Clostridium difficile
Authors: Hoonmo L. Koo, M.D., M.P.H. and Daniel M. Musher, M.D.
MICROBIOLOGY
Clostridium difficile is an anaerobic, gram-positive spore-forming rod-shaped bacterium. Culture of C. difficile requires strict anaerobic conditions and pre-reduced media. When cultivated on the selective CCFA (cycloserine-cefoxitin-fructose agar) media, colonies of C. difficile on CCFA are flat and yellow, with a ground-glass appearance and a surrounding yellow halo. Sporulation is important to the capacity of this organism to cause disease, as the spores persist on environmental surfaces, and are resistant to a number of disinfectants and antibiotics, thereby facilitating transmission.
EPIDEMIOLOGY
Several risk factors are known to predispose to C. difficile infection (CDI). Prior antibiotic therapy is foremost and, until recent years, cases not associated with prior antibiotics were almost unheard of. Advancing age, institutionalization and/or debilitation (e.g., hospitalization, significant comorbidities, a bed-ridden state, or residence in a long-term care or rehabilitation facility), and use of effective gastric antacids (H2 blockers and proton pump inhibitors) are other well-documented risk factors (48). C. difficile is also emerging as an important cause of healthcare- and community-associated diarrhea in children (149). In the past few years, cases of CDI have increasingly been documented in otherwise healthy outpatients who are not taking antibiotics (5). A full explanation for this phenomenon has not been provided, but increased use of laboratory testing in outpatients has certainly contributed.
The association of CDI with healthcare facilities reflects the facts that: elderly and debilitated persons reside in them; a varying proportion of these residents are incontinent of feces; antibiotics are used frequently; C. difficile is transmissible; and hospitals and other chronic-care institutions are major reservoirs for toxigenic strains of this organism (50). CDI is endemic in most tertiary care hospitals, and large outbreaks continue to occur in these settings (110). Surveillance of hospitalized patients indicates that C. difficile colonization is common, and careful epidemiological typing studies have demonstrated that the majority of these organisms are acquired exogenously (67, 91, 119). In fact, the risk of being colonized by C. difficile is directly associated with the length of hospital stay (26). In one study, after three weeks of hospitalization, one-third of initially non-colonized patients were culture-positive, the majority of whom were asymptomatic (26). However, a recent epidemiological study using more sensitive genetic analysis of C. difficile isolates with whole genome sequencing observed that only one-third of isolates were related to C. difficile from a previously symptomatic patient (42). Sources of the remaining two-thirds of acquired C. difficile were unclear but may reflect the importance of reservoirs in the community or asymptomatic hospitalized patients.
Clostridium difficile infections were originally recognized in patients who were treated with clindamycin and, for several decades thereafter, were almost exclusively associated with antimicrobial therapy (87). Disruption of indigenous bacterial flora in the intestinal tract by antimicrobial therapy (or, occasionally, by chemotherapy) is a critical element in the pathogenesis of infection (145). With the understanding that this infection is a complication of antimicrobial therapy, an important therapeutic intervention is discontinuation of the offending drug when possible (52). Simply discontinuing the offending agent may be the only intervention necessary in a small proportion of patients, generally those with mild infection (130). More commonly, however, in the absence of specific therapy, C. difficile infection is prolonged. Clindamycin remains the most likely antibiotic to cause CDI. The quinolones have also been implicated out of proportion to their use, followed by third-generation cephalosporins, but virtually all antibacterial drugs have been implicated. It is important to note, however, that increasing numbers of cases in recent years have occurred outside of health-care settings and have been unassociated with prior antibiotic use (3, 24). Contagion within the community has been documented (24, 102). Use of drugs that suppress gastric acid production has been particularly prominent in these subjects. Contamination of food may also have a role in transmission of C. difficile for these community acquired cases (56). It is uncertain whether unique strains of C. difficile are responsible for community acquired CDI in persons traditionally considered at low risk for this disease (5).
Geographically dispersed outbreaks caused by a putatively more virulent and previously uncommon strain of C. difficile were reported to occur first in North America beginning in 2000, followed by northern Europe (33, 89)). The strain was characterized by several methods, leading to its characterization as restriction endonuclease (REA) type BI, pulsed field gel electrophoresis (PFGE) type NAP1, polymerase chain reaction (PCR) ribotype 027, and toxinotype III; it is commonly referred to as BI/NAP1/027(89). Similar to other C. difficile, BI/NAP1 strains produce the two traditional toxins (A and B), but these strains produce much greater amounts of toxin, probably resulting from an 18-base pair deletion in a repressor tcdC gene (89). Warny and colleagues (141) demonstrated that 16- and 23-times more toxin A and B, respectively, were produced in vitro compared with the common toxinotype 0 strain used as a laboratory control. In addition to the two traditional toxins, BI/NAP1strains also harbor a previously uncommon binary toxin gene (noted to be present in 6% of a historical sample of clinical isolates) (89).The structure and function of this toxin is similar to that of other binary toxins, such as iota toxin found in C. perfringens. Nearly all BI/NAP1strains are resistant to fluoroquinolones (82,89).
Incidence and Mortality
The incidence of CDI and the associated mortality have steadily increased (40). Deaths associated with CDI in the United States rose from 5.7 per million persons in 1999 to 23.7 per million in 2004 (40, 117). Epidemiologic surveillance data that might demonstrate the continuation of these trends after 2005 are currently unavailable.
CLINICAL MANIFESTATIONS
Exposure to C. difficile may lead to asymptomatic colonization or infection. Infection is associated with a spectrum of clinical manifestations including non-specific watery diarrhea, severe diarrhea, fever, leukocytosis, pseudomembranous colitis and toxic megacolon (50). Asymptomatic colonization by toxin-producing C. difficile stimulates production of circulating antibody to C. difficile toxins that is thought to protect against clinical disease (74). Typically, CDI presents as watery, non-bloody diarrhea in persons who, based on epidemiologic considerations noted above, are at risk for infection. Fever, cramping abdominal pain, and leukocytosis are common. In the more severe manifestations of C. difficile disease, patients may also have abdominal distention and tenderness with or without ascites. Imaging studies may show thickened colonic wall, ascites, or marked colonic dilatation consistent with toxic megacolon. Endoscopic visualization of pseudomembranes in this setting is diagnostic of C. difficile disease. Some patients with severe C. difficile disease may not have diarrhea due to ileus or toxic megacolon and may be misdiagnosed with ischemic bowel or some other intra-abdominal catastrophe. Blood cultures are rarely positive for C. difficile (77), although patients with CDI may have transient bacteremia due to translocation by other colonic bacteria (133).
LABORATORY DIAGNOSIS
Laboratory diagnosis of CDI has evolved rapidly in the past few years. Culture of feces to detect toxigenic C. difficile in a symptomatic patient was the original diagnostic technique and remains is the most sensitive one, but it is most labor intensive and takes 4-5 days to yield a final result. Stool culture requires an additional toxin assay because both toxigenic and non-toxigenic C. difficile can be detected.
Soon after CDI was recognized, cytotoxicity assays were developed to detect C. difficile toxin in fecal samples. These were regarded as extremely reliable – in fact, for years, they were called the “gold standard.” This technique is also labor-intensive, requiring maintenance of tissue-cultured cells in vitro, a skilled technician and an inverted microscope, and the final reading can not be reported until 48 hours have elapsed. Accordingly, an enzyme-linked immunosorbent assay (EIA) was developed to detect C. difficile toxins. At first, several specimens had to be freshly obtained and sent for analysis, and the EIA was, at best, 75-80% sensitive compared to cytotoxicity. In more recent years, commercial EIA tests have been 92-97% sensitive and specific when compared to cytotoxicity, and rapid EIA tests based on individual kits similar to those used in pregnancy tests yield similar results (100). Some laboratories have found it advantageous to screen fecal samples for glutamate dehydrogenase, since this enzyme is uniformly produced by toxigenic and non-toxigenic C. difficile. The absence of glutamate dehydrogenase detection is associated with a high negative predictive value and allows for laboratories to study only GDH-positive samples for the presence of toxin.
A polymerase chain reaction (PCR) technique to detect the C. difficile toxin genes has been commercially available since 2010. Earlier studies reported greater sensitivity with PCR for detecting toxigenic C. difficile than ELISA and high specificity (123). Musher et al showed that PCR increased the diagnostic sensitivity of ELISA by about 50% (101). The cost for each individual PCR test is substantially greater than each EIA, but this cost may be offset because repeat PCR tests do not have to be done on negative samples. Not surprisingly, as an increasing number of clinical microbiology laboratories in the US have switched from ELISA to PCR, the reported rates of CDI have significantly risen (57). However, recent studies suggest that adverse clinical outcomes of CDI may be associated with the detection of toxin in feces rather than PCR or toxigenic culture identification of toxigenic C. difficile that have the potential to produce toxin (81, 116). Multi-step algorithms utilizing PCR or glutamate dehydrogenase as a screening test, followed by a confirmatory test such as a toxin ELISA have been proposed to increase the specificity and positive predictive value of C. difficile testing (144). Further studies are necessary to ascertain the optimal CDI diagnostic method.
PATHOGENESIS
C. difficile infection has been called a "three-hit" disease (Figure 1) (66). Patients are made susceptible by exposure to antimicrobials and/or other epidemiologic considerations (first hit). If they are then exposed to, and become colonized with, toxigenic strains of C. difficile (second hit), they may or may not develop symptomatic illness depending on the presence of another factor (third hit) which may relate to host susceptibility factors such as the immune response to toxin A and B (74).
C. difficile infection is a toxin-mediated disease and two, large, single-unit exotoxins are produced by most pathogenic strains. Both toxin A, an enterotoxin, and toxin B, a potent cytotoxin, stimulate fluid secretion and cause mucosal damage and inflammation. More recent studies indicate that toxin B acts synergistically with toxin A to cause CDI (86). These relatively homologous toxins probably have the same intra-cellular mode of action, but with different cell receptor specificity. Both toxins cause cell rounding and death as a consequence of glycosylation of small GTP-binding proteins of the Rho subfamily that are involved in the organization of the cell cytoskeleton (137). Subsequent pathogenic events may involve disruption of epithelial cell tight junctions and the pro-inflammatory effects of the toxins on leukocytes and monocytes.
SUSCEPTIBILITY IN VITRO AND IN VIVO
Treatment
The principal consideration in evaluation susceptibility of C. difficile to an antimicrobial agent is the achievable antimicrobial concentrations at the site of infection- the colonic mucosa. Nonabsorbable antibiotics such as vancomycin, rifaximin, and fidaxomicin are excellent examples. In an in vitro study of the activities of 15 antimicrobial agents against 110 clinical isolates of toxigenic C. difficile collected from 1983 to 2004, Hecht et al (60) showed uniform susceptibility to achievable colonic concentrations of metronidazole, vancomycin, nitazoxanide, fidaxomicin, tigecycline and ramoplanin. However, reports of resistance to metronidazole have appeared in Spain and the United Kingdom (12, 49, 111, 112). In the study by Hecht et al, 3 isolates were highly resistant to rifaximin; resistance has appeared during treatment with this drug when it was studied in a small numbers of patients (64, 106). Other studies have shown fusidic acid, bacitracin, linezolid, daptomycin, and tigecycline to be active against C. difficile in vitro (11, 105). It remains poorly understood why certain antibiotics to which C. difficile is susceptible in vitro, such as ampicillin, imipenem or doripenem, do not cure CDI and actually predispose to it.
Vancomycin inhibits and kills C. difficile at concentrations of <4 ug/mL (60). With oral therapy, fecal vancomycin concentrations are typically in the 100 - 1000 µg/gm range (68), at which concentration this drug is bacteriostatic but not bactericidal for C. difficile (79). This observation may partially explain the failure to eradicate C. difficile following initially effective therapy and may justify the use of tapered doses in treating CDI. In contrast, metronidazole is fully absorbed in the small intestine (10,61, 68) and is thought to appear in the lumen of the colon only through exudation during inflammation. This finding may explain the high relapse rate that is observed when patients are treated with this drug; as soon as inflammation comes under control, metronidazole no longer appears in the colon in a concentration sufficient to continue to act against the infecting organisms (17).
General Considerations and Treatment for First and Second Episodes
Treatment of a First Infection
Vancomycin
Vancomycin was the first effective therapy for C. difficile diarrhea and has been the drug to which all subsequent therapies have been compared (44). Cure rates of >90% have consistently been reported when vancomycin is used at a dosage of >125 mg given orally 3-4 times daily for >10 days (Table 1) (36, 39, 85, 97, 130, 142, 146). Until the very recent approval of fidaxomicin (see below), this drug remained the only FDA-approved one for treating CDI.
Metronidazole
Soon after vancomycin was shown to be effective, the possibility of treating with metronidazole was investigated; initial results suggested that this drug was just as effective (107, 130, 142). Throughout the 1980’s and 1990’s metronidazole was used extensively to treat CDI, even without an FDA indication. In fact, this drug was generally preferred because it was far less expensive and because its use was believed to reduce the risk that vancomycin-resistant bacteria would emerge in hospital settings (2, 43, 50)).
In the 1990’s, it became apparent that metronidazole therapy was associated with a substantial number of treatment failures, probably because hospitalized patients were older, more likely to be bedridden, and suffered from more severe comorbid conditions than in previous decades. In separate studies, Musher et al (96) and Pepin et al (113) presented remarkably similar results, showing that about 25% of patients treated with metronidazole failed therapy and another 25% relapsed within 1-2 months.
Vancomycin vs. Metronidazole: Current Recommendations
The Infectious Diseases Society of America (IDSA) currently recommends metronidazole as the therapeutic agent of choice for mild CDI and vancomycin for severe CDI (29). These recommendations are based upon two recent prospective randomized controlled trials, which demonstrated significantly greater cure rates with vancomycin compared to metronidazole for severe CDI. Zar et al demonstrated that patients who had mild disease responded equally well with >90% cure rate, whereas those with severe illness were more likely to fail metronidazole than vancomycin treatment. The severity of CDI was determined by age, height of fever, serum albumin, white blood cell (WBC) count, requirement for ICU care and the presence of pseudomembranes on endoscopy (148). Louie et al (83) showed that vancomycin provided a significantly higher treatment response rate compared to metronidazole (85% vs. 65%, respectively) for severe CDI. Severity for this second trial was defined by the number of bowel movements passed in 24 hours, WBC count and severity of abdominal pain. Although a validated classification system for CDI severity is lacking, the IDSA recommends considering patients with a white blood cell count of >15,000 cells/mm3 or a rise in serum creatinine level equal to or greater than 1.5 times the baseline level to have severe CDI (29). In the absence of an established severity scoring scheme, it is incumbent for each physician to use clinical judgment in evaluating the severity of an episode of CDI. The authors of this chapter recommend that factors considered by Zar et al (148) and Louie et al (83) be considered in determining the severity of CDI.
Fidaxomicin
Fidaxomicin (DIFICID®, formerly referred to as OPT-80) is a newly released, poorly absorbed macrolide that is highly active against C. difficile but has limited activity against other intestinal organisms. Two randomized, double-blind clinical trials (4,84) have compared fidaxomicin 200 mg twice daily to vancomycin 4 times daily for 10 days in patients with CDI. Similar cure rates were demonstrated for fidaxomicin and vancomycin in both studies (88% vs 86% and 88% vs 87%, respectively). However, the recurrence rate was significantly lower in fidaxomicin-treated patients (30% vs. 43%, respectively) over 25 days of followup. This lower recurrence rate was only observed in patients infected with non-BI/NAP1 strains. Lower recurrence with fidaxomicin may be associated with less disruption of the intestinal microbiome compared to vancomycin (129). Fidaxomicin is approved by the Food and Drug Administration of the United States for the treatment of CDI. The high cost of this antibiotic (the estimated cost of a 10-day course of therapy is $2,800) limits its use in clinical practice (1).
Other Options
Teicoplanin has demonstrated efficacy similar to that achieved with vancomycin and metronidazole in two prospective clinical studies when studied at two different doses given twice a day for 10 days (36, 142). Fusidic acid has been studied prospectively and although similar, high cure rates were demonstrated, the clinical recurrence rate was higher than for those treated with teicoplanin (142). Fusidic acid was also associated with at higher rate of side effects (gastrointestinal discomfort) compared with vancomycin and teicoplanin therapy. Clinical cure rates and C. difficile eradication rates with bacitracin are somewhat lower than with vancomycin (39, 146), and bacitracin should be considered as a second line agent in the treatment of C. difficile diarrhea. Nitazoxanide, an antiparasitic drug that blocks anaerobic metabolism, has also been shown to treat CDI, comparing favorably to metronidazole and vancomycin (Table 3) (97, 98) and even curing patients who fail with metronidazole or vancomycin (92). Rifaximin has been effective in the treatment of small series of patients with recurrent CDI but, even in these few cases, resistant organisms have appeared(47, 70).
Miscellaneous Considerations
The optimal dosage of most effective treatments for C. difficile diarrhea has not been determined, but duration of therapy for at least 10 days appears necessary to achieve cure rates of 90%. Based on the available data we recommend the following dosages; metronidazole 500 mg three times daily and vancomycin 125 mg orally four times daily. Patients who respond slowly or who have had relapsing disease should receive at least 14 days of treatment. Further studies will be necessary before teicoplanin (6, 142) and fusidic acid (31,142) dosage recommendations can be made. Nitazoxanide and fidaxomicin have been studied only at a single, twice-daily dosage. The efficacy of anion-exchange binding resins, such as cholestyramine and colestipol, in the treatment of primary C. difficile diarrhea has been poor, and these drugs are not recommended.
In general, because treatment of CDI relies on nonabsorbable antimicrobial agents, such treatment is given orally. For patients who cannot take oral therapy, intravenous metronidazole should be as effective based on the above comments relating to complete absorption of this drug in the small bowel after oral administration and the appearance of the drug in inflammatory secretions in the bowel. Based on a small number of anecdotal reports, many authorities have stated that antiperistaltic agents should not be administered to patients with CDI (29, 51), although Koo et al (73) have questioned the basis for, and the relevance of this conclusion when effective anti-C. difficile treatment is co-administered. Carefully designed prospective studies need to be conducted before general recommendations can be made regarding the use of antimotility agents for C. difficile induced diarrhea (73).
Treatment of a Relapsing Infection
Historically, patients whose first episode of CDI has responded to therapy but who develop a 2nd episode have been retreated with the same therapy, an approach also endorsed by IDSA guidelines (29). A recent study conducted in the era of BI/NAP1 confirmed that 1st and 2nd episodes of CDI can be effectively treated with metronidazole (114). However in this study, regardless of choice of therapy (metronidazole or vancomycin), complication rates were higher than previously reported for either drug. Caveats to the general principle of utilizing the same therapy to manage 1st and 2nd episodes of CDI, which may influence the clinician’s decision to use a different agent for the 2nd episode, include the presence of markers for severe disease and whether or not the gastrointestinal tract is functioning (e.g., presence of ileus, toxic megacolon). As with the primary infection, metronidazole can be used for recurrent CDI when the episode is mild, but vancomycin is recommended when the recurrence meets criteria for severe disease.
Treatment of Multiply Relapsing Infections ≥3rd Episode
Recurrent disease remains a challenge for clinicians, researchers, and particularly for the patients themselves. The majority of patients with CDI respond to a first or second course of treatment. Recurrence, however, can be seen in up to 25% of cases.
The mechanism for recurrent disease is uncertain and probably varies from case to case. As mentioned above, recurrence may be a result of decreasing metronidazole fecal levels during initial resolution of diarrhea or the bacteriostatic (rather than bactericidal) effect of vancomycin on C. difficile at the high concentrations achieved during therapy (79). Alteration of the colonic flora by the initial insult that led to CDI may be perpetuated by therapy given for this disease. Metronidazole is broadly active against anaerobic bacteria. Although vancomycin is often considered as being restricted in activity to Gram positive organisms, in concentrations achieved in the colon during oral therapy, this drug suppresses Bacteriodes species, as well. Of available drugs, fidaxomicin has the most limited antimicrobial spectrum, and preliminary studies have shown relatively little disruption of the microbial flora of the colon by treatment with this drug. Persistent diarrhea may also reflect a chronic abnormality of the bowel mucosa that has been triggered by CDI. Finally, diarrhea recurrence may be due to regrowth of the original infecting strain or exogenous reinfection with a new strain (65). There is no evidence that recurrence or failure of initial treatment in the case of metronidazole is due to acquisition of resistance to the initial therapeutic agent (120). An underlying host factor responsible for relapsing disease may be the failure to develop antibody to C. difficile toxins (76).
Several strategies have been explored for managing patients who have multiple recurrences (Table 2). When interpreting the efficacy of these strategies, one must consider a number of pitfalls that cloud the interpretation of the efficacy of the intervention and limits the widespread extrapolation of the results to a broader population. Chief among these are the introduction of type 2 error and investigator bias due in part to small sample sizes and the open-label nature of the treatments in these reports.
Biotherapy
If disruption of bowel flora is the principal explanation for relapsing CDI, it would be reasonable to replace the normal bacterial flora of the colon to the extent possible. Bacteria administered in this fashion are called probiotics. Of the biotherapeutic approaches, treatment with the yeast Saccharomyces boulardii is probably the most widely studied. In the initial report of 13 patients with multiple diarrhea recurrences who received vancomycin for 10 days and S. boulardii for 28 days, 11 patients had no further recurrences (127). A subsequent randomized, placebo-controlled study showed that S. boulardii in combination with standard therapy was more effective than standard therapy alone in preventing recurrences in patients who had a history of more than one C. difficile diarrhea episode (92). In a more recent study, however, S. boulardii failed to decrease CDI recurrence compared to placebo, but a subanalysis in which the standard therapy and therapeutic dose were stratified showed a significant decrease in recurrent episodes among patients treated with high-dose vancomycin (2 gm/day) and S. boulardii compared with to high-dose vancomycin and placebo (128). Neither low-dose vancomycin nor metronidazole with or without S. boulardii was effective. Overall, the data to support the use of Lactobacillus-containing and S. boulardii-containing regimens are poor and conflicting. The best study supporting the use of S. boulardii (92) did not convince the Food and Drug Administration to approve it for this use (14). With the heterogeneity of study methodologies and patient populations, it remains exceedingly difficult to reach conclusions from the probiotic literature. Critical examinations of an earlier meta-analysis (32) of probiotic use cast doubt upon the authors’ conclusions for related reasons (15). A systematic review of probiotic efficacy indicated that the current literature does not support the use of probiotics for CDI (38). Significant discrepancies from the reported probiotic contents, including probiotic concentrations, viability, and identification of the actual probiotic strains contained exist because of the current lack of regulation of probiotic manufacturing. Moreover, so-called non-pathogenic strains of the various fungi and bacteria used in the currently marketed probiotics have caused infectious complications both in immunocompetent and immunocompromised hosts; S. boulardii has especially been implicated (23, 41, 80, 118). As a result, there is currently insufficient evidence to recommend the use of probiotics for primary or recurrent CDI treatment or prevention.
The most specific biotherapy might be the administration of a non-toxigenic strain of C. difficile. Colonization with such an organism might fill an ecologic niche that would prevent relapse of CDI. Anecdotal reports showed some success with this approach (110, 114, 121), and Merrigan et al. have reported good results in preventing relapsing CDI in the hamster model (94). Trials in human subjects are currently underway.
Infusion of Normal Feces
Infusion of feces from healthy donors via enema, colonoscope, duodenoscope or nasogastric tube has been reported to be highly effective in treating CDI. In a review of all reported cases (55) the overall cure rate exceeded 90%. Importantly, the great majority of patients who received this treatment had already failed numerous courses of therapy with available antimicrobial agents. In an open-label, randomized trial, van Nood et al administered a filtrate of feces from normal subjects via nasoduodenal tube after a course of therapy with vancomycin, which was significantly more effective in resolving recurrent CDI than a 14-day course of vancomycin or vancomycin with bowel lavage (81% vs. 31% and 23%, respectively) (136). A second infusion of normal feces may cure recurrent CDI patients who fail to respond to the initial donor feces (136, 46). Although logistic and aesthetic considerations remain obstacles to widespread use of this treatment, the growing body of literature supporting the efficacy of this therapeutic intervention is mobilizing the medical community to adopt and provide this treatment. Given the potential for transmission of infections or other diseases from donors to recipients of fecal transplantation, development of specific bacterial microbiota therapy is a necessity.
Repeated Courses of Anti-C. difficile Antibiotics
The simplest approach to managing repeated relapses of CDI is to retreat with a different drug or prolonged therapy with a single agent with either metronidazole or vancomycin. Patients often have control of symptoms, but the disease may relapse promptly once therapy is discontinued. This sequence of events is consistent with persistence of C. difficile due to disruption in the microbiome or that C. difficile spores resistant to antibiotics persist in the colon rather than with the concept that CDI somehow alters the colonic mucosa. Two weeks of rifaximin therapy have been given to a total of 8 patients with relapsing disease after symptoms were brought under control by conventional antibiotics; 7 of the 8 appeared to be cured by this therapy. One patient required a second course of rifaximin before becoming asymptomatic but developed high-level resistance to rifaximin during therapy (70).
Tapering and Pulsed Doses of Antibiotics
In observational studies, a regimen of tapering doses of vancomycin has been said to be effective in treating relapsing CDI (90). After the initial 10-14 day course of therapy with 125 mg vancomycin four times daily, 125 mg may be given twice daily for a week, then once daily for a week, and finally every other day for 2- 8 weeks (29). A study of 163 patients evaluated a variety of strategies including treatment with different vancomycin doses for 10 days, treatment which was followed by tapering doses for a mean of 21 days, or treatment with vancomycin (or no treatment) immediately followed by pulsed dosed vancomycin (a single dose of 125, 250, or 500 mg given every 3 days) for a mean of 27 days (90). Treatment followed by tapered vancomycin and treatment followed by pulsed vancomycin resulted in a significantly reduced rate of recurrence when compared with treatment alone, with the latter regimen being associated with the least amount of recurrences. Tedesco and colleagues (131) also studied vancomycin tapered regimens in 22 patients. The regimen consisted of: week 1 (500 mg/d), week 2 (250 mg/d), week 3 (125 mg/d), weeks 4-6 (pulsed dosed vancomycin 125 mg every 3 days). Collectively, these descriptive studies demonstrate that tapered and pulsed dosed vancomycin regimens seem to be effective in reducing recurrences, and prospective controlled studies are warranted. Metronidazole has also been studied in a pulsed and tapered regimen but too few patients were evaluable to draw any conclusions. In addition, prolonged metronidazole administration should be avoided due to concerns of potential neurotoxicity. In one very small case series, a rifaximin taper was used with apparent success in a 5 of 6 cases (47). The basis for pulsed and tapered regimens is that current treatments (e.g., vancomycin, metronidazole) are only effective against vegetative forms (but not spore forms) of C. difficile. By allowing for a relatively antibiotic-free period in the gastrointestinal tract, it is theorized that spore forms will germinate into vegetative forms rendering them susceptible to subsequent therapy. The other theory for the success of pulsed or tapered dosing is to allow recovery the normal intestinal microbiome by decreasing antibiotic exposure.
Monoclonal Antibody to C. difficile Toxins
A single double-blind randomized control trial appeared to show benefit from treatment of CDI in humans with monoclonal antibody to toxins A and B. The stated primary outcome of the study was the prevention of recurrence of infection. A significant and meaningful difference was demonstrated for recipients of the monoclonal antibody, but only in outpatient subjects who were less ill and had a history of multiple previous CDI episodes. Secondary endpoints of the study were the rate of resolution of symptoms, the persistence of severe symptoms, and the initial response to therapy. For these endpoints, infusion of monoclonal antibody was not beneficial. In the opinion of the authors of this chapter, the initial response rate should have been used to determine the primary outcome. It is unclear why monoclonal antibodies should fail to help bring the disease under control, yet should help to prevent a recurrence 2-3 weeks later. These concerns, in addition to the anticipated high cost of the monoclonal antibodies, will likely narrow prospective recipients of this therapy to include CDI subjects who experience multiple recurrences who fail other treatment options if phase 3 clinical trials are similarly successful (Table 3).
Intravenous Immunoglobulins
Based on anecdotal reports and observational studies, intravenous administration of “immune globulin” (IVIG), a preparation of IgG isolated from healthy controls but not specifically containing antibody to C. difficile toxins, may benefit a subgroup of patients with multiple recurrences of CDI (59, 78, 140). In two of the published studies involving a total of 7 patients, patients had low levels of serum anti-toxin A IgG (78, 140) and the patient in the third study had selective IgG1 deficiency (59). In one observational study of 14 patients (93), 6 responded clinically with no further relapse reported. The doses used ranged from 150-400 mg/kg administered as a single-dose (one patient received a second dose). The median response time was 10 days. In another study, 3 of 5 patients treated with doses of IVIG between 300 to 500 mg/kg (usually 400 mg/kg), were deemed successes, with resolution occurring within 11 days (131). However, an equally large retrospective analysis at one institution, (71) found no benefit to this therapeutic approach. In summary, IVIG has been said by some investigators to possibly provide a therapeutic option for patients with severe/relapsing disease where no other therapeutic options are available. Unfortunately, marginal efficacy, lack of data regarding the optimal dose, cost (~$U.S. 1,500/dose for a 70kg patient) (145) and frequent shortages of the preparation are significant disadvantages (134).
Toxoid Vaccine
Toxoid vaccine (Acambis Pharmaceuticals) is currently in early developmental stages (Table 3). In a very small study of three patients with recurrent CDI who had received 7-22 months of continuous vancomycin therapy, a parenterally administered C. difficile vaccine containing toxoids A and B enabled discontinuation of the vancomycin without further recurrence (125). Two of the 3 patients demonstrated increased IgG antitoxin A antibodies (3- and 4-fold increases) and an increased IgG antitoxin B antibody response (20- and 50-fold).
When Oral Therapy Is Not Possible
Although several options exist for the treatment of C. difficile diarrhea (Table 1), all well-studied regimens employ oral therapy. When the oral route is compromised, intravenous therapy may be considered. After intravenous administration of vancomycin, colonic concentrations of vancomycin are insufficient to be active against C. difficile, and there is little support for this option. Metronidazole is fully absorbed in the small intestine and gets into the colon along with inflammatory exudates; therefore, fecal concentrations of metronidazole are similar whether metronidazole is given orally or intravenously (17). Clinical experience is supported by limited reports in the literature supporting the use of intravenous metronidazole therapy for CDI (17, 45, 54, 72). This therapy alone may be inadequate in patients with severe adynamic ileus, but that may relate to the advanced state of the disease, not the available concentration of metronidazole in the colonic lumen (58). Vancomycin, 500 mg every 6 hours in a volume of 100 ml saline, has been administered by enema, with success being reported in a few cases (8, 104). The 2013 Guidelines from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) (35) recommend intravenous metronidazole for 10 days for nonsevere CDI. For severe, complicated or refractory CDI, ESCMID recommends adding intracolonic vancomycin with or without vancomycin by nasogastric tube, while the IDSA recommends adding vancomycin orally or by nasogastric tube with or without intracolonic vancomycin (29). Unfortunately, the data supporting these recommendations are sparse.
FAILURE OF MEDICAL THERAPY
Fulminant CDI
Many C. difficile strains have been shown to be responsible for fulminant CDI. Some studies have found that the so-called hypervirulent C. difficile strain (BI/NAP1) has caused more severe disease, without regard to which treatment is used (5, 33, 88, 113, 115) although others dispute that finding, particularly in non-epidemic settings (28, 138). In cases of severe CDI, patients should be monitored daily for response to therapy, including the number and consistency of bowel movements as well as for markers that might indicate a need for surgical intervention.
Severe Ileus and Toxic Megacolon
The most serious manifestation of C. difficile disease is toxic megacolon which, paradoxically, may present without diarrhea (95, 107)). Treatment of patients with toxic megacolon or severe ileus is difficult and controversial, and the mortality remains high. Various attempts have been made to achieve effective antimicrobial concentrations at the site of infection when the oral route is compromised. Although clinical data are insufficient to evaluate optimal therapy for severe CDI complicated by ileus or toxic megacolon, expert opinion advocates treatment with intravenous metronidazole combined with vancomycin by nasogastric tube and rectal enema both at dosages of 2 grams per day (53, 107, 109, 124). This strategy was used successfully at one institution in six patients with severe ileus and included vancomycin administered by nasogastric tube and by retention enema plus intravenous metronidazole(53, 107).
When Medical Therapy Fails
Further intervention is indicated in patients with toxic megacolon who are not responding to medical treatment or when colonic perforation is suspected (95). The more conservative approach is decompressive colonoscopy (123). After decompression of the colon in 8 patients with ileus and toxic megacolon, a fenestrated tube was positioned over a guidewire and the tube was perfused with a vancomycin solution. This treatment was effective in treating the colitis in several patients, and complications such as perforation were not seen. Colonic diversions and partial colectomies have been performed but mortality is high (95). Although efficacy has been difficult to assess, subtotal colectomy with sparing of the rectal stump (total abdominal colectomy) appears to be the preferred surgical option (30, 95). At one medical center during a 12-year period, 64 patients died or underwent colectomy for complicated CDI (33). The mortality in patients who underwent colectomy in this series was 57%, and the authors state that early colectomy was associated with a better outcome. Clues to the need for surgical intervention include very high WBC counts, hypotension and lactate levels of >5 mmol/L (75).
ENDPOINTS FOR MONITORING THERAPY
The principal endpoint for monitoring the success of therapy for CDIis clinical evaluation of patient symptoms. In first treatment for uncomplicated cases, an anti-C. difficile drug (Table 1) should be given for 10 days and discontinued. Although some clinicians will continue specific therapy for longer than 10 days if the precipitating antimicrobial cannot be discontinued, there are no studies to support this practice. In addition, it is unclear how long C. difficile therapy would need to be continued in this situation because antibiotic disturbances of the intestinal microbiome can persist for weeks after the discontinuation of the offending antibiotic (147). More prolonged therapy in patients who have responded to treatment has potential disadvantages, because the treatments themselves (metronidazole and vancomycin) are not active against spore forms of C. difficile and are destructive to normal commensal flora; in rare instances, these treatments have been implicated as causes of CDI (108). CDI should be treated episodically and there is no role for “prophylaxis” by continuation of metronidazole or vancomycin once the patient has experienced clinical resolution (65). Clinical resolution of CDI has not been shown to correlate with clearance of C. difficile bacteria or toxins. Positive stool cultures for C. difficile at the completion of therapy is moderately predictive of recurrence (139), but it is strongly recommended that stool cultures and toxin assays not be performed if the patient’s symptoms have resolved (29) because repeat positive test results often lead clinicians to inappropriately prolong therapy or switch therapy to a different agent. Patients who have previously relapsed or who respond slowly to therapy should probably be treated for 14 days, although there are no data specifically to support this practice, and most guidelines recommend 10-14 days of therapy.
PREVENTION
Strategies employed to control and prevent C. difficile diarrhea can be classified into those that reduce the risk transmission of C. difficile and those that reduce the risk of clinical illness if the patient is exposed.
Infection Control Measures
Numerous methods have been employed in attempts to prevent transmission including barrier precautions, gloving, handwashing, environmental disinfection, replacement of electronic rectal thermometers and treatment of asymptomatic carriers of C. difficile. Although handwashing and especially barrier precautions are probably effective, decreased rates of C. difficile diarrhea have only been demonstrated to date with glove use (20, 67), replacement of electronic thermometers with single use disposables (20), and replacement of quaternary ammonium solution with unbuffered 1:10 hypochlorite solution for environmental disinfection (9, 88). C. difficile spores are resistant to alcohol. Handwashing with soap and water is believed to physically remove spores from contaminated hands. Differences in hand-to-hand transmission of C. difficile were noted between the two methods favoring the use of handwashing with soap and water over alcohol hand rubs (63)). As a result, handwashing with soap and water is preferred over alcohol hand rubs, as recommended by the CDC and the IDSA, when caring for patients with CDI during outbreaks. It is important to note that alcohol hand sanitizers have improved compliance with hand hygiene policies in healthcare settings and should continue to be encouraged when caring for patients without C. difficile. Isolation of patients in private rooms or cohorting of patients is also recommended to decrease transmission of C. difficile spores (22, 29, 126).
Complete prevention of transmission may be impossible because of compliance with barrier methods, practical issues with hypochlorite disinfectants, and other unidentified factors. Since many patients are either treated as outpatients or are initially treated in an acute care facility and then discharged when clinically improved, patients should be advised regarding proper hand hygiene and environmental cleaning (with regard to their bathrooms at home).
Antimicrobial Stewardship
Even if prevention of transmission is incomplete, methods that reduce risk of disease should also be implemented and are potentially effective. Control of antimicrobial use has successfully reduced rates of CDI and interrupted local epidemics caused by C. difficile. Restriction of clindamycin was repeatedly shown to be effective (27, 110) in settings characterized by heavy clindamycin use and the presence of highly clindamycin-resistant strains (69). Control of second and third generation cephalosporins was also effective, particularly when these drugs were replaced with apparently less-predisposing antimicrobials, such as piperacillin-tazobactam (122). Programs that encourage diversity in antimicrobial use, and reduce unnecessary use (by eliminating redundant antimicrobials, promoting shorter courses of therapy, using a “careful watch and wait” approach to outpatient upper respiratory tract infections, as examples) may be part of a “bundled” strategy for healthcare facilities or clinics to adopt. A recent Cochrane Review emphasized that reduction in the incidence of CDI was one of the major benefits of an antimicrobial stewardship program (34). Guidelines for establishing a programmatic approach to enhance antimicrobial stewardship were recently published jointly by the IDSA and SHEA (37).
Other measures. Several studies have implicated proton pump inhibitors as predisposing to CDI (25,60), although one recent case control series has questioned this association (103). In the majority of patients, there has been no recorded indication in the medical record for the proton pump inhibitors. Cadle et al found that the incidence of recurrent CDI was 4 times greater in patients receiving a proton pump inhibitor (21). It seems prudent to avoid unnecessary usage of proton pump inhibitors in hospitalized patients.
SUMMARY
In summary, diarrhea and colitis due to C. difficile are, most often, complications of antimicrobial therapy. Unnecessary antibiotics should be avoided, especially in hospitalized persons. Once CDI is suspected, it may be helpful, if possible to discontinue the offending antibiotic (50). In hospitalized patients who have diarrheal disease and in whom CDI is suspected, empiric therapy should be started while awaiting the results of toxin assay results. This approach will decrease the degree of debilitation produced by the infection and decrease the likelihood of spread of infectious organisms. Barrier precautions and contact isolation, with handwashing with soap and water before and after patient contact, should be meticulously observed if the diagnosis is established. Metronidazole is recommended for mild CDI, but vancomycin should be used to treat severe cases. The expected time to response is 3-4 days, but many patients may respond more slowly. The usual duration of treatment is 10 days, but one might consider a longer course of therapy – e.g., 14 days – in patients who have slowly responded or who have previous relapsed. No test of cure assay is indicated in patients who have responded to therapy. In patients who fail to respond, less traditional therapeutic agents, biotherapeutic approaches, and even immunotherapy should be considered. Extensive experience with fecal replacement favors that approach when it is practicable.
REFERENCES
1. FirstWord. Optimer Pharmaceuticals to price Dificid at twice the cost of other C. Diff treatments. [online resource]. http://www.firstwordplus.com. [Accessed June 14, 2011].
2. Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44(RR-12):1–13 [PubMed]
3. Centers for Disease Control and Prevention (CDC). Surveillance for community-associated Clostridium
difficile--Connecticut, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:340-3.
4. DIFICID (fidaxomicin) [prescribing information]. 2011.
5. Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep 2005;54:1201-5. [PubMed]
6. Treatment of Clostridium difficile associated diarrhea and colitis with an oral preparation of teicoplanin; a dose finding study. The Swedish CDAD Study Group. Scand J Infect Dis 1994;26:309-16. [PubMed]
7. Aas JC, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003;36:580-5. [PubMed]
8. Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis 2002;35:690-6. [PubMed]
9. Apisarnthanarak A, Zack JE, Mayfield JL, Freeman J, Dunne WM, Little JR, Mundy LM, Fraser VJ. Effectiveness of environmental and infection control programs to reduce transmission of Clostridium difficile. Clin Infect Dis 2004;39:601-2. [PubMed]
10. Arabi Y, Dimock F, Burdon DW, Alexander-Williams J, Keighley MR. Influence of neomycin and metronidazole on colonic microflora of volunteers. J Antimicrob Chemother 1979;5:531-7. [PubMed]
11. Baines SD, Noel AR, Huscroft GS, Todhunter SL, O'Connor R, Hobbs JK, Freeman J, Lovering AM, Wilcox MH. Evaluation of linezolid for the treatment of Clostridium difficile infection caused by epidemic strains using an in vitro human gut model. J Antimicrob Chemother 2011;66:1537-46. [PubMed]
12. Baines SD, O'Connor R, Freeman J, Fawley WN, Harmanus C, Mastrantonio P, Kuijper EJ, Wilcox MH. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. Antimicrob Chemother 2008;62:1046-52. [PubMed]
13. Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009;15:285-9. [PubMed]
14. Bartlett JG. New drugs for Clostridium difficile infection. Clin Infect Dis 2006;43:428-31. [PubMed]
15. Beckly J, Lewis S. Probiotics and antibiotic associated diarrhoea. The case for probiotics remains unproved. BMJ 2002;325:901; author reply 901. [PubMed]
16. Boero M, Berti E. Morgando A, Verme G. Treatment for colitis caused by Clostridium difficile: results of a randomized, open-label study of rifaximin vs. vancomycin. Microbiologia Medica 1990;5:74-77. [PubMed]
17. Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. 1986;Gut 27:1169-72. [PubMed]
18. Borody,TJ. "Flora Power"-- fecal bacteria cure chronic C. difficile diarrhea. Am J Gastroenterol 2000;95:3028-9. [PubMed]
19. Bowden TA Jr, Mansberger AR Jr, Lykins LE. Pseudomembraneous enterocolitis: mechanism for restoring floral homeostasis. Am Surg 1981;47:178-83. [PubMed]
20. Brooks SE, Veal RO, Kramer M, Dore L, Schupf N, Adachi M. Reduction in the incidence of Clostridium difficile-associated diarrhea in an acute care hospital and a skilled nursing facility following replacement of electronic thermometers with single-use disposables. Infect Control Hosp Epidemiol 1992;13:98-103. [PubMed]
21. Cadle RM, Mansouri MD, Logan N, Kudva DR, Musher DM. Association of proton-pump inhibitors with outcomes in Clostridium difficile colitis. Am J Health Syst Pharm 2007;64:2359-63. [PubMed]
22. Cartmill TD, Shrimpton SB, Panigrahi H, Khanna V, Brown R, Poxton IR. Nosocomial diarrhoea due to a single strain of Clostridium difficile: a prolonged outbreak in elderly patients. Age Ageing 1992;21:245-9. [PubMed]
23. Cassone MP, Serra F, Mondello A, Girolamo S, Scafetti E, Pistella, Venditti M. Outbreak of Saccharomyces cerevisiae subtype boulardii fungemia in patients neighboring those treated with a probiotic preparation of the organism. J Clin Microbiol 2003;41:5340-3. 24. [PubMed]
24. Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, Farley MM, Dumyati GK,
Wilson LE, Beldavs ZG, Dunn JR et al. Epidemiology of community-associated Clostridium difficile infection,
2009 through 2011. JAMA Intern Med 2013;173:1359-67.
25. Choudhry MN, Soran H, Ziglam HM. Overuse and inappropriate prescribing of proton pump inhibitors in patients with Clostridium difficile-associated disease. QJM 2008;101:445-8. [PubMed]
26. Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis 1992;166:561-7. [PubMed]
27. Climo MW, Israel DS, Wong ES, Williams D, Coudron P, Markowitz SM 1998. Hospital-wide restriction of clindamycin: effect on the incidence of Clostridium difficile-associated diarrhea and cost. Ann Intern Med 1998;128:989-95. [PubMed]
28. Cloud J, Noddin L, Pressman A, Hu M, Kelly C. Clostridium difficile strain NAP-1 is not associated with severe disease in a nonepidemic setting. Clin Gastroenterol Hepatol 2009;7:868-73.
29. Cohen, SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431-55. [PubMed]
30. Cone JB, Wetzel W. Toxic megacolon secondary to pseudomembranous colitis. Dis Colon Rectum 1982;25:478-82. [PubMed]
31. Cronberg SB, Castor, Thoren A. Fusidic acid for the treatment of antibiotic-associated colitis induced by Clostridium difficile. Infection 1984;12:276-9. [PubMed]
32. D'Souza AL, Rajkumar C, Cooke J, Bulpitt C J. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. Bmj 2002;324:1361. [PubMed]
33. Dallal RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, Lee KK, Simmons RL. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg 2002;235:363-72. [PubMed]
34. Davey PE, Brown L, Fenelon R, Finch I, Gould G, Hartman A, Holmes C, Ramsay E, Taylor M, Wilcox M, Wiffen P. 2005. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2005;CD003543. [PubMed]
35. Debast SB, Bauer MP, Kuijper EJ; The Committee. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): update of the treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect 2013. [Epub ahead of print].
36. de Lalla F, Nicolin R, Rinaldi E, Scarpellini P, Rigoli R, Manfrin V, Tramarin A. Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother 1992;36:2192-6. [PubMed]
37. Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159-77. [PubMed]
38. Dendukuri N, Costa V, McGregor M, Brophy JM, Probiotic therapy for the prevention and treatment of Clostridium difficile-associated diarrhea: a systematic review. CMAJ 2005;173:167-70. [PubMed]
39. Dudley MN, McLaughlin JC, Carrington G, Frick J, Nightingale CH, Quintiliani R. Oral bacitracin vs vancomycin therapy for Clostridium difficile-induced diarrhea. A randomized double-blind trial. Arch Intern Med 1986;146:1101-4. [PubMed]
40. Elixhauser A, e. a. Healthcare Cost and Utilization Project: Statistical Brief #50. April 2008. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb50.pdf. Accessed March 10, 2010.
41. Enache-Angoulvant A, Hennequin C, Invasive Saccharomyces infection: a comprehensive review. Clin Infect Dis 2005;41:1559-68. [PubMed]
42. Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O'Connor L, Ip CL, Golubchik T, Batty EM, Finney JM, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med 2013;369:1195-205.
43. Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 1997;92:739-50. [PubMed]
44. Fekety R, Shah AB. Diagnosis and treatment of Clostridium difficile colitis. JAMA 1993;269:71-5. [PubMed]
45. Friedenberg F, Fernandez A, Kaul V, Niami P, Levine GM. Intravenous metronidazole for the treatment of Clostridium difficile colitis. Dis Colon Rectum 2001;44:1176-80. [PubMed]
46. Garborg K, Waagsbo B, Stallemo A, Matre J, Sundoy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 42:857-61. [PubMed]
47. Garey, KW, Jiang ZD, Bellard A, Dupont HL. Rifaximin in Treatment of Recurrent Clostridium difficile-associated Diarrhea: An Uncontrolled Pilot Study. J Clin Gastroenterol. 2008;43(1):91-3.[PubMed]
48. Garey, KW, Sethi S, Yadav Y, DuPont YL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect 2008;70:298-304. [PubMed]
49. George WL, Volpicelli NA, Stiner DB, Richman DD, Liechty EJ, Mok HY, Rolfe RD, Fd SM. 1979. Relapse of pseudomembranous colitis after vancomycin therapy. N Engl J Med 1979;301:414-5. [PubMed]
50. Gerding DN, Johnson S, Peterson LR, Mulligan ME, and Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol 1995;16:459-77. [PubMed]
51. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis 46 Suppl 2008;1:S32-42. [PubMed]
52. Gerding ND, Olson MM, Johnson S, Peterson LR, and Lee JT Jr. Clostridium difficile diarrhea and colonization after treatment with abdominal infection regimens containing clindamycin or metronidazole. Am J Surg 1990;159:212-7. [PubMed]
53. Goodpasture HC, Dolan PJ Jr, Jacobs ER, Meredith WT. Pseudomembranous colitis & antibiotics. Kans Med 1986;87:133, 146. [PubMed]
54. Gorschluter MA,. Glasmacher C, Hahn F, Schakowski, Ziske C, Molitor E, Marklein G, Sauerbruch T, Schmidt-Wolf IG. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis 2001;33:786-91. [PubMed]
55. Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011;53:994-1002.
56. Gould LH, Limbago B. Clostridium difficile in food and domestic animals: a new foodborne pathogen? Clin Infect Dis 2010;51:577-82. [PubMed]
57. Gould CV, Edwards JR, Cohen J, Bamberg WM, Clark LA, Farley MM, Johnston H, Nadle J, Winston L, Gerding DN, et al. Effect of nucleic acid amplification testing on population-based incidence rates of Clostridium difficile infection. Clin Infect Dis. 2013;57:1304-7
58. Guzman RJ, Kirkpatrick K, Forward, and Lim F. Failure of parenteral metronidazole in the treatment of pseudomembranous colitis. J Infect Dis 1988;158:1146-7. [PubMed]
59. Hassett J, Meyers S, McFarland L, Mulligan ME. Recurrent Clostridium difficile infection in a patient with selective IgG1 deficiency treated with intravenous immune globulin and Saccharomyces boulardii. Clin Infect Dis 20 Suppl 1995;2:S266-8. [PubMed]
60. Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. In vitro activities of 15 antimicrobial agents against 110 toxigenic clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents 61. Hoverstad TB. Carlstedt-Duke E, Lingaas T, Midtvedt KE, Norin H, Saxerholt, Steinbakk M. Influence of ampicillin, clindamycin, and metronidazole on faecal excretion of short-chain fatty acids in healthy subjects. Scand J Gastroenterol 1986;21:621-6. [PubMed]
62. Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784-90. [PubMed]
63. Jabbar U, Leischner J, Kasper D, Gerber R, Sambol SP, Parada JP, Johnson S, Gerding DN. Effectiveness of alcohol-based hand rubs for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol 2010;31:565-70. [PubMed]
64. Jiang ZD, Dupont HL, La Rocco M, Garey KW. In vitro activity of rifaximin and rifampin against clinical isolates of Clostridium difficile in Houston, Texas. 2009;Anaerobe. [PubMed]
65. Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis 1989;159:340-3. [PubMed]
66. Johnson S, Gerding DN. Clostridium difficile--associated diarrhea. Clin Infect Dis 1998;26:1027-34; quiz 1035-6. [PubMed]
67. Johnson S, Gerding DN, Olson MM, Weiler MD, Hughes RA, Clabots CR, Peterson LR. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med 1990;88:137-40. [PubMed]
68. Johnson S, Homann SR, Bettin KM, Quick JN, Clabots CR, Peterson LR, Gerding DN. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann Intern Med 1992;117:297-302. [PubMed]
69. Johnson S, Samore MH, Farrow KA, Killgore GE, Tenover FC, Lyra D, Rood JI, DeGirolami P, Baltch AL, Rafferty ME, Pear SM, Gerding DN. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med 1009;341:1645-51. [PubMed]
70. Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007;44:846-8. [PubMed]
71. Juang P, Skledar SJ, Zgheib NK, Paterson DL, Vergis EN, Shannon WD, Ansani NT, Branch RA. Clinical outcomes of intravenous immune globulin in severe clostridium difficile-associated diarrhea. Am J Infect Control 2007;35:131-7. [PubMed]
72. Kleinfeld DI, Sharpe RJ, Donta ST. Parenteral therapy for antibiotic-associated pseudomembranous colitis. J Infect Dis 1988;157:389. [PubMed]
73. Koo HL, Koo DC, Musher DM, DuPont HL. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis 2009;48:598-605. [PubMed]
74. Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 342:390-7. [PubMed]
75. Lamontagne F, Labbe AC, Haeck O. Lesur O, Lalancette M, Patino C, Leblanc M, Laverdiere M, Pepin J. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg 2007;245:267-72. [PubMed]
76. Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, Gerding DN, Kelly CP, Katchar K, Baxter R, Ambrosino D, Molrine D. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine 28:965-9. [PubMed]
77. Lee NY, Huang YT, Hsueh PR, Ko WC. Clostridium difficile bacteremia, Taiwan. Emerg Infect Dis 16:1204-10. [PubMed]
78. Leung DY, Kelly CP, Boguniewicz M, Pothoulakis C, LaMont JT, Flores A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr 1991;118:633-7. [PubMed]
79. Levett, P. N. 1991. Time-dependent killing of Clostridium difficile by metronidazole and vancomycin. J Antimicrob Chemother 27:55-62. [PubMed]
80. Lherm T, Monet C, Nougiere B, Soulier M, Larbi D, Le Gall C, Caen D, Malbrunot C. Seven cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive Care Med 2002;28:797-801. [PubMed]
81. Longtin Y, Trottier S, Brochu G, et al. Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program. Clin Infect Dis 2013;56:67-73
82. Loo VG, Poirier L, Miller MA, Oughton M,. Libman MD, Michaud S, A. M. Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353:2442-9. [PubMed]
83. Louie T. Clinical Success by Disease Severity: Tolevamer Phase III Results. [Abstract K-425-a]. In: Program and abstracts of the 47th Annual ICAAC Meeting, Chicago, IL, Sept. 17-20, 2007. [PubMed]
84. Louie TJ, Mille MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 364:422-31.[PubMed]
85. Louie TJ, Peppe J, Watt CK, Johnson D, Mohammed R, Dow G, Weiss K, Simon S, John JF Jr., G. Garber G, S. Chasan-Taber S, and D. M. Davidson DM. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006;43:411-20. [PubMed]
86. Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. Toxin B is essential for virulence of Clostridium difficile. Nature 2009;458:1176-9. [PubMed]
87. Manabe YC, Vinetz JM, Moore RD, Merz C, Charache P, Bartlett JG. Clostridium difficile colitis: an efficient clinical approach to diagnosis. Ann Intern Med 1995;123:835-40. [PubMed]
88. Mayfield JL, Leet T, Miller J, Mundy LM. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis 2000;31:995-1000. [PubMed]
89. McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, S. P. Sambol SP, S. Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005;353:2433-41. [PubMed]
90. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002;97:1769-75. [PubMed]
91. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 1989;320:204-10. [PubMed]
92. McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z, and et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994;271:1913-8. [PubMed]
93. McPherson S, Rees CJ, Ellis R, Soo S, Panter SJ. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum2006;49:640-5.[PubMed]
94. Merrigan MM, Sambol SP, Johnson S, Gerding DN. New approach to the management of Clostridium difficile infection: colonisation with non-toxigenic C. difficile during daily ampicillin or ceftriaxone administration. Int J Antimicrob Agents 33 2009;Suppl 1:S46-50. [PubMed]
95. Morris JB, Zollinger RM Jr., Stellato TA. Role of surgery in antibiotic-induced pseudomembranous enterocolitis. Am J Surg1990;160:535-9. [PubMed]
96. Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis 2005;40:1586-90. [PubMed]
97. Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis 2009;48:e41-6.[PubMed]
98. Musher DM, Logan N, Hamill RJ, Dupont HL, Lentnek A, Gupta A, Rossignol JF. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006;43:421-7. [PubMed]
99. Musher DM, Logan N, Mehendiratta V, Melgarejo NA, Garud S, Hamill RJ. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J Antimicrob Chemother 2007;59:705-10. [PubMed]
100. Musher DM, Manhas A, Jain P, Nuila F, Waqar A, Logan N, Marino B, Graviss EA. Detection of Clostridium difficile toxin: comparison of enzyme immunoassay results with results obtained by cytotoxicity assay. J Clin Microbiol 2007;45:2737-9. [PubMed]
101. Musher DM, Stager, C. Diagnosis of Clostridium difficile infection. Clin Infect Dis 2012; 54:1675-6.
102. Musher DM, Musher BL. Contagious acute gastrointestinal infections. N Engl J Med 2004;351:2417-27.
103. Naggie S, Mille BA, Zuzak KB, Pence BW, Mayo AJ, Nicholson BP, Kutty PK, McDonald LC, Woods CW. A case-control study of community-associated Clostridium difficile infection: no role for proton pump inhibitors. Am J Med 124:276 e1-7.[PubMed]
104. Nathanson DR, Sheahan M, Chao L, Wallack MK. Intracolonic use of vancomycin for treatment of clostridium difficile colitis in a patient with a diverted colon: report of a case. Dis Colon Rectum 2001;44:1871-2. [PubMed]
105. Noren T, Alriksson I, Akerlund T, Burman LG, Unemo M. In vitro susceptibility to 17 antimicrobials among clinical Clostridium difficile isolates collected 1993 - 2007 in Sweden. Clin Microbiol Infect. 2009[PubMed]
106. O'Connor JR, Galang MA, Sambol SP, Hecht DW, Vedantam G, Gerding DN, Johnson S. Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile. Antimicrob Agents Chemother 2008;52:2813-7. [PubMed]
107. Olson MM, Shanholtzer CJ, Lee JT Jr., Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991. Infect Control Hosp Epidemiol 1994;15:371-81.[PubMed]
108. Owens RC Jr., Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis 46 Suppl 2008;1:S19-31. [PubMed]
109. Pasic M, Jost R, Carrel T, Von Segesser V, Turina M. Intracolonic vancomycin for pseudomembranous colitis. N Engl J Med 1993;329:583. [PubMed]
110. Pear SM, Williamson TH, Bettin KM, Gerding DN, Galgiani JN. Decrease in nosocomial Clostridium difficile-associated diarrhea by restricting clindamycin use. Ann Intern Med 1994;120:272-7. [PubMed]
111. Pelaez T, Alcala L, Alonso R, Rodriguez-Creixems M, Garcia-Lechuz JM, Bouza E. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother 2002;46:1647-50. [PubMed]
112. Pelaez T, Cercenado E, Alcala L, Marin M, Martin-Lopez A, Martinez-Alarcon J, Catalan P, Sanchez-Somolinos M, Bouza E. Metronidazole resistance in Clostridium difficile is heterogeneous. J Clin Microbiol 2008;46:3028-32. [PubMed]
113. Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, Godin D, Bourassa C. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis 2005;40:1591-7. [PubMed]
114. Pepin JS, Routhier S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis 2006;42:758-64. [PubMed]
115. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. 2005;Cmaj. [PubMed]
116. Planche TD, Davies KA, Coen PG, et al. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis 2013;13936-45.
117. Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999-2004. Emerg Infect Dis 2007;13:1417-9. [PubMed]
118. Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, Jarvinen A. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis 2004;38:62-9. [PubMed]
119. Samore MH, DeGirolami PC, Tlucko A, Lichtenberg DA, Melvin ZA, Karchmer AW. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis 1994;18:181-7.[PubMed]
120. Sanchez JL, G. D., Olson MM, Johnson S. Metronidazole susceptibility in Clostridium difficile isolates recovered from cases of C. difficile-associated disease treatment failures and successes. Anaerobe 1999;5:205-208. [PubMed]
121. Seal D, Borriello SP, Barclay F, Welch A, Piper M, Bonnycastle M. Treatment of relapsing Clostridium difficile diarrhoea by administration of a non-toxigenic strain. Eur J Clin Microbiol 1987;6:51-3. [PubMed]
122. Settle CD, Wilcox MH, Fawley WN, Corrado OJ, Hawkey PM. Prospective study of the risk of Clostridium difficile diarrhoea in elderly patients following treatment with cefotaxime or piperacillin-tazobactam. Aliment Pharmacol Ther 1998;12:1217-23. [PubMed]
123. Shetler K, Nieuwenhuis R, Wren SM, Triadafilopoulos G. Decompressive colonoscopy with intracolonic vancomycin administration for the treatment of severe pseudomembranous colitis. Surg Endosc 2001;15:653-9. [PubMed]
124. Silva J Jr. Update on pseudomembranous colitis. West J Med 1989;151:644-8. [PubMed]
125. Sougioultzis S, Kyne L, Drudy D, Keates S, Maroo S, Pothoulakis C, Giannasca PJ, Lee CK, Warny M, Monath TP, Kelly CP. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology 2005;128:764-70. [PubMed]
126. Struelens MJ, Maas A, Nonhoff C, Deplano A, Rost F, Serruys E, Delmee M. Control of nosocomial transmission of Clostridium difficile based on sporadic case surveillance. Am J Med 1991;91:138S-144S. [PubMed]
127. Surawicz CM, McFarland LV, Elmer G, Chinn J. Treatment of recurrent Clostridium difficile colitis with vancomycin and Saccharomyces boulardii. Am J Gastroenterol 1989;84:1285-7. [PubMed]
128. Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, Garcia RJ, Brandmarker S, Bowen K, Borjal D, Elmer GW. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 2000;31:10pubmed.asp?link=12-7. [PubMed]
129. Tannock GW, Munro K, Taylor C, Lawley B, Young W, Byrne B, Emery J, Louie T. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 2010;156:3354-9.[PubMed]
130. Teasley DG, Gerding DN, Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, Lee JT Jr. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet 19832:1043-6. [PubMed]
131. Tedesco FJ, Gordon D, Fortson WC. Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am J Gastroenterol 1985;80:867-8. [PubMed]
132. Tenover FC, Novak-Weekley S, Woods CW, Peterson LR, Davis T, Schreckenberger P, Fang FC, Dascal A, Gerding DN, Nomura JH, Goering RV, Akerlund T, Weissfeld AS, Baron EJ, Wong E, Marlowe EM, Whitmore J, Persing DH. Impact of strain type on detection of toxigenic Clostridium difficile: comparison of molecular diagnostic and enzyme immunoassay approaches. J Clin Microbiol 2010;48:3719-24. [PubMed]
133. Thomas JA, Newman KC, Doshi S, Logan N, Musher DM. Secondary invasion of the bloodstream by colonic
bacteria during Clostridium difficile infection. Scand J Infect Dis 2011; 43:269-74.
134. Thorpe CM, Gorbach SL. Update on Clostridium difficile. Curr Treat Options Gastroenterol 2006;9:265-71. [PubMed]
135. Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 1989;1:1156-60. [PubMed]
136. van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368:407-15.
137. von Eichel-Streiber C, Boquet P, Sauerborn M, Thelestam M. Large clostridial cytotoxins--a family of glycosyltransferases modifying small GTP-binding proteins. Trends Microbiol 1996;4:375-82. [PubMed]
138. Walk ST, Micic D, Jain R, Lo ES, Trivedi I, Liu EW, Almassalha LM, Ewing SA, Ring C, Galecki AT, Rogers
MA, Washer L, Newton DW, Malani PN, Young VB, Aronoff DM. Clostridium difficile ribotype does not predict
severe infection. Clin Infect Dis 2012;55:1661-8.
139. Walters BA, Roberts R, Stafford R, Seneviratne E. Relapse of antibiotic associated colitis: endogenous persistence of Clostridium difficile during vancomycin therapy. Gut 1983;24:206-12. [PubMed]
140. Warny M, Denie C, Delmee M, Lefebvre C. Gamma globulin administration in relapsing Clostridium difficile-induced pseudomembranous colitis with a defective antibody response to toxin A. Acta Clin Belg 1995;50:36-9. [PubMed]
141. Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005;366:1079-84. [PubMed]
142. Wenisch C, Parschalk B, . Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 1996;22:813-8. [PubMed]
143. Wilcox MH. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother 2004;53:882-4. [PubMed]
144. Wilcox MH. Overcoming barriers to effective recognition and diagnosis of Clostridium difficile infection. Clin Microbiol Infect 2012;18 Suppl 6:13-20.
145. Wilson KH. The microecology of Clostridium difficile. Clin Infect Dis 16 Suppl 1993;4:S214-8.[PubMed]
146. Young GP, Ward PB, Bayley N, Gordon D, Higgins G, Trapani JA, McDonald MI, Labrooy J, Hecker R. Antibiotic-associated colitis due to Clostridium difficile: double-blind comparison of vancomycin with bacitracin. Gastroenterology 1985;89:1038-45. [PubMed]
147. Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol 2004;42:1203-6. [PubMed]
148. Zar FA, Bakkanagari SR, Moorth KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45:302-7. [PubMed]
149. Zilberberg MD, Tillotson GS, McDonald C. Clostridium difficile infections among hospitalized children, United States, 1997-2006. Emerg Infect Dis 16:604-609, 2010.
Tables
Table 1: Randomized, Comparative Trials of Oral Therapy for Clostridium difficile Diarrhea
| Agent | Regimen | Patients Studied | Cure Rate (%) | Time to Resolution | Relapse Rate (%) | References |
|---|---|---|---|---|---|---|
| Vancomycin | 125 mg qid x 7d | 21 | 86 | 4.2 d | NS | Young 1985 (133) |
| 125 mg qid x 10d | 80 | 91 | 2.0 d | 19 | Louie 2006 (78) | |
| 125 mg qid x 10d | 134 | 81 | 5.0 d | 23 | Louie 2007 (76) | |
| 125 mg qid x 10d | 71 | 97 | 7 | Zar 2007 (135) | ||
| 125 mg qid x 10d | 27 | 74 | 7 | Musher 2009 (90) | ||
| 125 mg qid x 10d | 309 | 86 | 3.3 d | 25 | Louie 2011 (77) | |
| Metronidazole | 500 mg tid x 10d | 31 | 94 | 3.2 d | 16 | Wenisch 1996 (130) |
| 250 mg qid x 10d | 76 | 87.95 | 2.4 d, NS | 42 | Teasley 1983 (121) Musher 2006 (91) |
|
| 375 mg qid x 10d | 143 | 72 | 5.0 d | 27 | Louie 2007 (76) | |
| 250 mg qid x 10d | 79 | 84 | 14 | Zar 2007 (135) | ||
| Fidaxomicin | 200 mg bid x 10d | 287 | 88 | 2.4 d | 15 | Louie 2011 (77) |
| Teicoplanin | 400 mg x bid x 10d | 28 | 96 | 2.8 d | 8 | Wenisch 1996 (130) |
| 100 mg bid x 10d | 26 | 96 | 3.4 d | 28 | daLalla 1992 (33) | |
| Fusidic acid | 500 mg tid x 10 d | 29 | 93 | 3.8 d | 28 | Wenisch 1996 (130) |
| Bacitracin | 20k-25k U qid x 7-10 d | 36 | 78 | 2.5-4.1 d | 23 | Dudley 1986, Young 1985 (36, 133) |
| Nitazoxanide | 500 mg bid x 7 d | 40 | 90 | NS | 23 | Musher 2006 (91) |
| 500 mg bid x 10 d | 36 | 89 | NS | 10 | Musher 2006 (91) | |
| Rifaximin | 200 mg tid x 10 d | 10 | 90 | NS | NS | Boero 1990 (16) |
Table 2. Empirical Treatment Strategies for Patients with Multiple Recurrences of C. difficile Diarrhea
Strategy |
|---|
Repeated courses of antimicrobial agents (metronidazole, vancomycin, nitazoxanide, rifaximin) |
Tapering doses of antimicrobial agents (vancomycin, rifaximin) |
Biotherapy |
Monoclonal antibody to C. difficile toxins |
Intravenous immunoglobulin |
Table 3. Potential Future Treatment Options
| Product | Type | Stage of Development | Company |
|---|---|---|---|
| C. difficile vaccine |
Vaccine |
Phase I |
Acambis |
| Monoclonal Antibody | Antibody | Phase III | Merck |
| Nitazoxanide | Antibiotic | Phase III | Romark |
| Ramoplanin | Antibiotic | Phase III | Oscient |
| Rifaximin | Antibiotic | Phase III | Salix |
Figure 1: Three Hit Disease
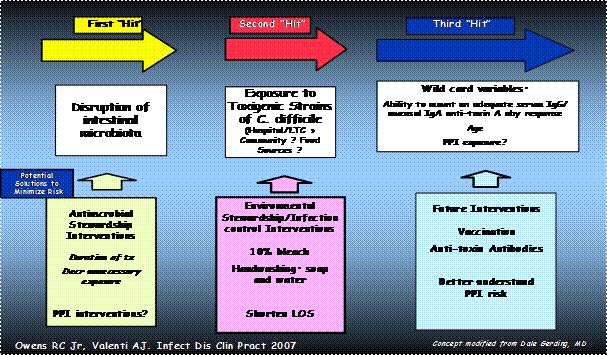
What's New
Kamboj M, et al. Relapse Versus Reinfection: Surveillance of Clostridium difficile Infection. Clinical Infectious Diseases. 2011;53(10): 1003-1006.
Lee NY, et al. Clostridium difficile Bacteremia, Taiwan. Emerg Infect Dis 2010;16:1204-1210.
Guided Medline Search For:
Reviews
McMaster-Baxter NL, Musher DM. Clostridium difficile: Recent Epidemiological Findings and Advances In Therapy. Pharmacotherapy 2007;27(7):1029-1039.
Ward SE, Dubberke ER. Clostridium difficile Infection in Solid Organ Transplant Patients
Guided Medline Search For Recent Reviews
History
Bartlett JG. Historical Perspectives on Studies of Clostridium difficile and C. difficile infection. Clin Infect Dis 2008;46(Suppl 1):S4-S11.
Guided Medline Search For Historical Aspects
Table of Contents
- Microbiology
- Epidemiology
- Clinical Manifestations
- Laboratory Diagnosis
- Pathogenesis
- Susceptibility In Vitro and In Vivo
- Failure of Medical Therapy
- Endpoints for Monitoring Therapy
- Prevention
- Summary
