Fever and Abdominal Pain
Authors: Christopher J Grace, M.D., FACP, Thomas L. Husted, M.D., Joseph S. Solomkin, M.D.
The patient presenting with fever and abdominal pain generates a broad differential diagnosis involving infections of the gastrointestinal tract, solid organs of the abdominal cavity, gynecologic organs and referred pain from infections outside of the abdominal cavity. The approach to diagnosis and antibiotic selection will follow that outlined in the chapter “Empiricism-Philosophy and Practice” (Figure 1). The first step is to develop a problem list. From that problem list, a clinical differential diagnosis can be generated. Based on the clinical diagnosis, a microbiologic differential diagnosis can then be created. The severity of illness, where the infection was acquired (community or hospital based setting), underlying co-morbidities and drug allergies also need to be accounted for. Finally, empiric antibiotic selection can occur only after careful consideration of the presumed microbiology, severity of illness, and site of infection acquisition.
PROBLEM: Abdominal Pain
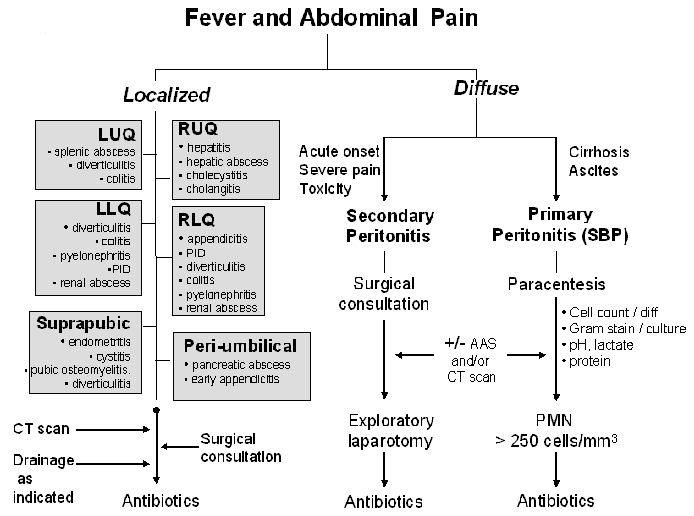
The first division point is the general nature of the pain being either diffuse or localized. See Figures 1 and 2. Diffuse abdominal pain raises the concern of a generalized peritonitis. Peritoneal irritation causes moderate to severe pain that is aggravated by motion, forcing the patient to lay still. Bowel sounds are hypoactive or absent. There is voluntary and involuntary guarding and rebound tenderness. Patients are often toxic appearing. Immune compromised patients, especially those on high dose corticosteroids, warrant heightened vigilance since the anti-inflammatory effects of the corticosteroids may mask both fever and the irritative signs of peritonitis.
Pain localized to a specific area or quadrant of the abdomen may incriminate a specific organ infection involving the liver, spleen, pancreas, kidney, bladder, colon, ovaries/salpinx or uterus (Table 2).
DIFFERENTIAL DIAGNOSIS
Diffuse Abdominal Pain (Figures 1 and 2, Table 1)
Diffuse abdominal pain may be due to bacterial infection of preexisting ascitic fluid, usually in the setting of hepatic cirrhosis, referred to as primary or spontaneous bacterial peritonitis (SBP). Organ perforation with spillage of gastrointestinal contents into the peritoneal cavity, referred to as secondary peritonitis, will also cause diffuse abdominal pain though the onset is often more acute and the patient more toxic than in those with SBP. In the cirrhotic patient with ascites presenting with diffuse abdominal pain, it is often challenging to differentiate primary from secondary peritonitis. Acute abdominal series or computerized tomography (CT) may reveal free peritoneal air in the latter condition. Secondary peritonitis is polymicrobic in nature while primary peritonitis is monomicrobic.
Table 1 outlines the clinical presentation, risk factors and diagnostic evaluation for diffuse abdominal pain (Problem # 1 from Figure 1). Patients suspected of having SBP should undergo paracentesis. Fluid should be sent for cell count and differential, protein content, lactate level, pH and Gram stain and culture. Some studies have suggested that the yield of ascitic fluid culture may be increased by injecting the fluid into blood culture bottles for incubation. SBP is diagnosed by the presence of bacteria on gram stain or culture and/or a fluid white blood cell count > 250 cells/mm3. Supporting evidence includes a protein > 3g/dl and a lactate > 25 mg/dl. Less commonly, diffuse abdominal pain may be referred into the abdominal area from pneumonia, vertebral body infection or dermatomal Herpes zoster.Clostridium difficile associated colitis should be considered in those who present with diarrhea, diffuse (or local) abdominal pain (often with associated leukocytosis) and have recent exposure to antibiotics or have been recently hospitalized. Non-infectious syndromes such as inflammatory bowel diseases, ischemic colitis, pancreatitis, polyarteritis nodosa (PAN), familial Mediterranean fever (FMF), porphyria, sickle cell crisis and malignancies can present with fever and diffuse abdominal pain.
Some infectious processes such as secondary peritonitis from gastrointestinal perforation are immediately life threatening and require urgent diagnosis and surgical intervention. Others are more often subacute in nature (viral hepatitis) and workup can proceed along at a less critical pace.
Localized Abdominal Pain (Figures 1 and 2, Table 2)
Localized abdominal pain may be due to many causes including 1) organ perforation with localized containment such as diverticulitis, 2) infections of obstructed organs such as occurs in cholecystitis and appendicitis, 3) bacteremic spread resulting in abscess formation in the kidney, liver or spleen and 4) spread of bacteria into otherwise sterile organs such as the pancreas (infected pseudocyst or pancreatic abscess) or kidney (pyelonephritis). The differential diagnosis of localized abdominal pain can be approached by location of the pain: right upper quadrant (RUQ), left upper quadrant (LUQ), right lower quadrant (RLQ) and left lower quadrant (LLQ). See Figure 2 Table 2 outlines the clinical presentation, risk factors and diagnostic evaluation for localized abdominal pain (Problem # 2 from Figure 1). As with diffuse abdominal pain non-infectious illnesses such as those summarized above in addition to acute alcoholic hepatitis, acute myocardial infarction, ectopic pregnancy and ruptured ovarian cyst may present with localized abdominal symptoms that may mimic infection.
MICROBIOLOGY
Decisions regarding antibiotic selection most often have to be made prior to the identification of the offending pathogen(s). In many instances, microbial pathogens are never isolated or cultures are performed after antibiotics have been initiated causing false negative results. In addition, an isolated pathogen may not fully represent the full microbiology of an infection. For example, Escherichia coli may be the only isolate from a blood culture in a patient with a diverticular abscess despite the polymicrobic nature of the infection. Therefore, the microbiology of an infection must be based on the clinical diagnosis.
Another important factor in thinking through the microbiology includes careful consideration of where the patient acquired the infection. Infections acquired in the community are more often caused by less resistant aerobic gram negative rods (GNR) such as E. coli,Klebsiella and Proteus spp.. Hospital acquired infections are more often caused by more resistant GNR Enterobacter spp, Serratia spp, Morganella spp, Pseudomonas aeruginosa and vancomycin resistant enterococci (VRE).
Table 3 summarizes the microbiology of peritonitis causing diffuse abdominal pain. Primary bacterial peritonitis is classically mono-bacterial. The bacteriology of secondary peritonitis depends on the site of the ruptured organ. Gastric and upper small bowel flora often includes salivary organisms such as streptococci, lactobacillus and candida spp. The distal jejunum, ileum and colon are polymicrobic including aerobic GNR, anaerobes such as bacteroides and clostridia spp., and enterococci. In the acutely ill patient with secondary peritonitis, it is often difficult to determine the origin of the perforation prior to laparotomy. Therefore it is best to assume the worst microbial case scenario and assume the perforation involves colonic bacteria.
Table 4 summarizes the microbiology of localized intraabdominal infections. Those infections related to biliary or gastrointestinal sources (diverticulitis, cholecystitis appendicitis, pancreatitic abscess) are polymicrobic. Pelvic inflammatory disease (PID) is usually a community acquired sexually transmitted illness. Pyelonephritis and renal abscesses are most often monobacterial. Abscesses in the liver can be polymicrobic (if originating from the gallbladder, appendix or intestine) or monomicrobic (if due to bacteremic seeding) depending on the source, while splenic abscesses are most often of bloodstream origin and monomicrobial.
DIAGNOSIS
In the absence of physical findings of diffuse peritonitis, diagnostic imaging with either computed tomography (CT) or ultrasound should be routinely performed in seriously ill patients with intra-abdominal infection. The urgency of investigation is dictated by the degree of hemodynamic instability present. Most patients should be evaluated within hours of clinical diagnosis. This initial imaging study has become central to therapeutic decision-making since interventional radiology has replaced operative treatment for many localized processes, including diverticular abscesses. Double contrast CT is the single best modality for fully evaluating the extent of disease in most situations. Ultrasound is also quite versatile and has the added advantage of being portable, thus allowing certain procedures to be performed in the ICU. However, ultrasonography is limited by bowel gas, body habitus, and a lower sensitivity for retroperitoneal processes or parenchymal infection. Usually the choice of modality is based on the experience and preference of the interventional radiologist.
INTERVENTIONAL THERAPY
Surgical Management of Diffuse Peritonitis
Procedures used for management of intraabdominal infection have at least five possible components: (A) drainage of any fluid collections, (B) closure of perforations of the GI tract by resection or diversion, (C) debridement of devitalized tissue, (D) drain placement, and (E) surgical wound management. Each of these elements is the subject of some debate (18, 19, 20).
Under certain circumstances, the procedure performed may not be optimal for control of infection. This may occur because of anatomic conditions do not allow the procedure of choice to be performed (e.g. extensive adhesions or tumor infiltration preventing mobilization of the bowel for resection or ostomy creation), unrecognized disease elements (e.g. multiple abscesses), misdiagnosis, or technical error such as inadvertent and unrecognized bowel perforation. Inadequate procedures may therefore be significant determinants of outcome that may make clinical cure less likely even with aggressive supportive and appropriate anti-infective therapy. These patients are a critical subgroup because they may in fact disproportionately benefit from highly effective antibiotic and anti-sepsis therapy.
A complex mix of factors affect a decision to perform a specific procedure, including variables such as the underlying condition of the patient, the acute physiologic response to infection, the duration of infection prior to diagnosis and treatment, the anatomic extent of disease, patient anatomy, and the availability of both post-operative intensive care support and radiographic reassessment. The complexity of this decision-making process makes the development of an algorithm quite difficult.
Operative management of secondary peritonitis involves immediate evacuation of all purulent collections, with particular attention to subphrenic, subhepatic, interloop, and pelvic collections. It is well established that the perforated bowel should be resected. This notion has evolved from studies over several decades of mortality following surgical treatment of perforated diverticulitis. Resection with end-colostomy was shown to decrease mortality significantly as compared to transverse loop colostomy and drainage. Despite recent reports of low rates of anastomotic dehiscence with primary anastomosis, surgeons have not universally accepted this concept because previously reported complication rates from primary anastomosis are staggering.
Controversies in the operative management of secondary peritonitis primarily surround wound closure techniques and scheduled re-laparotomy. Abdominal wall edema typically develops in patients with diffuse peritonitis secondary to colonic perforation or anastomotic dehiscence as part of a generalized syndrome of increased capillary permeability. This syndrome is worsened by the accepted need to provide aggressive restoration and in many cases supranormal expansion of intravascular volume. Primary closure of the abdominal incision in such patients may be difficult or even unwise. Increased intraabdominal pressure can result in compression of mesenteric and renal veins, leading in some instances to acute renal failure or bowel necrosis. This clinical entity is commonly referred to asabdominal compartment syndrome. To avoid this early postoperative complication, insertion of fascial prostheses can be performed. A variety of materials have been used, including Marlex, Silastic, polytetrafluoroethylene, or more recently an opened 3-L sterile intravenous bag. Each approach has its own virtues and problems (21).
Impermeable materials can exacerbate peritonitis and should be used only if planned re-laparotomy is to be undertaken. However, multiple laparotomies for abdominal sepsis have been correlated with increased mortality and poor outcomes, especially because of increased incidence of fistula formation (22). Reoperation to control intra-abdominal pathology has also been shown to cause substantial hypotension in the perioperative period due to increased cytokine release; demonstrating an inverse correlation between serum interleukin-6 levels and postoperative mean arterial pressure.
The mesh materials, particularly in patients with diffuse peritonitis, effectively create an open abdominal wound that allows continual abdominal drainage. However, these patients require extensive wound care. An alternative to definitive closure of the abdominal wall incision includes temporary abdominal closure using the clear Bogota bag. This clear 3-L saline intravenous solution bag is drained and opened to offer a one-ply impermeable dressing. This bag is sutured at the seam to the fascial edges to prevent contraction and provide a non-adherent surface to prevent development of adhesion. Additionally, with a clear bag on each side of the fascia, the edges can be closed together to provide a semi-sterile environment for the abdominal wound.
The advantages of such a temporary closure are two-fold. One, the fascia edges remain fresh, avoiding the injury encountered with re-laparotomy. Two, the bag can be cinched periodically to preclude fascial retraction, which may lead to difficult hernia closure in the future. This method has been used with success at the University of Cincinnati, often allowing for beside washout of intra-abdominal fluid collection and frequent re-assessment of the peritoneal cavity, while preserving and extending the viability of the abdominal fascia.
These techniques are often used when the patient is so hemodynamically unstable that bowel anastamoses are not performed (if resections have been done). Because of concerns for absence of sufficient mesenteric blood flow to allow for anastamotic healing, bowel ends may be left stapled. Anastamoses are then performed when shock has been reversed.
Percutaneous Abscess Drainage
Percutaneous abscess drainage and operative intervention are best viewed as complementary rather than competitive techniques. When feasible, non-operative (i.e., percutaneous) drainage of abscesses is preferable to open surgical intervention due to the initial patient condition decline that nearly universally accompanies operative manipulation of intra-abdominal infection. The exact basis for this is unclear, but a substantial proportion of patients undergoing emergency operation for intra-abdominal infection experience acute hemodynamic compromise in the early post-operative period. When used for appropriate indications, percutaneous abscess drainage is at least as effective as operation and is associated with less morbidity.
Inflammation may manifest as a phlegmon (viable inflamed tissue), a liquefied abscess, infected necrotic (nonviable) tissue, or a combination of all. Liquefied abscesses are drainable, whereas phlegma and necrotic tissue are not. Decisions regarding which modality to utilize are largely based on CT findings and require experience, clinical judgment, and careful consideration of underlying and coexistent disease processes. Close cooperation between the surgeon, interventional radiologist and other physicians involved in the patient’s care is mandatory. Specific indications for percutaneous abscess drainage have expanded significantly and now include many conditions that were previously thought undrainable, such as multiple or multiloculated abscesses, abscesses with enteric communication and infected hematomas (23, 24).
It is important to define the goals of the procedure in evaluating indications and success. Potential outcomes include cure, temporization, palliation and failure. A cure is achieved when the abscess is resolved by the drainage procedure. Temporization allows resolution of an abscess and clinical improvement, with operative intervention needed to treat the underlying cause or resect necrotic tissue. The benefits of temporizing relate to the improved physiologic condition of the patient and the reduction in the extent of infection as initial healing occurs. Palliation is achieved with improvement in the patient’s condition due to abscess drainage, despite the presence of a fatal underlying condition. We consider temporizing and palliative results to represent success.
The basic requirements for percutaneous abscess drainage include a safe route of percutaneous access and the presence of a fluid collection of drainable consistency. Bleeding dyscrasias are a relative contraindication, similar for any interventional procedure. Safe percutaneous access is attainable in most cases. It is generally possible to distinguish drainable fluid from phlegmon or necrotic tissue using a combination of imaging and fine-needle aspiration. Not all fluid collections require drainage, although it is generally required for those that are infected and for sterile collections that cause symptoms due to mass effect. This determination must be made on an individual basis.
Technical Aspects of Percutaneous Abscess Drainage
It is important that the drainage route not cross a sterile fluid collection or other infected space because of the risk of cross-contamination. Crossing the pleural space for thoracic and upper abdominal drainage carries the risk of empyema formation. It is acceptable to cross the peritoneal space to drain an extraperitoneal abscess. Placement of a catheter through the small bowel or colon should always be avoided. Transgastric drainage of lesser sac pseudocysts has been advocated by some authors and appears to be safe, although this approach remains controversial. Lesser sac collections also can be approached trans-hepatically through the left lobe of the liver, although traversing solid organs should be avoided whenever possible. Obviously, it is important to be aware of, and avoid, major vascular structures.
After catheter placement, the cavity should be evacuated as completely as possible and irrigated with saline until the fluid is clear. Initial manipulation of the catheter(s) and irrigation should be done as gently as possible to minimize the induction of transient bacteremia and subsequent potential hemodynamic instability. Immediate imaging determines the need for repositioning of the catheter, placing a larger-bore catheter or placing additional drains. For cavities that are completely evacuated at the initial drainage and for which there are no abnormal communications to viscera, simple gravity drainage generally suffices. For larger or more viscous collections and those with ongoing output due to fistulous connections, suction drainage with sump catheters is more effective. Thoracic drains always should be placed to water-seal suction to avoid the complication of simple or tension pneumothorax.
Proper catheter management following the initial placement is a critical determinant of success and requires the interventional radiologist to become an active member of the management team. Drains should be checked regularly (at least daily) to monitor the volume and nature of the output, ensure adequate function and clinical response, and quickly recognize and correct any catheter-related problems. Most authorities recommend periodic irrigation of the drains, once or several times per day, with sterile saline. This can be performed by either physicians or trained nurses. In general, irrigation with proteolytic agents or antibiotics is of no value, although fibrinolytic agents may be useful for evacuation of fibrinous or hemorrhagic collections.
No standard protocol has been established for follow-up imaging. Repeat imaging studies and catheter injections are frequently used to document progress and identify problems. It is occasionally necessary to replace or reposition drains or add additional catheters. The need for follow-up imaging studies should be determined on a case-by-case basis by monitoring clinical progress and drainage output.
Catheters should be removed when criteria for abscess resolution are met. Clinical criteria of success include resolution of symptoms and indicators of infection (fever and leukocytosis). Catheter-related criteria include a decrease in daily drainage to less than 10 mL and a change in the character of the drainage from purulent to serous. Radiographic criteria include documentation of abscess resolution and closure of any fistulous communications. If catheters are maintained until these criteria are satisfied, the likelihood of recurrence of the abscess is minimized. Although some authorities recommend gradual catheter removal over several days, we usually remove the drain in one step and have had no significant problem with recurrence. For sterile fluid collections, the drain should be removed as soon as possible, generally within 24 to 48 hours, to minimize the risk of superinfection.
Causes of Percutaneous Abscess Drainage Failure
In evaluating the causes of percutaneous abscess drainage failure, a number of factors are consistently identified. Among these factors is fluid that is too viscous for drainage or the presence of phlegma or necrotic debris. Technical modifications such as increasing the drain size and irrigation can salvage some of these drainage procedures. Recognition of phlegmon or necrotic tissue on follow-up imaging studies may lead to cessation of attempts at percutaneous abscess drainage or a modification of the expected goal. Multi-loculated collections and multiple abscesses are another cause of failure that can be minimized by using an adequate number of catheters along with mechanical disruption of adhesions with a guidewire. Fistulous communications, either unrecognized or persistent, are yet another potential cause of failure, as is drainage of a necrotic tumor mistaken by imaging to represent an abscess. Recognition of a significant soft tissue component, maintenance of a high index of suspicion and the use of percutaneous biopsies can minimize the risk of failing to appreciate the presence of tumor. Suspicious fluid also can be sent for cytologic assessment. The success rate for percutaneous abscess drainage tends to be lower in immunocompromised patients.
Low pelvic abscesses in contact with the rectum or vagina can be treated surgically by incision and drainage through these organs. The same approach can be taken using sonographic guidance, and advances in endoluminal ultrasound techniques have facilitated such procedures. Experience with ultrasound-guided transrectal and transvaginal drainage is growing, and these procedures appear to be effective and well tolerated. Good success also has been achieved in the management of tubo-ovarian abscesses complicating pelvic inflammatory disease that are refractory to medical management. In most cases, the need for hysterectomy and oophorectomy due to pelvic abscess has been outdated.
ANTIBIOTIC THERAPY (Tables 5, 6, 7)
The majority of infections addressed in this chapter require surgical intervention and/or drainage. Surgical consultation is strongly encouraged. Two sets of blood cultures should be drawn prior to initiation of antibiotic therapy.
The goals of antibiotic therapy for intra-abdominal infections that are to be treated by either percutaneous or operative intervention are: (1) to hasten the elimination of infecting microorganisms, (2) minimize the risk of recurrent intra-abdominal infection, (3) (perhaps) shorten the clinical manifestations of infection, and (4) limit the extension of abdominal wound infection (e.g., necrotizing fasciitis). In patients with localized abscesses, antibiotics reduce fever and other manifestations of systemic response, but only after a 24- to 36-hour interval. Antibiotics should be administered after fluid resuscitation has been initiated to restore adequate visceral perfusion and provide better drug distribution. Moreover, antimicrobial side effects may be exacerbated with impaired organ perfusion.
The selection of antimicrobials must take into account not only the presumed microbiology but also the general location of the acquisition of the infection, the severity of the patient’s illness and recently used antibiotics. Those who are more severely ill, especially if in septic shock, should be treated more aggressively since the margin for error is very thin. In these grave situations, it is best to select agents with activity against the most concerning pathogens such as Pseudomonas aeruginosa or VRE. If the patient is in septic shock, it may be prudent to select two agents that have activity against P. aeruginosa in addition to covering for both VRE (with daptomycin, quinupristin-dalfopristin (Synercid) or linezolid) and anaerobes (metronidazole). If a patient is on or has recently been on antibiotics it should be assumed, at least initially, that the pathogens causing the intraabdominal infection are resistant to those recently used antimicrobials and these antibiotics should be avoided. Once cultures are available, antibiotic empiricism can be more accurately tailored.
Although the appropriate role of anti-enterococcal therapy is controversial, most authorities believe that specific therapy directed towards this organism should be given only when Enterococci are the only organisms isolated from abdominal samples or are isolated from blood. Numerous prospective, blinded and randomized trials have compared regimens active against routine isolates of Enterococcus for community-acquired infections. In at least six of these studies, the comparator regimen did not have similar coverage (25, 26). Nonetheless, none of these trials demonstrated an advantage to treatment for Enterococci. Routine coverage of Enterococcus is therefore not necessary for patients with community acquired intra-abdominal infections.
Infections that are monomicrobial such as primary peritonitis or splenic or renal abscesses can be treated with a single antibiotic aimed at a specific pathogen. Polymicrobic infections often need several antibiotics each aimed at a different pathogen. For example the classic “surgical triple” antibiotic regimen of ampicillin, gentamicin and clindamycin prescribed for secondary peritonitis is aimed at enterococcus, aerobic GNR and anaerobes. Table 5 summarizes commonly used antibiotics that may cover aerobic GNR, anaerobes, enterococci and Pseudomonas species. Some drugs have activity against a single “column” of bacteria on Table 5 such as aminoglycosides against aerobic GNR. Other drugs cover more than one type of pathogen (the agent shows activity in more than one column on Table 5) and can be used singly as an equivalent of two or three antibiotics. For example, ampicillin-sulbactam will cover community acquired aerobic GNR, anaerobes and enterococci while piperacillin-tazobactam or imipenem-cilastatin can cover nosocomial aerobic GNR, anaerobes and most enterococci.
Several attempts have been made to identify clinical features in patients with peritonitis that increases the risk of adverse outcomes. These analyses have identified parameters prognostic of mortality rather than the risk of recurrent infection, including higher APACHE II scores, poor nutritional status, significant cardiovascular disease, and inability to obtain adequate source control. Similarly, patient’s immunosuppressed by medical therapy for transplantation, cancer, or inflammatory disease should receive a broader spectrum of therapy. Patients with other acute and chronic diseases may also be immunosuppressed although this is difficult to define. For such patients, antimicrobial regimens with expanded spectra may be warranted, including meropenem, imipenem/cilastatin, piperacillin/tazobactam, a quinolone plus metronidazole, or a third/fourth generation cephalosporin plus metronidazole.
Prolonged pre-hospital length of stay and prolonged (> 2 days) pre-operative antimicrobial therapy are significant predictors of failure due to recurrent infection, and suggest that organisms resistant to the standard empiric antimicrobial regimen may be responsible (16,17). Such patients should be treated for healthcare-associated infection. Appropriate regimens for such patients will mirror therapy provided for other ICU-acquired infections such as ventilator-associated pneumonia, and typically include a carbapenem and vancomycin (or coverage for VRE). Individual units may harbor multi-resistant organisms and require even more focused therapy.
Candida species often colonize the bowel lumen. Empiric therapy for community acquired infections is generally not indicated. Consideration of adding fluconazole empirically may be considered for patients with nosocomial infection and those in septic shock.Candida albicans or other Candida species are cultured from about 20% of patients with acute perforations of the gastrointestinal tract. Even when fungi are recovered, antifungal agents are unnecessary unless the patient has recently received immunosuppressive therapy for neoplasm, transplantation, or inflammatory disease, or has post-operative or recurrent intra-abdominal infection. If Candida albicans is found, fluconazole is an appropriate therapy. For fluconazole-resistant Candida species, therapy with amphotericin B, echinocandins (caspofungin, anidulofungin, micafungin) or a triazole (voriconazole or posaconazole) is appropriate. The latter two agents cause substantially less toxicity than amphotericin B and should be utilized before amphotericin B.
When selecting antibiotics for intra-abdominal infections, it is often useful to think through the most likely pathogens (the columns across Table 5) and select a single antibiotic to treat each one. Then, if one wishes, the antibiotic “triple” can be consolidated into one or two agents that will cover all the presumed pathogens.
Table 6 summarizes the antibiotic empiricism for diffuse peritonitis. Table 7 summarizes the empiricism for localized intra-abdominal infections. Hospital acquired infections are more often caused by resistant bacteria requiring more broad spectrum antibiotics. Those patients in septic shock due to an intra-abdominal infection should be treated most aggressively since the margin for error is thin. Several choices are given in each diagnosis to give the clinician options for patients with antibiotic intolerances. Keep in mind that there is no one correct antibiotic or combinations of antibiotics to treat these infections. There are many ways to skin a bacterium.
Duration of Therapy
Antimicrobial therapy for established infections should be continued until resolution of clinical signs of infection occurs, including normalization of temperature and white blood cell count, and return of gastrointestinal function. The risk of subsequent treatment failure appears to be quite low in patients who have no clinical evidence of infection at the time of cessation of antimicrobial therapy (15).
In patients who have persistent or recurrent clinical evidence of intra-abdominal infection after five to seven days of therapy, appropriate diagnostic investigation should be undertaken. This should include CT or ultrasound imaging, and antimicrobial therapy effective against the organisms initially identified should be continued. Patients with persistent or recurrent intra-abdominal infections will likely require additional intervention to achieve source control. If a patient has persistent clinical symptoms and signs, but no evidence of a new or persistent infection is uncovered after a careful investigation, termination of antimicrobial therapy is warranted. Therapy can be changed to oral antibiotics when patients are clinically improved and tolerating oral feeds.
Reading List
1. Aslam S, Musher DM. An update on diagnosis, treatment, and prevention of Clostridium difficile-associated disease. Gastroenterology Clinics of North America. 2006;35(2):315-35. [PubMed]
2. Beigi RH, Wiesenfeld HC. Pelvic inflammatory disease: new diagnostic criteria and treatment. Obstetrics & Gynecology Clinics of North America. 2003;30(4):777-93. [PubMed]
3. Blot S, De Waele JJ. Critical issues in the clinical management of complicated intra-abdominal infections. Drugs, 2005;65(12):1611-20. [PubMed]
4. Caruntu FA, Benea L. Spontaneous bacterial peritonitis: pathogenesis, diagnosis, treatment. Journal of Gastrointestinal & Liver Diseases.2006;15(1):51-6. [PubMed]
5. Green BT. Splenic abscess: report of six cases and review of the literature. American Surgeon. 2001;67(1):80-5. [PubMed]
6. Haggerty CL, Ness RB. Epidemiology, pathogenesis and treatment of pelvic inflammatory disease. Expert Review of Antiinfective Therapy. 2006;4(2):235-47. [PubMed]
7. Hyman N, Grace C. Intra-Abdominal Infections. In Medical Management of Infectious Diseases. Editor, Grace C. Marcel Decker, 2003.
8. Solomkin JS, Mazuski JE, Baron EJ, Sawyer RG, Nathens AB, DiPiro JT, Buchman T, Dellinger EP, Jernigan J, Gorbach S, Chow AW, Bartlett J; Infectious Diseases Society of America. Guidelines for the Selection of Anti-infective Agents for Complicated Intra-abdominal Infections. Clinical Infectious Diseases. 2003;37:997-1005. [PubMed]
9. Kurland JE, Brann OS. Pyogenic and amebic liver abscesses. Current Gastroenterology Reports. 2004;6(4):273-9. [PubMed]
10. Liu H, Mulholland SG. Appropriate antibiotic treatment of genitourinary infections in hospitalized patients. American Journal of Medicine. 2005;118 Suppl 7A:14S-20S. [PubMed]
11. Piccoli GB, Consiglio V, Colla L, Mesiano P, Magnano A, Burdese M, Marcuccio C, Mezza E, Veglio V, Piccoli G. Antibiotic treatment for acute 'uncomplicated' or 'primary' pyelonephritis. International Journal of Antimicrobial Agents. 2006;28 Suppl 1:S49-63. [PubMed]
12. Simpson J, Speake W. Appendicitis. Clinical Evidence. 2005;(14):529-35. [PubMed]
13. Whetsone D, Hazey J, Pofahl WE, Roth JS. Current management of diverticulitis. Current Surgery. 2004; 61(4):361-5. [PubMed]
14. Wong PF, Gilliam AD, Kumar S, Shenfine J, O'Dair GN, Leaper DJ. Antibiotic regimens for secondary peritonitis of gastrointestinal origin in adults. Cochrane Database of Systematic Reviews. 2005;(2):CD004539. [PubMed]
15. Solomkin JS, Wilson SE, Christou NV et al. Results of a clinical trial of clinafloxacin versus imipenem/cilastatin for intraabdominal infections. Ann Surg 2001:79;233. [PubMed]
16. Welschbillig-Meunier K, Pessaux P, Lebigot J, et al. Percutaneous cholecystostomy for high-risk patients with acute cholecystitis. Surg Endosc 2005:19;:1256. [PubMed]
17. Macri A, Scuderi G, Saladino E, et al. Acute gallstone cholecystitis in the elderly: treatment with emergency ultrasonographic percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Surg Endosc 2006:20;88.[PubMed]
18. Wachs ME and Wolfgang HS. Primary intestinal anastomosis is unsafe in the presence of generalized peritonitis, in Simmons RL, Udekwu AO (eds): Debates in Clinical Surgery. St. Louis, Mosby–Year Book, p228, 1991.
19. Liebert C and Deweese BM. Primary resection without anastomosis for perforation of acute diverticulitis. Surg Gynecol Obstet 1981:152;30-32. [PubMed]
20. Debas H and Thomson FB. A critical review of colectomy with anastomosis. Surg Gynecol Obstet 1972:35;747.[PubMed]
21. Buck JR, Fath JJ, Chung SK, et al. Use of absorbable mesh as an aid in abdominal closure in the emergent setting. Am Surg 1995:61;655. [PubMed]
22. Sautner T, Gotzinger P, Red-Wenzel EM, et al. Does reoperation for abdominal sepsis enhance the inflammatory host response? Arch Surg 1997:132;250. [PubMed]
23. vanSonnenberg E, D’Agostino HB, Casola G, et al. Percutaneous abscess drainage: current concepts. Radiology 1991:81;617. [PubMed]
24. Solem CA and Loftus EV. Management of refractory inflammatory bowel disease. Gastroenterol Clin North Am 2004:33;319. [PubMed]
25. Ohlin B, Cederberg A, Forssell H, Solhaug JH, Tveit E. Piperacillin/tazobactam compared with cefuroxime/ metronidazole in the treatment of intra-abdominal infections. Eur J Surg 199:65;1999. [PubMed]
26. Cohn SM, Lipsett PA, Buchman TG et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg 2000:232;254. [PubMed]
Tables
Table 1: Diagnosis of patients with diffuse abdominal pain
|
Risk Factors |
Presentation |
Evaluation |
|---|---|---|---|
Primary Peritonitis |
· Ascites due to cirrhosis, severe hypoalbuminemia from nephrotic syndrome, CHF, malignancy · CAPD catheter · VP shunts |
· Diffuse abdominal pain, fever, nausea, vomiting1 · Leukocytosis3 · Other signs and symptoms of hepatic failure5 |
· Blood cultures4 · AAS · Abdominal CT scan · Paracentesis2 · Liver enzymes7 · Amylase, lipase |
Secondary Peritonitis |
· Appendicitis · Diverticulitis · Peptic ulcer disease · Abdominal injury · GI neoplasm · Bowel obstruction · Surgical anastomotic leak · GU infections6 |
· Acute onset of diffuse abdominal pain, fever, nausea, vomiting · Abdomen rigid, hypoactive or absent bowel sounds, guarding and rebound tenderness · Leukocytosis3 |
· Blood cultures4 · AAS · Abdominal CT scan · Liver enzymes7 · Amylase, lipase · Exploratory laparotomy
|
Lower Lobe Pneumonia |
· Aspiration · Smoking · COPD |
· Upper abdominal pain · Cough, hypoxia may be present · Localized rales on chest examination |
· Blood cultures · Chest x-ray |
CT, computerized tomography; CHF, congestive heart failure; COPD, chronic obstructive lung disease; GI, gastrointestinal; GU, genitourinary; CAPD, continuous peritoneal dialysis catheter; VP, ventriculoperitoneal; AAS, acute abdominal series
1 Patients with cirrhosis and primary peritonitis may occasionally present without fever or abdominal pain. Consideration should be given to perform paracentesis inpatients with ascites.
2 Ascitic fluid should be sent for white blood cell count and differential, protein, Gram stain and culture, lactate level and pH. Fluid may also be inserted into a blood culture bottle for culture. The yield of ascitic fluid Gram stain and culture is poor. A negative test result does not exclude spontaneous bacterial peritonitis (SBP). A fluid white blood cell count > 250 cells/mm3 is diagnostic of SBP.
3 Patients with overwhelming infections may have leukopenia and marked bandemia
4 Two sets should be obtained prior to the start of antibiotics. The yield of blood cultures in secondary peritonitis approaches 75%, while it is substantially poorer in patients with SBP.
5 Encephalopathy, variceal bleeding
6 septic abortion, salpingitis, post partum endometritis
7 Liver enzymes; aspartate amino transferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, bilirubin
Table 2: Diagnosis of patients with localized abdominal pain
| Risk Factors | Presentation | Evaluation | |
|---|---|---|---|
| Hepatitis | · Alcohol ingestion · IDU · Ingestion of contaminated food | · RUQ pain · Fever, nausea, vomiting · Jaundice | · Liver enzymes7 · Serologic tests for viral hepatitis3 · Serologic tests for less common causes as indicated4 |
| Hepatic abscess | · Appendicitis · Diverticulitis · Cholecystitis · Bacteremia | · RUQ and epigastric pain · Fever, nausea, vomiting · Leukocytosis | · Blood cultures · Liver enzymes7 · RUQ ultrasound · CT scan |
| Cholecystitis1 | · Gallstones · Trauma, burns | · Postprandial RUQ and epigastric pain · Fever, nausea, vomiting · (+) Murphy’s sign2 · Leukocytosis | · Blood cultures · RUQ ultrasound5 · Liver enzymes7 · Amylase, lipase |
| Cholangitis | · Obstruction of the biliary tree from gallstones, malignancy, surgery | · RUQ pain6 · Fever, nausea, vomiting · Jaundice · Leukocytosis | · Blood cultures · RUQ ultrasound · Liver enzymes7 · Amylase, lipase |
| Appendicitis | · Generally none · Foreign bodies · Tumor · Strictures · Parasitic infection8 | · Periumbilical pain migrating to RLQ · Fever, nausea, vomiting · Leukocytosis | · CT scan |
| Diverticulitis | · Diverticulosis | · LLQ pain9 · Fever, nausea, vomiting · Leukocytosis | · Blood cultures · CT scan |
| Splenic abscess | · Bacteremia · Endocarditis · Sickle cell disease · IVDA | · LUQ pain referred to left shoulder · Fever, nausea, vomiting · Leukocytosis | · CT scan · CXR11 |
| Colitis10 | · Contaminated food and water · Antibiotics · Recent hospitalization | · Diarrhea, hematochezia · RLQ, LLQ pain · Fever · Leukocytosis | · Stool culture10 · Fecal leukocytes · Clostridium difficiletoxin assay |
| Pelvic Inflammatory Disease | · Young age and sexual active12 · New sexual partner · Bacterial vaginosis · IUD | · RLQ, LLQ pain · Fever · Leukocytosis | · Bimanual pelvic examination · Pelvic ultrasound · CT scan |
| Endometritis | · Pregnancy13 | · Suprapubic pain · Fever · Leukocytosis | · Bimanual pelvic examination |
| Pancreatic abscess | · Pancreatitis | · Periumbilical and back pain · Fever · Leukocytosis | · Blood cultures · Liver enzymes7 · Amylase, lipase · CT scan |
| Renal abscess | · Kidney stones · Ureteral obstruction · DM · Bacteremia | · Flank and back pain · Fever · Leukocytosis | · Blood cultures · Urine culture · Renal ultrasound · CT scan |
| Pyelonephritis | · Kidney stones · Ureteral obstruction · DM | · Flank and back pain · Fever, nausea, vomiting · Leukocytosis | · Blood cultures · Urine culture · Renal ultrasound |
IDU, injection drug use; RUQ, right upper quadrant; RLQ, right lower quadrant; LLQ, Left lower quadrant; LUQ, left upper quadrant; CT, computerized tomography; DM, diabetes mellitus; CXR, chest x-ray; IUD, Intrauterine contraceptive devices
1 95% due to gallstones. Acalculous cholecystitis can be seen after trauma, surgery, burns and in those with HIV infection, immune suppression and DM
2 Murphy’s sign: inspiratory arrest during palpation of the RUQ. Named after John B. Murphy (1857- 1918), a Chicago, Illinois surgeon.
3 Hepatitis A virus IgG and IgM antibody, Hepatitis B surface antigen (HBsAg), Hepatitis B surface antibody (HBsAb), Hepatitis B core antibody (HBcAb), Hepatitis C virus antibody.
4 IgM, IgG antibody for cytomegalovirus (CMV), monospot for Epstein Barr Virus (EBV) infection, antibody for human immunodeficiency virus (HIV) infection
5 Thickened gallbladder wall, pericholecystic fluid, (+) sonographic Murphy’s Sign
6 The classic Charcot’s triad of RUQ pain, fever and jaundice is seen in less than 20% of patients.
7 Liver enzymes: aspartate amino transferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, bilirubin
8 Enterobius vermicularis, Ascaris lumbricoides, Strongyloides stercoralis
9 Pain may be RLQ or suprapubic depending on the position of the colon and location of the inflamed diverticula.
10 Including food borne bacterial colitis from Campylobacter, Salmonella, Shigella, Escherichia. Coli 015H7 and antibiotic related Clostridium difficile.
11 May reveal left lower lobe atelectasis, effusion, elevated left hemidiaphragm
12 Pelvic inflammatory Diseases (PID) are often due to sexually transmitted pathogens such as Neisseria gonorrhoeae or Chlamydia trachomatis.
13 Seen more often with cesarian section, ruptured membranes for > 6 hours, multiple cervical examinations and chorioamnionitis.
Table 3: Microbiology of diffuse peritonitis
Clinical Diagnosis |
Community acquired |
Hospital acquired |
|---|---|---|
Primary Peritonitis1 |
· E. coli, Klebsiella pneumoniae,Proteus spp, Enterobacter spp OR OR · Streptococci, enterococci |
· Resistant E. coli, Klebsiella spp, Proteus spp, Enterobacter spp OR
|
Secondary Peritonitis2 |
· E. coli, Klebsiella spp, Proteus spp, Enterobacter spp AND AND · Anaerobes including Bacteroides,Clostridium, Prevotella
|
· Resistant E. coli, Klebsiella spp, Proteus spp, Enterobacter spp, P. aeruginosa, Serratia, Acinetobacter AND AND · Anaerobes including Bacteroides, Clostridium, Prevotella AND · Candida spp. |
1Primary peritonitis is most often monomicrobial. One third of patients have negative cultures from paracentesis. Anaerobic bacteria are uncommon and isolation should raise the concern of secondary peritonitis.
2 Secondary peritonitis is polymicrobic involving aerobic gram negative rods (GNR), enterococci and anaerobes.
Table 4: Microbiology of localized intra-abdominal infections
Clinical Diagnosis |
Community Acquired |
Hospital Acquired |
|---|---|---|
Diverticulitis |
· Enteric GNR1 AND AND · Anaerobes3 |
· Resistant Enteric GNR2 AND · Enterococci including VRE AND · Anaerobes3 |
Appendicitis |
Same as diverticulitis |
Same as diverticulitis |
Pancreatic abscess |
Same as diverticulitis |
Same as diverticulitis |
· Enteric GNR1 · Anaerobes3,10 |
· Resistant Enteric GNR2 · Anaerobes3,10 |
|
Hepatic abscess4 |
· Enteric GNR1 AND/OR · Enterococci AND/OR · Anaerobes3 OR · S. aureus, Streptococci,Candida, Yersinia5 OR |
· Resistant Enteric GNR2 AND/OR · Enterococci including VRE AND/OR · Anaerobes3 OR · S. aureus, Streptococci, Candida, |
Splenic abscess7 |
· S. aureus, Streptococci |
· Resistant E. coli, Klebsiella spp, Proteus spp, Enterobacter spp,P. aeruginosa, Serratia,Acinetobacter |
Colitis |
· Campylobacter jejuni,Salmonella spp, Shigella spp, E. coli 0157:H7, Vibrio parahaemolyticus, Yersinia enterocolitica 8 |
· Clostridium difficile9 |
Pelvic Inflammatory Disease |
· Enteric GNR1 and Anaerobes3 |
Not applicable |
Endometritis |
· Enteric GNR1 and Anaerobes3 |
· Resistant Enteric GNR2 and Anaerobes3 · Streptococcus agalactiae |
Renal abscess |
· E. coli, Proteus mirabilis, Klebsiella pneumoniae · S. aureus, streptococci |
· Resistant Enteric GNR2 · Enterococci including VRE · Candida spp |
· E. coli, Proteus mirabilis, Klebsiella pneumoniae |
· Resistant Enteric GNR2 · Enterococci including VRE · Candida spp |
|
Hepatitis |
· Viral hepatitis A, B,C |
· CMV |
GNR, Gram negative rod; CMV, cytomegalovirus; VRE, vancomycin resistant enterococci; MRSA, methicillin resistant Staphylococcus aureus
1 E. coli, Klebsiella spp, Proteus spp, Enterobacter spp
2 Resistant E. coli, Klebsiella spp, Proteus spp, Enterobacter spp, P. aeruginosa, Serratia, Acinetobacter
3 Bacteroides, Clostridium, Prevotella, anaerobic Streptococcus
4 Most often polymicrobic originating from infections of the hepatobiliary tree, appendicitis, diverticulitis.
5 May be monobacterial due to endocarditis or bacteremia.
6 Related to travel outside of the United States
7 Most often monobacterial related to bacteremia and endocarditis. 25% are polymicrobic.
8 Foodborne
9 Related to antibiotic usage.
10 Less often isolated except in patients with biliary-intestinal anastomosis.
Table 5: Relative activities1 of antimicrobial agents used to treat intra-abdominal infections
|
Non-Pseudomonas Gram-Negative Aerobes |
Gram-Negative Anaerobes |
||
|---|---|---|---|---|
Aminoglycoside |
+++ |
- |
+++ |
- |
++ |
++ |
- |
+++ |
|
+ |
- |
- |
+++ |
|
+++ |
- |
+++ |
- |
|
++ |
++ |
- |
- |
|
++ |
- |
- |
- |
|
++ |
- |
- |
- |
|
+++ |
- |
++ |
- |
|
- |
+++ |
- |
- |
|
Co-trimoxazole |
++ |
- |
- |
- |
++ |
+++ |
- |
- |
|
Imipenem-cilastatin |
+++ |
+++ |
+ |
++2 |
+++ |
+++ |
++ |
++ |
|
- |
+++ |
- |
- |
|
+++ |
++ |
+ |
++ |
|
++ |
++ |
++ |
++ |
|
+++ |
+++ |
+++ |
+++ |
|
- |
- |
- |
+++ |
Adapted from Medical Management of Infectious Diseases, Ed C Grace, Marcel Dekker, 2003
1- No activity, + Limited activity, ++ Moderate activity, +++ High activity
2 activity against E. faecalis but not E. faecium
Table 6: Antibiotic empiricism for diffuse peritonitis
Clinical Diagnosis |
Community Acquired |
Hospital Acquired |
|
|---|---|---|---|
Primary Peritonitis |
OR OR OR OR · Cotrimoxazole |
· Cefepime OR · Levofloxacin |
· Cefepime AND · Levofloxacin AND |
Secondary Peritonitis due to gastrointestinal perforation |
· Ampicillin-sulbactam OR
OR
· Ceftriaxone AND Metronidazole
|
· Cefepime AND AND Vancomycin OR · Piperacillin-tazobactam OR · Imipenem-cilastatin +/- |
· Cefepime AND AND Metronidazole AND Vancomycin AND Fluconazole |
Table 7: Antibiotic empiricism for localized intra-abdominal infections
Clinical Diagnosis |
Community Acquired |
Hospital Acquired |
|
|---|---|---|---|
Diverticulitis |
OR AND
OR
OR
|
· Cefepime AND Metronidazole AND
OR OR · Imipenem-cilastatin |
· Cefepime AND Ciprofloxacin AND Metronidazole AND Vancomycin |
Appendicitis |
same as diverticulitis |
same as diverticulitis |
same as diverticulitis |
Pancreatic abscess |
same as diverticulitis |
same as diverticulitis |
same as diverticulitis |
same as diverticulitis |
same as diverticulitis |
same as diverticulitis |
|
Hepatic abscess |
same as diverticulitis |
same as diverticulitis |
same as diverticulitis |
Splenic abscess |
· Vancomycin AND Ceftriaxone
OR
· Ampicillin-sulbactam
OR · Ertapenem |
· Vancomycin AND Cefepime
OR · Piperacillin-tazobactam
OR · Imipenem-cilastatin |
· Vancomycin AND Cefepime
OR · Piperacillin-tazobactam
OR · Imipenem-cilastatin |
Colitis |
· Ciprofloxacin
OR
· Moxifloxacin
OR
· Co-trimoxazole
OR
· Metronidazole1 |
· Metronidazole1 |
· Metronidazole1 |
Pelvic Inflammatory Disease |
· Ampicillin-sulbactam AND
OR · Cefotetan AND Doxycycline
OR · Levofloxacin AND Metronidazole |
· Piperacillin-tazobactam AND Doxycycline
OR · Levofloxacin AND Metronidazole |
· Piperacillin-tazobactam AND Doxycycline
OR · Levofloxacin AND Metronidazole |
Endometritis |
· Ceftriaxone orGentamicin AND
OR · Ampicillin-sulbactam |
· Piperacillin-tazobactam
OR · Imipenem-cilastatin |
· Cefepime AND Metronidazole AND Vancomycin
OR · Piperacillin-tazobactam
OR · Imipenem-cilastatin |
Renal abscess |
· Ceftriaxone |
· Cefepime AND Vancomycin |
· Cefepime AND Ciprofloxacin AND Vancomycin |
Pyelonephritis |
Same as renal abscess |
Same as renal abscess |
Same as renal abscess |
1 for Clostridium difficile colitis
Figure 1: Approach to the Patient with fever and Abdominal Pain
RUQ, right upper quadrant; RLQ, right lower quadrant; LLQ, Left lower quadrant; LUQ, left upper quadrant; AAS. Acute abdominal series; CT, computerized tomography; PID, pelvic inflammatory disease
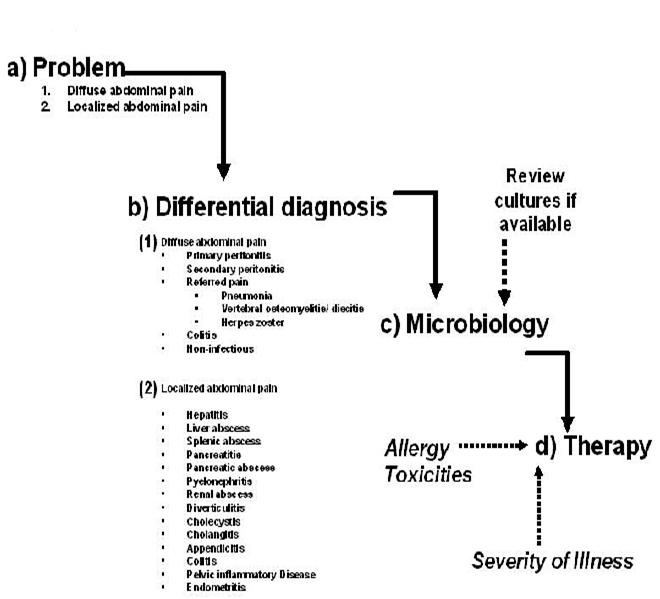
Figure 2: Sequential process for thinking through the diagnosis, microbiology and therapy of a patient with fever and abdominal pain
What's New
Ho VP. et al. Antibiotic regimen and the timing of prophylaxis are important for reducing surgical site infection after elective abdominal colorectal surgery. Surg Infect (Larchmt). 2011 Aug;12(4):255-60.
Guided Medline Search For:
Reviews
Solomkin JS, et al. Diagnosis and Management of Complicated Intra-Abdominal Infection In Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Disease Society of America. Clin Infect Dis 2010;50:133-164.

