. Escherichia coli, Proteus, Klebsiella, Pseudomonas, Serratia spp. and enterococci are the usual strains found in cultures. Enterobacteriaceae predominate (60-75%), withE. colias the most common pathogen, particularly if the UTI is a first infection. Otherwise, the bacterial spectrum may vary from time to time and from one hospital to another.
Complicated UTIs Associated with Urinary Stones
In the subset of complicated UTIs related to urinary stones, the frequency ofE. coliand enterococci infection seems less important pathogens. In contrast, a greater portion of Proteus spp. and Pseudomonas is found. Of the urease-producing organisms, Proteus, Providencia, Morganella spp., and Corynebacterium urealyticum are predominant, but Klebsiella, Pseudomonas, Serratia and staphylococci are also urease producers to a certain extent. Among patients with staghorn calculus disease, 88% were found to have a UTI at the time of diagnosis, with 82% of patients infected with urease-producing organisms. The enzyme, urease, splits urea into carbon dioxide and ammonia. The resulting increase in ammonia in the urine injures the glycosaminoglycan (GAG) layer, which in turn increases bacterial adherence and enhances the formation of struvite crystals. These aggregate to form renal stones and incrustations on urinary catheters.
The pathogenic potential of coagulase-negative staphylococci and non-group D streptococci is controversial. Under certain circumstances, such as the presence of a stone or foreign bodies, staphylococci can be relevant pathogens. Otherwise, staphylococci are not so common in complicated UTIs (0-11%).
Treatment
General Principles
Treatment strategy depends on the severity of the illness. Appropriate antimicrobial therapy and the management of the urological abnormality are mandatory. If needed, supportive care is given. Hospitalization is often necessary depending on the severity of the illness.
Choice of Antibiotics
Empirical treatment of a symptomatic complicated UTI requires a knowledge of the spectrum of possible pathogens and local antibiotic resistance patterns, as well as assessment of the severity of the underlying urological abnormality (including the evaluation of renal function). Bacteraemia is usually reported too late to influence the choice of antibiotics. However, suspicion of bacteraemia must influence the empirical treatment. Most important for the prognosis is still the severity of the associated illness and of the underlying urological condition. Many therapeutic trials have been published on the use of specific antimicrobial therapies in complicated UTIs. Unfortunately, most reports are of limited use for the practical management of the patient in a day-to-day situation because of limitations such as:
• poor characterization of the patient populations
• unclear evaluation of the severity of the illness
• nosocomial and community-acquired infections are not accurately distinguished
• urological outcome is seldom taken into consideration.
Intense use of any antimicrobial, especially when used on an empirical basis in this group of patients with a high likelihood of recurrent infection, will lead to the emergence of resistant micro-organisms in subsequent infections. Whenever possible, empirical therapy should be replaced by a therapy adjusted for the specific infective organism(s) identified in the urine culture. Therefore, a urine specimen for culture must be obtained prior to initiating therapy and the selection of an antimicrobial agent should be re-evaluated once culture results are available.
So far, it has not been shown that any agent or class of agents is superior in a case where the infective organism is susceptible to the drug administered. Agents appropriate for empiric therapy of complicated UTI are shown in Table 7 and 9.
Hsueh PR. Consensus review of the epidemiology and appropriate antimicrobial therapy of
complicated urinary tract infections in Asia-Pacific region.
Follow-up After Treatment
The greater likelihood of the involvement of resistant micro-organisms in complicated UTIs is another feature of these infectious diseases. This is not a priori related to the urinary abnormality, but is related more to the fact that patients with a complicated UTI tend to have recurrent infection. For these reasons, prior to and after the completion of the antimicrobial treatment, urine cultures must be obtained for the identification of the micro-organisms and the evaluation of susceptibility testing.
(Printable Version of Complicated UTIs due to Urological Disorders)
Suggested Reading
1. Carson C, Naber KG. Role of fluoroquinolones in the treatment of serious bacterial urinary tract infections. Drugs 2004;64: 1359-1373. [PubMed]
2. Dumanski AJ, Hedelin H, Edin-Liljergen A, Beauchemin D, McLean RJ. Unique ability of the Proteus mirabilis capsule to enhance mineral growth in infectious urinary calculi. Infect Immun 1994; 62: 2998-3003. [PubMed]
3. Naber KG, Bishop MC, Bjerklund-Johansen TE, Botto H, Cek M, Grabe M, Lobel B, Palou J, Tenke P. EAU guidelines on the management of urinary and male genital tract infections. EAU Working Group on Urinary and Male Genital Tract Infections. European Association of Urology (EAU) Guidelines Office. 2006 edition. 1-126. [PubMed]
4. National Institute on Disability and Rehabilitation Research. The prevention and management of urinary tract infections among people with spinal cord injuries. National Institute on Disability and Rehabilitation Research Consensus Statement. January 27-29, 1992. J Am Paraplegia Soc 1992;15: 194-204. [PubMed]
5. Nicolle LE. A practical guide to the management of complicated urinary tract infection. Drugs 1997;53: 583-592. [PubMed]
6. Reid G. Biofilms in infectious disease and on medical devices. Int J Antimicrob Agents 1999; 11: 223-226. [PubMed]
7. Sharifi R, Geckler R, Childs S. Treatment of urinary tract infections: selecting an appropriate broadspectrum antibiotic for nosocomial infections. Am J Med 1996;100(Suppl 6A): 76-82. [PubMed]
8. Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med 1993; 329: 1328-1334. [PubMed]
9. Yoshikawa TT, Nicolle LE, Norman DC. Management of complicated urinary tract infection in older patients. JAGS 1996;44: 1235-1241. [PubMed]
CATHETER-ASSOCIATED UTIs
Background
Forty per cent of nosocomial infections originate in the urinary tract. The majority of patients have chronic indwelling catheters (80%). In the 1920s, Foley introduced the self-retaining catheter. However, initially it was used with open drainage, and bacteriuria was virtually universal by the end of the fourth day. With the introduction and development of plastics technology and the design of suitable receptacles, closed-catheter systems were introduced. Development of bacteriuria was delayed but still universal after 30 days. A control trial comparing open with closed catheters was never performed and quite soon it became clear that there was little point in stating the obvious and closed systems became the standard.
Risk of Bacteriuria
An indwelling catheter bypasses normal urethral host defences, so allowing continuous access of organisms to the urinary tract. Multivariate analyses have emphasized that the duration of catheterization is the most important risk factor in the development of catheter-associated bacteriuria.
Other risk factors include the following:
(a) colonization of the drainage bag, catheter and periurethral segment
(b) diabetes mellitus
(c) female patient
(d) renal function impairment
(e) poor quality of catheter care.
Pathogenesis
The urethral catheter can inhibit or bypass some defence mechanisms which would normally prevent or minimize bacteria-epithelial cell interactions, e.g. GAG layer, biofilm formation. Bacteria can enter the urinary tract in catheterized patients through the following routes:
Review Article: Tabibian JH, et al. Uropathogens and Host Characteristics. J Clin Microbiol 2008;46: 3980-3986.
At the Time of Catheter Insertion
This may be a consequence of inadequate cleansing of the introitus, distal urethra and perineum. There is unlikely to be any consequence in otherwise healthy individuals. It would be responsible for the bacteriuria seen in patients on intermittent clean catheterization, where very little attempt is made to cleanse the ‘entry points’ before introduction of the catheter. It is doubtful whether such cleansing is of any significant benefit, but introduction of organisms at the time of catheterization could be critical in hospitalized patients. Up to 20% of individuals will be colonized immediately after catheterization.
After Catheter Insertion
Long-term catheterization will promote the development of a mucous sheath developing loosely between the catheter and urethral mucosa. This provides a favourable environment for bacterial invasion and perforation.
Arguably, it is responsible for a greater proportion of bacteriuria in women (70-80%) than in men (20-30%).
In males the predominant route is through the lumen of the catheter and collecting system by retrograde spread, i.e. ascending infection against the flow of urine. The taps of the drainage bags commonly become contaminated and regular opening of these and the connecting points, which may become disconnected for the purposes of bladder washout or urine collection, will promote entry of bacteria into the system.
Biofilm Infection
Biofilm is an accumulation of micro-organisms and their nucleic acid fragments within a mucopolysaccharide medium, which together form a structured community on a solid surface. Biofilms are ubiquitous. In the context of urological practice they can be demonstrated on catheters, drainage bags and other foreign bodies and prostheses. They can also be found within renal scars at sites of chronic infection (e.g. prostatitis, epididymitis).
Methods of Catheterization and Risk of UTI
Single Catheterization - ‘in-out’
Bacteriuria develops in 1-5% of patients. The risk is increased in females, patients with retention, peripartum catheterization, prostatic obstruction, diabetes mellitus, debilitation and the elderly.
Short-term Catheterization
Between 15% and 25% of patients admitted to hospital may be catheterized between 2 and 4 days during their stay. Between 10% and 30% will develop bacteriuria. Most episodes of short-term catheter-associated bacteriuria are asymptomatic and are caused by single organisms. Most catheter-associated bacteriurias are accompanied by pyuria.
Clec'h C,et al. Does Catheter-Associated Urinary Tract Infection Increase Mortality in Critically Ill Patients. Infect Control Hosp Epidemiol 2007;28(12): 1367-73.
Long-term Catheterization
Bacteriuria with at least one strain is universal, while most patients are infected with two or more strains. The commonest infecting organism is E. coli. Another organism rarely found outside of the catheterized urinary tract is Providencia stuartti.Other associated flora include Pseudomonas, Proteus, Morganella and Acinetobacter species. Bacteriuria is polymicrobial in up to 95% of urine specimens. One-quarter of organisms in catheter urine are not present in urine simultaneously obtained by suprapubic bladder puncture, suggesting that some organisms only colonize the catheter.
Up to 50% of patients undergoing catheterization for more than 28 days’ experience recurrent encrustation and catheter blockage. Intermittent urinary retention can lead to vesicoureteral reflux and ascending complicated infection. Infecting organisms often include P. mirabilison account of its properties as a potent producer of urease, which promotes the development of struvite stones by mechanisms which include hydrolysis of urea to ammonium. Bladder catheterization for more than 10 years as in patients with a spinal injury suggests that there is an increased risk of bladder cancer.
Muder RR, Brennen C, Rihs JC, Wagener MM, Obman A, Stout JE, Yu VL. Isolation ofStaphylococcus aureusfrom the Urinary Tract: Association of Isolation with Symptomatic Urinary Tract Infection and Subsequent Staphylococcal Bacteremia. Clin Infect Dis 2006;42: 46-50.]
Alternative Methods of Urine Drainage
Prevention of catheter-associated infection may be best accomplished by finding alternatives to indwelling catheterization and perhaps treatment of bacteriuria.
- Intermittent catheterization
- Suprapubic catheterization
- Condom catheters
Treatment(Table 9)
Treatment of asymptomatic bacteriuria
Asymptomatic bacteriuria should in general not be treated as it would only select complications of resistant organisms. Obviously there are occasional exceptions:
(a) treatment may be part of a plan to control nosocomial infection by a particularly virulent organism which is prevalent in a treatment unit
(b) patients who have a high risk of serious complications (granulocytopenia)
(c) patients undergoing urological surgery or implantation of prostheses
(d) patients with recurrent catheter obstruction and persistent infection with Proteus spp.
(e) patients infected with strains causing a high incidence of bacteraemia, e.g. Serratia marcescens.
Usually, after catheter removal, the urinary tract will clear bacteria spontaneously. However, elderly females may need treatment, since bacteriuria in these patients may not resolve spontaneously.
Treatment of Symptomatic UTI
Parenteral antibiotics should be administered to catheterized patients who are febrile and ill, particularly if the blood culture is positive, though the results of culture may not become available in time to influence treatment decisions. Of course, other causes for pyrexia should be considered. Catheter removal should be considered as part of the treatment of symptomatic catheter-associated bacteriuria with the rationale that bacteria are sequestered within the biofilm coating the external and internal catheter surfaces.
After the initial administration of empirical treatment, the choice of antibiotic may need to be adjusted based on culture results of the urine and the catheter itself. Therefore before any antibacterial therapy is initiated, a urine sample for culture has to be taken. Long-term antibiotic treatment is not effective because the catheter acts as a foreign body. Urine cannot be permanently sterilized.
Prevention of Cross-infection
Periurethral bacterial flora in the mucous sheath, surfaces of the catheter and drainage system, the reservoir of contaminated urine contained within it and the skin of the patient provide a source of infection to be readily transmitted on the hands of the medical and nursing staff. This may be reduced by treating the catheterized urinary tract as an open wound, and therefore using gloves after hand washing in antiseptic solutions.
(Printable Version of Catheter-Associated UTIs)
Suggested Reading
1. Andersen JT, Heisterberg L, Hebjorn S, Petersen K, Stampe Sorensen S, Fischer-Ramussen W, Molsted Pedersen L, Nielsen NC. Suprapubic versus transurethral bladder drainage after colposuspension/vaginal repair. Acta Obstet Gynecol Scand 1985;64: 139-143. [PubMed]
2. Carapeti EA, Andrews SM, Bentley PG. Randomized study of sterile versus non-sterile urethral catheterization. Ann R Coll Surg Engl 1994;78: 59-60. [PubMed]
3. Delnay KM, Stonehill WH, Goldman H, Jukkola AF, Dmochowski RR. Bladder histological changes associated with chronic indwelling urinary catheter. J Urol 1999;161: 1106-1109. [PubMed]
4. Duffy LM, Cleary J, Ahern S, Kuskowski MA, West M, Wheeler L, Mortimer JA. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc 1995;43: 865-870. [PubMed]
5. Garibaldi RA, Burke JP, Dickman ML, Smith CB. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 1974;291: 215-219. [PubMed]
6. Goto T, Nakame Y, Nishida M, Ohi Y. In vitro bactericidal activities of beta-lactamases, amikacin and fluoroquinolones against Pseudomonas aeruginosa biofilm in artificial urine. Urology 1999;53: 1058- 1062. [PubMed]
7. Harding CK, Nicolle LE, Ronald AR, Preiksatis JK, Forward KR, Low DE, Cheang M. How long should catheter-acquired urinary tract infection in women be treated? A randomized controlled study. Ann Intern Med 1991;114: 713-719. [PubMed]
8. Jain P, Parada JP, David A, Smith LG. Overuse of the indwelling urinary tract catheters in hospitalized medical patients. Arch Intern Med 1995;155: 1425-1429. [PubMed]
9. Krieger JN, Kaiser DL, Wenzel RP. Urinary tract etiology of bloodstream infections in hospitalized patients. J Infect Dis 1983;148: 57-62. [PubMed]
10. Kunin CM, Chin QF, Chambers S. Formation of encrustations on indwelling urinary catheters in the elderly: comparison of different types of catheter materials in ‘blockers’ and ‘nonblockers’. J Urol 1987;138: 899-902. [PubMed]
11. Kunin CM, McCormack RC. Prevention of catheter-induced urinary-tract infections by sterile closed drainage. N Engl J Med 1966;274: 1155-1161. [PubMed]
12. Naber KG, Bishop MC, Bjerklund-Johansen TE, Botto H, Cek M, Grabe M, Lobel B, Palou J, Tenke P. EAU guidelines on the management of urinary and male genital tract infections. EAU Working Group on Urinary and Male Genital Tract Infections. European Association of Urology (EAU) Guidelines Office. 2006 edition. 1-126. [PubMed]
13. Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med 1982;307: 637-642. [PubMed]
14. Platt R, Polk BF, Murdock B, Rosner B. Risk factors for nosocomial urinary tract infection. Am J Epidemiol 1986;124: 977-985. [PubMed]
15. Prieto-Fingerhut T, Banovac K, Lynne CM. A study comparing sterile and nonsterile urethral catheterization in patients with spinal cord injury. Rehabil Nurs 1997;22: 299-302. [PubMed]
16. Sedor J, Mulholland SG. Hospital-acquired urinary tract infections associated with the indwelling catheter. Urol Clin of North Am 1999;26: 821-828. [PubMed]
17. Tambyah PA, Maki DG. Catheter-associated UTI is rarely symptomatic. A prospective study of 1,497 catheterized patients. Arch Intern Med 2000;160: 678-682. [PubMed]
18. Warren J, Bakke A, Desgranchamps F, Johnson JR, Kumon H, Shah J, Tambyah P. Catheterassociated bacteriuria and the role of biomaterial in prevention. In: Naber KG, Pechere JC, Kumazawa J, Khoury S, Gerberding IL, Schaeffer AJ, eds. Nosocomial and health care associated infections in urology. Plymouth: Health Publications Ltd, 2001, pp. 153-176. [PubMed]
19. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents 2001;17: 299-303. [PubMed]
20. Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982;146: 719-723. [PubMed]
Table 1. Recommended Antimicrobial Regimens for the Treatment of Acute Uncomplicated Bacterial Cystitis in Adult Premenopausal, Non-pregnant Women [Download PDF]
Substance |
Dosage |
Duration |
|---|---|---|
100 mg bid |
3 days |
|
250 mg bid |
3 days |
|
CiproXR* |
500 mg od |
3 days |
Fosfomycin trometamol |
3000 mg SD |
1 day |
250 mg od |
3 days |
|
50-100 mg tid, |
5-7 days |
|
100 mg SR bid |
||
400 mg bid |
3 days |
|
200 mg bid |
3 days |
|
Pivmecillinam |
200 mg bid |
7 days |
200 mg bid |
5-7 days |
|
160/800 mg bid |
3 days |
*Resistance rates of E.coli vary considerably within countries. These substances are only recommended for empirical therapy when the resistance rate of E. coli is < (10%-)20%.
CiproXR = ciprofloxacin sustained release; SMX = sulphamethoxazole; od = once daily; bid = twice daily; qid = four times daily; SD = single dose; SR = sustained release.
Table 2. Oral Treatment Options of Acute Uncomplicated Pyelonephritis in Adult Pre-menopausal Non-pregnant Women. [Download PDF]
Substance |
Dosage |
Duration |
|---|---|---|
500 mg bid |
7 days |
|
CiproXR |
1000 mg od |
7-10 days |
200 mg bid |
10 days |
|
400 mg od |
10 days |
|
250 mg od |
10 days |
|
400 mg od |
10 days |
|
160/800 mg bid |
14 days |
*Cefpodoxime proxetil.
TMP = trimethoprim; SMX = sulphamethoxazole; bid = twice daily; od = once daily
Table 3. Recommendations for Antimicrobial Prophylaxis of Recurrent Uncomplicated UTI in Women [Download PDF]
Agent1 |
Dose |
|---|---|
Standard regimen: |
|
| 50 mg/day | |
• Nitrofurantoin macrocrystals |
100 mg/day |
| 40/200 mg/day or three times weekly | |
| 100 mg/day | |
• Fosfomycin trometamil |
3 g/10 day |
‘Breakthrough’ infections: |
|
| 125 mg/day | |
| 200-400 mg/day | |
• Pefloxacin |
800 mg/week |
During pregnancy: |
|
| 125 mg/day | |
• Cefaclor |
250 mg/day |
1 Taken at bedtime.
Table 4. Criteria of UTI in Children
Urine specimen from suprapubic bladder puncture |
Urine specimen from bladder catheterization |
Urine specimen from midstream void |
|---|---|---|
Any number of cfu/mL (at least 10 identical colonies) |
≥ 1,000-50,000 cfu/mL |
≥ 104 cfu/mL with symptoms |
≥ 105 cfu/mL without symptoms |
Table 5. Dosing of Antimicrobial Agents in Children Aged 3 Months to 12 Years* [Download PDF]
Antimicrobial agent |
Application |
Age |
Total dosage per day |
Doses per day |
|---|---|---|---|---|
Intravenous |
3-12 months |
100-300 mg/kg BW |
3 |
|
Ampicillin |
Intravenous |
1-12 years |
60-150 (-300) mg/kg BW |
3 |
Oral |
3 months to 12 years |
50-100 mg/kg BW |
2-3 |
|
Intravenous |
3 months to 12 years |
60-100 mg/kg BW |
3 |
|
Amoxicillin/clavulanate |
Oral |
3 months to 12 years |
37.5-75 mg/kg BW |
2-3 |
Cephalexin Treatment |
Oral |
3 months to 12 years |
50-100 mg/kg BW |
3 |
Prophylaxis Cefaclor |
Oral |
1-12 years |
10 mg/kg BW |
1-2 |
• Treatment |
Oral |
3 months to 12 years |
50-100 mg/kg BW |
3 |
• Prophylaxis |
Oral |
1-12 years |
10 mg/kg BW |
1-2 |
Oral |
3 months to 12 years |
8-12 mg/kg BW |
1-2 |
|
Intravenous |
3 months to 12 years |
50-100 mg/kg BW |
1 |
|
Intravenous |
3 months to 12 years |
(50)-100 mg/kg BW |
3 |
|
Intravenous |
3-12 months |
5-7.5 mg/kg BW |
1-3 |
|
Gentamicin |
Intravenous |
1-2 years |
5 mg/kg BW |
1-3 |
• Treatment |
Oral |
1-12 years |
6 mg/kg BW |
2 |
• Prophylaxis |
Oral |
1-12 years |
1-2 mg/kg BW |
1 |
• Treatment |
Oral |
1-12 years |
3-5 mg/kg BW |
2 |
• Prophylaxis |
Oral |
1-12 years |
1mg/kg BW |
1-2 |
BW = body weight.
Table 6. Factors That Suggest A Potential Complicated UTI
|
• The presence of an indwelling catheter, stent or splint (urethral, ureteral, renal) or the use of intermittent bladder catheterization • A post-void residual urine of > 100 mL • An obstructive uropathy of any aetiology, e.g. bladder outlet obstruction (including neurogenic urinary bladder), stones and tumour • Vesicoureteric reflux or other functional abnormalities • Urinary tract modifications, such as an ileal loop or pouch • Chemical or radiation injuries of the uroepithelium • Peri- and post-operative UTI • Renal insufficiency and transplantation, diabetes mellitus and immunodeficiency |
Table 7. Antimicrobial Treatment Options for Empiric Therapy of Complicated UTI [Download PDF]
|
Antibiotics recommended for initial empirical treatment • Fluoroquinolones • Aminopenicillin plus a BLI • Cephalosporin (Groups 2 or 3a) • Aminoglycoside Antibiotics recommended for empirical treatment in case of initial failure or for severe cases • Fluoroquinolone (if not used for initial therapy) • Ureidopenicillin (piperacillin) plus BLI • Cephalosporin (Group 3b) • Carbapenem • Combination therapy: - Aminoglycoside + BLI - Aminoglycoside + fluoroquinolone Antibiotics not recommended for empirical treatment • Aminopenicillins, e.g. amoxicillin, ampicillin • Trimethoprim-sulphamethoxazole (only if susceptibility of pathogen is known) • Fosfomycin trometamol BLI = ß-lactam inhibitor |
Table 8. Criteria for The Diagnosis of A UTI, as Modified According to IDSA/ESCMID Guidelines [Download PDF]
Category |
Description |
Clinical features |
Laboratory Investigations |
|---|---|---|---|
1 |
Acute uncomplicated UTI Dysuria, in women; acute uncomplicated cystitis in women |
urgency, frequency, suprapubic pain, no urinary symptoms in 4 weeks before this episode |
≥ 10 WBC/mm3 ≥ 103 cfu/mL* |
2 |
Acute uncomplicated Fever, pyelonephritis |
chills, flank pain; other diagnoses excluded; no history or clinical evidence of urological abnormalities (ultrasonography, radiography) |
≥ 10 WBC/mm3 ≥ 104 cfu/mL* |
3 |
Complicated UTI |
Any combination of symptoms from categories 1 and 2 above; one or more factors associated with a complicated UTI (see text) |
≥ 10 WBC/mm3 ≥ 105 cfu/mL* in women ≥ 104 cfu/mL* in men, or in straight catheter urine in women |
4 |
Asymptomatic bacteriuria |
No urinary symptoms |
≥ 10 WBC/mm3 ≥ 105 cfu/mL* in two consecutive MSU cultures ≥ 24 hours apart |
5 |
Recurrent UTI (antimicrobial prophylaxis) |
At least three episodes of uncomplicated infection documented by culture in last 12 months: women only; no structural/functional abnormalities |
< 103 cfu/mL* |
MSU = mid-stream sample of urine; UTI = urinary tract infection; WBC = white blood cells. All pyuria counts refer to unspun urine. *Uropathogen in MSU culture. |
|||
Table 9. Recommendations for Antimicrobial Therapy in Urology [Download PDF]
Diagnosis |
Most frequent pathogen/species |
Initial, empirical antimicrobial therapy |
Therapy duration |
|---|---|---|---|
Cystitis acute, uncomplicated |
• E. coli • Proteus |
3 days |
|
• Fluoroquinolone* |
(1-)3 days |
||
• Fosfomycin trometamol |
1 day |
||
• Pivmecillinam |
(3-)7 days |
||
(5-)7 days |
|||
Pyelonephritis acute, uncomplicated |
• E. coli • Proteus • Klebsiella • Other enterobacteria • Staphylococci |
• Fluoroquinolone* • Cephalosporin (group 3a) Alternatives: • Aminopenicillin/BLI • Aminoglycoside |
7-10 days |
UTI with complicating factors Nosocomial UTI Pyelonephritis acute, complicated |
• E. coli • Staphylococci • Klebsiella • Proteus • Enterobacter • Other enterobacteria |
• Fluoroquinolone* • Aminopenicillin/BLI • Cephalosporin (group 2) • Cephalosporin (group 3a) • Aminoglycoside In case of failure of initial therapy within 1-3 days or in clinically severe cases: Anti-Pseudomonas active: • Fluoroquinolone, if not used initially • Acylaminopenicillin/BLI • Cephalosporin (group 3b) • Carbapenem • ± Aminoglycoside |
3-5 days after defeverescence or control/elimination of complicating factor |
• (Candida) |
|||
Prostatitis acute, chronic |
• E. coli • Other enterobacteria • Pseudomonas |
Fluoroquinolone* Alternative in acute bacterial prostatitis: • Cephalosporin (group 3a/b) |
Acute: 2-4 weeks Chronic: 4-6 weeks or longer |
Epididymitis acute |
• Enterococci • Staphylococci |
In case of Chlamydia or Ureaplasma: • Macrolide |
10 days |
Urosepsis |
• E. coli • Other enterobacteria After urological interventions – multi-resistant pathogens: • Pseudomonas • Proteus • Serratia • Enterobacter |
• Cephalosporin (group 3a/b) • Fluoroquinolone* • Anti-Pseudomonas active acylaminopenicillin/BLI • Carbapenem • Aminoglycoside |
3-5 days after defeverescence or control/elimination of complicating factor |
BLI = ß-lactamase inhibitor; UTI = urinary tract infection. *Fluoroquinolone with mainly renal excretion (see text). °Only in areas with resistance rate < 20% (for E. coli).
Figure 1. Clinical Management of Acute Pyelonephritis [Download PDF]
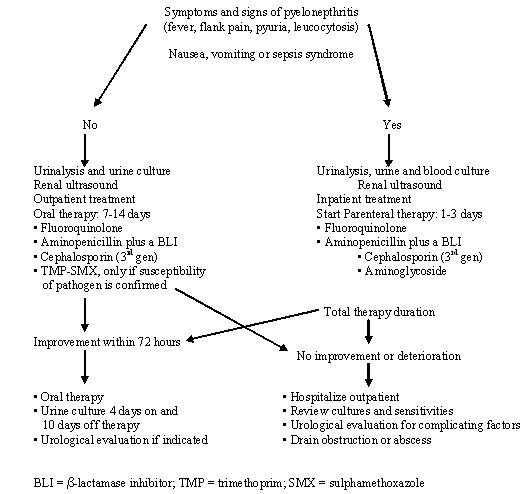
Figure 2. Schedule of Investigation of A UTI in A Child [Download PDF]
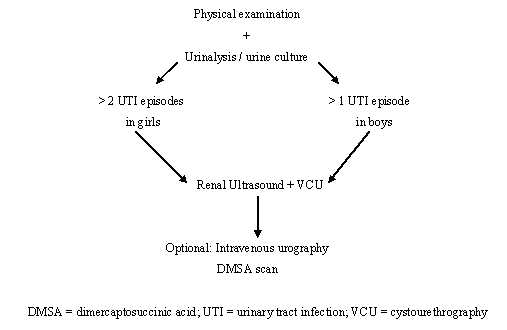
Figure 3. Treatment of Febrile UTIs in Children [Download PDF]
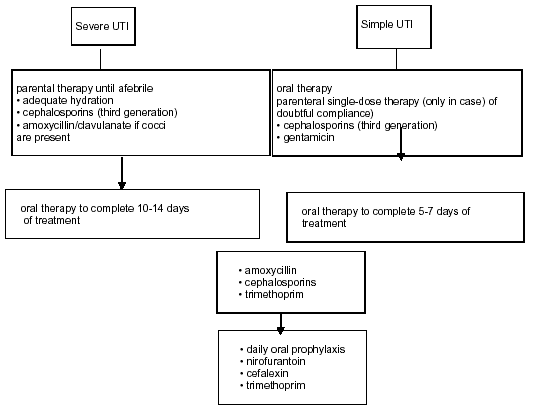
What's New
None
Reviews
None
History
None
