Chlamydophila (Chlamydia) pneumoniae
Authors: Francesco Blasi, M.D. Paolo Tarsia, M.D.
Previous author: Lisa A. Jackson, M.D., J. Thomas Grayston, M.D., 1997 Edition, 2002 Edition
MICROBIOLOGY
Chlamydia is an obligate intracellular bacteria that has a unique biphasic developmental cycle. The genus contains three species which are human pathogens; C. psittaci, C. trachomatis, and C. pneumoniae (TWAR). Chlamydiae have cell walls with inner and outer membranes, replicate by binary fission, contain DNA, RNA, and ribosomes, and synthesize some proteins. They cannot however synthesize ATP or GTP and must rely on the host cell for ATP. The bacterium presents in two developmental forms: infective elementary bodies and reproductive reticulate bodies. The small, dense elementary body is the metabolically-inactive infectious form of the organism. Elementary bodies have a rigid cell wall resulting from disulfide cross-linking of envelope proteins, allowing survival outside of the host cell. After infection of the host cell by receptor-mediated endocytosis, the elementary bodies differentiate into reticulate bodies. The reticulate body is the larger, metabolically active form of the organism. Inside the host cell, the reticulate body divides by binary fission, forming a microcolony referred to as a chlamydial inclusion. After a period of growth and division, the reticulate bodies reorganize and condense to form new elementary bodies. After host cell lysis, the elementary bodies are released to initiate new infectious cycles.
The first C. pneumoniae isolates were serendipitously obtained from conjunctival cultures of children during trachoma vaccine studies in the 1960s. The organism is not, however, associated with eye infection and its role as a human pathogen was not fully defined until 1983, when the first respiratory isolate was obtained (26). Since that time C. pneumoniae has been identified as an important cause of community-acquired respiratory infections, such as community-acquired pneumonia and acute bronchitis.
The first C. pneumoniae isolates were obtained in yolk-sac cultures, the only method then available for isolation of Chlamydia, and were thought to represent strains of C. psittaci based on inclusion morphology. After development of cell culture methods which allowed further characterization, the organism was observed to have a characteristic pear-shaped elementary body surrounded by a periplasmic space that is morphologically distinct from the round elementary bodies of C. trachomatis and C. psittaci. DNA homology studies have revealed that C. pneumoniae isolates have less than 5% DNA homology with C. trachomatis and less than 10% with C. psittaci. C. pneumoniae isolates are highly (> 95%) related to each other, however. Since only one strain or serovar of C. pneumoniae has been identified, at this time the strain name, TWAR (after the designation of two of the initial isolates, TW-183 and AR-39), is synonymous with C. pneumoniae.
In 1999 a new taxonomic classification of Chlamydia pneumoniae was proposed, renaming the bacterium as Chlamydophila pneumoniae (19). The proposal failed to encounter universal acceptance and both names are currently in use by different authors.
EPIDEMIOLOGY
C. pneumoniae is a common cause of respiratory infections worldwide, with seroprevalence rates of over 50% among adults in the United States and many other countries. Infection appears to be uncommon before age 5 years in industrialized countries but is increasingly common in older children, with a peak incidence of acute infection, as demonstrated by antibody conversion, among children 5 through 14 years of age. By age 20, approximately 50% of persons have detectable levels of antibody to the organism, and the seroprevalence increases to approximately 75% in the elderly. Most infections are asymptomatic. Although the exact mode of transmission is not known, spread via droplets has been proposed. Persistent antibody positivity for C. pneumoniae, identification of the organism in different body sites and the difficulty in obtaining effective clearance of the bacterium, has lead to the hypothesis that, in addition to acute airway infection, this microorganism may involved in maintaining or worsening of diverse chronic conditions such as COPD, asthma, atherosclerotic disease, multiple sclerosis, and Alzheimer’s disease.
CLINICAL MANIFESTATIONs
Chlamydia pneumoniae is an important cause of both lower and upper respiratory tract infections. Pneumonia and bronchitis are the most common, while upper respiratory infections, including sinusitis and pharyngitis, may also occur, either in isolation or in conjunction with a lower respiratory infection. The incubation period of infection due to C. pneumoniae is about 21 days. Upper respiratory signs and symptoms, such as rhinitis, sore throat, or hoarseness, may be reported initially. These signs and symptoms may then subside over days to weeks, followed by the onset of cough, which is a predominant feature of C. pneumoniae respiratory infections, resulting in a biphasic pattern of illness symptoms. In addition to having a gradual onset, symptoms due to C. pneumoniae respiratory infections may be of prolonged duration, with persistence of cough and malaise for several weeks or months despite appropriate antibiotic therapy. C. pneumoniae has also associated with acute respiratory exacerbations in patients with cystic fibrosis and acute chest syndrome in children with sickle cell disease. Other associated syndromes include adult-onset asthma, acute exacerbations among adults with asthma and reactive airway disease in children. Several chronic diseases have been presumptively associated with C. pneumoniae infection, including atherosclerotic cardiovascular disease, chronic obstructive pulmonary disease, multiple sclerosis, and Alzheimer’s disease.
Pneumonia
C. pneumoniae is reported to account for a relatively large number (6-20%) of community-acquired pneumonia (CAP) cases (39), although data are largely based on serological determinations alone. The clinical course may vary from mild, self-limiting illnesses to severe forms of pneumonia, particularly in elderly patients, and with coexisting cardiopulmonary diseases.
Presenting symptoms most frequently reported by patients with C. pneumoniae pneumonia are sore throat and hoarseness (27). After a period of up to a week, dry persistent cough often sets in (28). Body temperature is generally slightly increased, seldom going higher than 38-39°C.
Fever may be often missed if the patient is not seen early in the course of infection. Physical examination does not often show abnormalities and, if present, physical findings are generally not specific. Pulmonary rales, ronchi or signs of pulmonary consolidation are sometimes found.
Chest X-ray generally reveals small pulmonary infiltrates, sublobar or segmental at presentation. Multiple infiltrates may sometimes be seen and are often bilateral. Extensive lobar involvement is uncommon, whereas pleural effusion may be present in up to 20% of cases. This agent is part of a coinfection involving other bacterial agents in approximately 30% of adult CAP cases (57).
Asthma
Asthma, a chronic inflammatory disease of the airways. Its etiology is complex, involving interactions between genetic susceptibility, exposure to allergens and external aggravating factors such as smoking, air pollution and respiratory tract infections. It has been suggested that there may be an association between acute asthma exacerbations and infection with or reactivation of the atypical bacterium C. pneumoniae. An association between asthma and C. pneumoniae infection was first put forward by Hahn et al. in the early 90’s (32). Since then, evidence has accumulated both for paediatric and adult populations.
Children:
A relationship between acute infection with atypical pathogens and acute asthma exacerbations in children has been sought for in controlled and uncontrolled studies (17). The vast majority of studies were concordant in finding an association between atypical bacterial infection and asthma exacerbations. Rates of C. pneumoniae identification varied between 4.5% and 25% of asthma episodes (17).
Adults:
Acute atypical infection has been studied in adults with asthma exacerbations (52). A recent study found that patients with acute asthma exacerbation and evidence of C. pneumoniae acute infection exhibited more severe functional impairment on admission. In addition, these patients also showed a slower FEV1 rise during follow-up when compared with the group without acute infection (14). The relevance of C. pneumoniae in the pathogenesis of chronic asthma has also been extensively investigated. Accumulating evidence from sero-epidemiological studies has shown that many asthmatics have elevated antibody levels to C. pneumoniae (84).
Use of Antibiotics in Asthma Patients with C. pneumoniae Infection: Should the role of C. pneumoniae infection in asthma patients be proven, C. pneumoniae eradication from the airways would become an important aspect of asthma treatment. Antibiotics exerting activity towards atypical bacteria, such as macrolides, the ketolide telithromycin, tetracyclines and fluoroquinolones would be logical candidate drugs. To date, clinical trials in children and adults with asthma have mostly employed macrolides due to their favorable tolerability/safety profile and excellent intracellular accumulation characteristics (6, 18, 33, 47).
A Cochrane review of macrolide usage in chronic asthma found an overall positive effect on symptoms and eosinophilic markers of inflammation following macrolide therapy (71).
A recent multicenter, double-blind, randomized, placebo-controlled clinical study assessed oral telithromycin as a supplement to standard of care treatment for adults with acute asthma exacerbations (42). Ketolide treatment was associated with statistically significant and clinically substantial benefits. In this population, 61% of patients had evidence of C. pneumoniae and/or M. pneumoniae infection and the effect of telithromycin on FEV1 was statistically significant in patients with documented infection at baseline and not in those without evidence of infection. However, there were no differences between infection-positive and -negative groups in terms of other study outcomes, so that the mechanisms of benefit remain unclear. The main limits of the above studies are related to patient populations size and prevalent use of serology as the applied diagnostic test. There is a need for further well designed studies to define both the importance of C. pneumoniae involvement in acute and chronic asthma and the usefulness of antibiotics in this disease.
Chronic Obstructive Pulmonary Disease (COPD)
In patients with COPD persistence of micro-organisms in the respiratory tract may facilitate access of different pathogens to the lower airways, and longstanding infection might trigger what is traditionally described as the vicious circle of chronic bronchitis. Chronic C. pneumoniae infection has been found to be common in chronic bronchitis and could contribute to disease progression by a toxic effect on bronchial epithelial cells, impairing ciliary function, and increasing chronic inflammation via proinflammatory cytokine production (7, 83). The possibility of chronic colonization with C. pneumoniae in patients with COPD is suggested by serology, electron microscopy and immunohistochemistry (81, 89). Chronic colonization with C. pneumoniae is significantly associated with more severe functional impairment and colonization is associated with a greater propensity to develop acute exacerbations (8). Moreover, long-term antibiotic treatment, delivered over a 6 week period, is insufficient to eradicate the organism.
Atherosclerosis
Known risk factors are felt to account for approximately 50-70% of the pathogenesis or cardiovascular diseases. Attention has been focused on investigating hypothetical additional risk factors and on a fuller understanding of the development of atherosclerosis, the main pathologic process involved in coronary heart disease. The first indication that C. pneumoniae has an association with atherosclerosis and coronary heart disease dates back to 1986 (26). This association has been shown by seroepidemiology, immunohistochemistry, PCR, electron microscopy and tissue culture (75, 88). Animal models of atherosclerosis have been used to study the role of C. pneumoniae in the initiation and progression of atherosclerotic disease (63). The results of some treatment trials using antibiotics for the prevention of cardiovascular events in animal models of atherosclerosis encouraged secondary prevention trials in humans (22, 62).
Small-scale studies indicated that antibiotic treatment may prevent adverse cardiovascular events (31). However, large clinical trials failed to demonstrate any effect (2, 16, 25, 29, 41, 67). Two studies have evaluated the effect of antibiotics on the rate of expansion of abdominal aortic aneurysms. One small study found a non-significant decrease in this rate with doxycycline treatment (60) and the other found a similar difference with roxithromycin treatment that did reach statistical significance (82).
A comprehensive review analysed the epidemiological and experimental evidence accumulated over the last 20 years linking C. pneumoniae to atherosclerosis (85). The authors conclude that, considering present evidence, C. pneumoniae is neither sufficient in itself nor necessary to cause atherosclerosis or its clinical consequences in humans. However, C. pneumoniae is highly likely to be a modifiable risk factor that may be a target of future therapies (46).
Multiple Sclerosis
C. pneumoniae is detected with higher frequency in the cerebrospinal fluid of MS patients than in that of neurological controls. The original findings of Sriram et al. (76) have been partially replicated by some, but not all, studies (12,44,45,51), because of genetic heterogeneity, differences in patient selection, and techniques of DNA extraction or amplification. C. pneumoniae infection seems to characterize a subgroup of MS patients with an anticipated onset of disease and more pronounced evidence of CNS inflammation/demyelination (30). It was also found that C. pneumoniae-infected patients who were positive according to PCR had more active lesions than C. pneumoniae-infected patients who were PCR-negative, suggesting a role for C. pneumoniae in fostering a chronic inflammatory stimulation within the CNS. These findings led to the hypothesis that C. pneumoniae might act as a cofactor that is able to fuel already established inflammatory and demyelinating processes and promote more active disease.
Published data suggest the need for longitudinal observations, and clinical trials with C. pneumoniae-specific antibiotics to clarify the exact role of C. pneumoniae infection in MS patients (78).
Alzheimer’s Disease (AD)
Studies that have used PCR to detect C. pneumoniae in the brains of AD patients have yielded conflicting results (4,24,66). A recent study demonstrated that brain tissue samples from a high proportion of patients with AD are PCR-positive for C. pneumoniae, but those from age-/sex-matched non-AD controls are not. Moreover, the organism is viable within the brains of patients with AD, indicating metabolic activity of the organism in those tissues (23). A randomized, placebocontrolled, multicentre clinical trial has been performed to determine whether a 3-month course of doxycycline and rifampin can reduce the decline of cognitive function in patients with AD (55). This study showed significantly less cognitive decline at 6 months in the antibiotic group than in controls. Antibiotic therapy was also associated with less dysfunctional behaviour at 3 months. There was no clear relationship between the results and treatment in terms of eradication of chronic C. pneumoniae infection, suggesting that the activity of the two drugs may be related to non-antibiotic effects. None of these observations demonstrates a causal relationship between CNS infection with C. pneumoniae and the neuropathogenesis characteristic of AD, but they do open the way to further investigations.
Development of a non-transgenic animal model was undertaken to address how infection could play a role in the pathogenesis of AD (54). Following intranasal infection with C. pneumoniae, analysis of pathology in the brain revealed Aβ1–42 deposits that resembled amyloid plaques found in human AD. The induction of amyloid deposits in the brains of non-transgenic BALB/c mice supports the hypothesis that infection with C. pneumoniae is capable of accelerating or inducing AD-like pathology, and it may be a trigger in the pathogenesis of the disease. In a recent review, a further animal model on antibiotic treatment to treat or limit the pathology induced following infection in the CNS has been reported (5). In this study different groups of mice were treated with moxifloxacin hydrochloride at days 7–21, 28–42, 56–70, or 84–98 post-infection. Intriguingly, at the earliest time of antibiotic treatment (i.e., 7–21 days post infection), the number of amyloid plaques was equivalent to the baseline level as observed in uninfected mice. However, in the infected mice in which the antibiotic treatment was delayed until 56 days post-infection, the number of amyloid plaques was 8–9 fold higher than baseline; this number of plaques was comparable to the number found in the brains of infected animals that received no antibiotics. These data suggest that early antibiotic intervention after infection is effective in limiting the number of amyloid plaques that arise as a result of infection, even though complete antigen eradication may not be achieved. However, a review from Stallings (77) analyzing the published papers on the possible involvement of C. pneumoniae in Alzheimer’s disease, concludes that whether C. pneumoniae is an underlying agent in the pathogenesis of Alzheimer’s disease or one of the brain’s intruders found there by chance is not apparent from the existing literature.
LABORATORY DIAGNOSIS
Reliable diagnosis of infection due to C. pneumoniae and investigation of its role in chronic diseases remain difficult because of the paucity of well-standardized and commercially available diagnostic tests that are accurate and reliable () (48). Laboratory methods for the diagnosis of C. pneumoniae infection include culture, antigen detection, serology and PCR. Different cell lines have been evaluated for culturing C. pneumoniae, and HL and Hep-2 have been found to yield the best results. So far, successful culture of C. pneumoniae has been obtained in a limited number of laboratories. The main problems encountered are easy inactivation during transport, and low yield, often requiring repeated blind passages.
The sensitivity of antigen detection using direct fluorescent antibodies on respiratory specimen smears is estimated to be 20–60% as compared to that of culture; the specificity should approach 95%, but this is highly operator dependent. This technique is mostly employed for culture confirmation.
The development of PCR technology has brought major advantages to the diagnosis of C. pneumoniae infection. It has been successfully employed with respiratory specimens, lung and vascular biopsy specimens, and blood. Several studies have found PCR to be a more sensitive technique than culture (48). Nested PCR assays involve significant problems with contamination, which may result in the overestimation of disease attributed to C. pneumoniae. Real-time assays are reported to have distinct advantages over conventional assays (3,79). A multiplex PCR has been developed that allows simultaneous identification of C. pneumoniae, Legionella pneumophila and M. pneumoniae in respiratory specimens (87). The overall diagnostic utility of PCR techniques is currently hampered by the lack of standardization of extraction procedures, primer definition, etc., and by the limited number of commercially available tests. Some are now marketed in Europe, but none has been approved by the US Food and Drug Administration.
Serology testing for C. pneumoniae currently includes microimmunofluorescence (MIF) assays, ELISAs and enzyme immunoassays, each of which exists in a variety of in-house and commercial versions. MIF is considered by the CDC to be the only currently acceptable serological test, and is considered to be the reference standard for serodiagnosis, despite significant limitations (15). On the basis of available techniques, the most convincing evidence of acute infection is obtained when IgM antibodies or a four-fold rise in IgG antibodies can be shown. However, the need for paired sera to show a four-fold rise in antibody titres is a limitation of the MIF technique. In primary infections, IgM antibodies appear 2–3 weeks after infection and IgG antibodies appear 6–8 weeks after infection, whereas in cases of re-infection, IgM antibodies may be absent, or of low titre if present, and IgG antibodies appear earlier, within 1–2 weeks after infection.
Recently, a new method for rapid diagnosis of C. pneumoniae pneumonia has been published (59). It is an immunochromatographic test for the detection of C. pneumoniae-specific IgM antibodies. The results obtained with serum samples from 140 patients (41 with C. pneumoniae pneumonia) using this test were compared with those obtained using two other serological tests (MIF and enzyme immunoassay). The reported sensitivity and specificity of the test were 100% and 92.9%, respectively. However, as it detects only the IgM response, the usefulness of this test may be limited to primary infection.
PATHOGENESIS
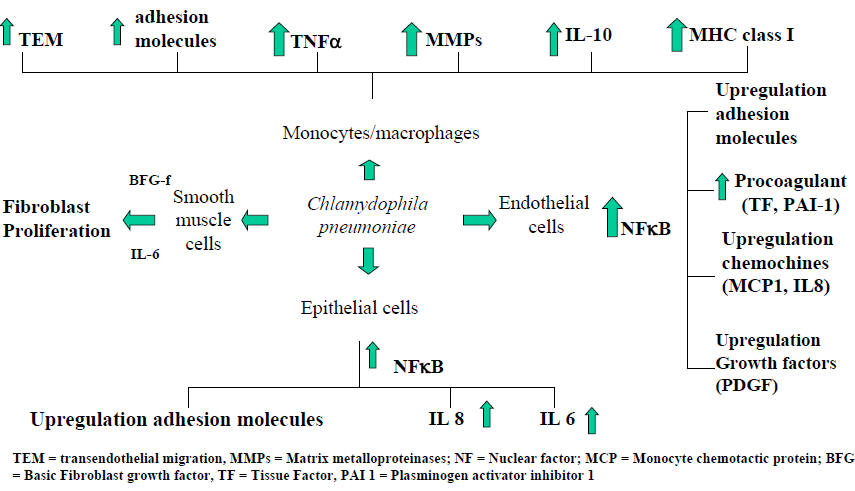
C. pneumoniae has been shown to thrive and replicate within most cell types within airway and vascular walls (epithelial cells, endothelial cells, smooth muscle cells, and macrophages). All these cell types may contribute both to acute infection and to progression of chronic respiratory diseases and atheroma formation. Current evidence indicates that C. pneumoniae gene products (mainly heat shock protein-60), through the activation of transcription factors (notably nuclear factor kappa-B), are responsible for the activation of most cellular elements in bronchial tissue, resulting in a cascade of cytokine release and adhesion molecule upregulation, which favours cellular influx into the airways, persistent infection and airway remodelling. Considering that C. pneumoniae infection is extremely common in the population, there must be individual predisposing factors involved in associating this infection with chronic airway conditions (64). Figure 1 summarizes the interactions between C. pneumoniae and the different cell types present in airways.
In a recent animal model, mice were inoculated intranasally with C. pneumoniae (9). C. pneumoniae infection caused both sustained airway hyperresponsiveness and airway inflammation. This has clinical implications, as changes in airway responsiveness and inflammation status induced by this bacterium may worsen and/or provoke breathlessness in individuals with asthma and COPD. Gieffers et al. (25) reported an animal model showing that intratracheal infection with C. pneumoniae is followed by systemic dissemination of the infection mediated by peripheral blood mononuclear cells (PBMCs). Infection of the lung is characterized by an early phase dominated by granulocytes, and a late phase dominated by alveolar macrophages. Alveolar macrophages, infected by granulocytes, would migrate through the mucosal barrier, using lymphatic tissue, and gain access to the systemic circulation as PBMCs reaching the spleen and the vasculature. The interesting finding of the Gieffers’ study (25) is the demonstration that granulocytes are one of the main target of C. pneumoniae infection and that these cells can act as “infecting” cells and reservoir of the pathogen. These data confirm the excellent capacity of C. pneumoniae to survive in different immune cells, use the immune cells as carriers for breaching the blood-tissue barriers, and potentially cause chronic/persistent infections. In chronic infection C. pneumoniae has been shown to successfully escape from programmed cell death by inhibiting apoptotic signaling cascades within various host cell populations (20), e.g. monocytes (1), epithelial cells (70) or microglial cells (11). By gaining control over the host cell apoptotic regulation, chlamydiae can thus create the appropriate environment for replication or persistence.
SUSCEPTIBILITY IN VITRO AND IN VIVO
The in vitro methods used for the susceptibility testing of C. pneumoniae were adapted from those used for C. trachomatis and involve inoculating cell monolayers with the organism and incubating in the presence of serial dilutions of antibiotic. The majority of published studies of in vitro susceptibility have used cycloheximide-treated HeLa or McCoy cells although some studies have used HL or Hep-2 cells. After the cell monolayers have been inoculated, an overlay medium containing twofold dilutions of the test antimicrobial agent is added. The infected cell monolayers are then incubated for a period of several days and stained, usually with a genus-specific fluorescent monoclonal antibody. The minimum inhibitory concentration (MIC) for this first pass is defined as the lowest antibiotic concentration resulting in the absence of inclusions.
Most investigators also determine the MIC for second passage by harvesting the cells from duplicate plates run in parallel and disrupting and passing the cells onto new monolayers. These cells are cultured in antimicrobial agent free media and stained with fluorescent antibody for inclusion counts. The MIC for second passage is defined as the lowest antibiotic concentration that results in no inclusions after passage. Some investigators term the MIC for second passage as the MCC (minimum chlamydicidal concentration) or the MLC (minimal lethal concentration). It is important to note that the methods for in vitro susceptibility testing of C. pneumoniae are not yet standardized and testing methods including the type of tissue culture system, treatment of cells prior to testing, inoculum size, and method for detection of inclusions may vary between investigators.
A summary of the results of antimicrobial susceptibility testing from previous published reports is presented in Table 1. The results of in vitro testing indicate that, unlike C. trachomatis, C. pneumoniae is resistant to sulfonamides. These results also indicate that tetracyclines, macrolides, and azalides are active against C. pneumoniae (13, 35, 37, 49, 86). Although older quinolones such as oflaxacin are not highly active against C. pneumoniae, the MIC ranges of newer quinolone agents, such as sparfloxacin, grepafloxacin, gatifloxacin, and gemifloxacin, are comparable to macrolide agents (13, 34, 50, 73).
Available data on the susceptibility of C. pneumoniae to beta-lactams indicates that ampicillin and penicillin have high MICs on first passage but relatively low MICs on second passage. This suggests that beta-lactams inhibit the production of infectious particles by inhibition of the maturation of reticulate bodies to elementary bodies but do not completely inhibit the replication of reticulate bodies. Accordingly, although high concentrations of antibiotic are required to produce the complete absence of inclusions for the MIC on first passage, the inclusions seen in the presence of antibiotics were not viable on passage and so much lower concentrations of antibiotic are required on second passage into antibiotic free medium. Although resistance of C. trachomatis to tetracycline and erythromycin in vitro has been reported (43, 61), resistance of C. pneumoniae to these agents has not been described.
ANTIMICROBIAL THERAPY
General
As previously described, erythromycin, tetracycline, and doxycycline demonstrate in vitro activity against the organism and these agents are recommended as first line therapy of acute respiratory infections due to C. pneumoniae. The organism is not susceptible in vitro to penicillin, ampicillin, or sulfa drugs; therefore, these agents are not recommended for treatment of suspected C. pneumoniae infection. Clinical experience shows that symptoms of C. pneumoniae infection frequently recur after short or conventional courses of appropriate antibiotics, and persistent infection has been documented by culture after treatment. Consequently, a relatively intensive long-term course of treatment is recommended.
The drugs of choice for treatment of adults include tetracycline 500 mg four times daily for 14 days; doxycycline 100 mg twice daily for 14 days; or erythromycin 500 mg four times daily for 14 days. Erythromycin 250 mg four times daily for 21 days may be used if the higher dose is not tolerated. Tetracyclines should not be given routinely to children under 8 years of age; therefore, erythromycin (30-50 mg/kg/day divided q 6h) is the drug of choice in younger children.
If symptoms such as cough or malaise persist after one course of antibiotics, a second course may be useful. Unless the drug is contraindicated, tetracycline or doxycycline is recommended for the second course. Although C. pneumoniae infections may be prolonged, serious sequelae are rare and most patients are expected to completely recover from their infection.
Chlamydia are slow-growing, obligate intracellular bacteria which are difficult to isolate from clinical specimens. As a result, many of the studies of respiratory infections due to C. pneumoniae have identified infected persons using acute and convalescent serology. This method accurately retrospectively identifies infected persons; however, it limits the ability of investigators to document eradication of the bacteria as a consequence of antimicrobial therapy. In the original report documenting C. pneumoniae as a cause of respiratory infection, infected patients were identified by both nasopharyngeal culture and serology; however, in general, cultures were not repeated post-treatment to document eradication. In that study, many patients treated with 1 gm of erythromycin orally per day for 5 to 10 days did not have resolution of symptoms, suggesting that this therapy was inadequate. Grayston et al. then recommended either 2 g of tetracycline per day for 7 to 10 days or 1 gm per day for 21 days (26).
Subsequently, Lipsky et al. reported the clinical response of patients with pneumonia and bronchitis caused by C. pneumoniae to ofloxacin treatment (53). Infection was defined by acute and convalescent sera. The four patients with C. pneumoniae infection treated with ofloxacin (400 mg twice a day for 10 days) appeared to respond clinically.
More recently, the results of several clinical trials which have included nasopharyngeal culture positivity as an endpoint have been reported. These types of studies allow determination of the microbiologic efficacy of antimicrobial therapy against C. pneumoniae infections. The first such trial was a randomized controlled trial of treatment with clarithromycin (15 mg/kg per day for 10 days) vs. erythromycin suspension (40 mg/ kg of body weight per day for 10 days) among children 3 to 12 years of age with radiographically demonstrated community-acquired pneumonia (10). Of the 260 children enrolled in the study, 74 (28%) had evidence of infection with C. pneumoniae by isolation from or PCR of throat swab samples. Of the 33 evaluable patients with C. pneumoniae isolated from pre-treatment nasopharyngeal cultures, bacteriologic eradication was documented in 79% (15/19) of those treated with clarithromycin vs. 86% (12/14) of those treated with erythromycin. The MICs to erythromycin and clarithromycin of isolates obtained from children positive both before and after therapy did not change during treatment (74). All of the children with persistent infection improved clinically, with complete resolution of the chest x rays.
In a similar study of azithromycin for treatment of community-acquired pneumonia in children 6 months through 16 years of age, 36 of 456 (8%) of children had C. pneumoniae isolated from pre-treatment nasopharyngeal cultures (72). The organism was eradicated after treatment in 19 of the 23 (83%) patients evaluable after treatment.
In an open study of azithromycin (1.5 g total oral dose over 5 days) for the treatment of community-acquired pneumonia in adults (72), C. pneumoniae infection was identified by culture in 10 of 48 (21%) patients at enrollment. Seven of those 10 patients were culture-negative after treatment. The isolate obtained from one of the persistently infected patients had an MIC four times higher (0.25 µg/mL vs. 0.062 µg/mL) than the isolate obtained at enrollment, although the higher MIC was still within the range considered to represent antibiotic susceptibility. All patients improved clinically. Studies of moxifloxacin for treatment of community-acquired pneumonia have also shown persistence of C. pneumoniae in nasopharyngeal specimens in a minority of patients following treatment (38).
The ability of C. pneumoniae to persist despite antimicrobial therapy with agents to which it is susceptible in vitro is well documented. The above referenced studies demonstrate persistence of the organism after azithromycin, clarithromycin, erythromycin and moxifloxacin treatment. In these studies, the patients showed improvement of clinic symptoms despite persistent culture positivity. Hammerschlag et al. have also reported several patients with acute respiratory illness and positive C. pneumoniae nasopharyngeal cultures who remained culture positive and symptomatic after 2 weeks of erythromycin or 30 days of tetracycline or doxycycline treatment (10). Thus the correlations between in vitro susceptibility and nasopharyngeal eradication, and between eradication and clinical response, are not completely defined.
These studies do suggest, however, that azithromycin and clarithromycin are likely to be at least as effective as doxycycline or erythromycin therapy for C. pneumoniae respiratory infections. These agents achieve high tissue and intracellular levels, have been demonstrated effective against C. pneumoniae in vitro, and are better tolerated than erythromycin, with fewer gastrointestinal side effects. They may be preferable in certain situations for the treatment of C. pneumoniae infections. If these agents are used, the recommended dose for respiratory infections for adults is 500 mg on day one then 250 mg a day on days 2 through 5 for azithromycin and 500 mg twice a day for 10 to 14 days for clarithromycin. The recommended pediatric dosage of azithromycin is 5-12 mg/kg once daily and of clarithromycin is 7.5 mg/kg/day divided twice a day. In adults, there is also a role for use of newer quinolone agents for empiric treatment of respiratory infections potentially due to C. pneumoniae.
Special Circumstances
Pneumonia and bronchitis are the most common manifestations of acute infection with C. pneumoniae; however, the organism is also associated with other acute respiratory illnesses. It has been associated with non-purulent sinusitis (80) and the organism has been isolated from patients with purulent sinusitis as well as from children with otitis media (68). Primary pharyngitis due to C. pneumoniae has also been reported (40). Other reported clinical syndromes include endocarditis (56), and lumbosacral meningoradiculitis (58). Prospective assessments of treatment of upper respiratory and non-respiratory syndromes due to C. pneumoniae have not been performed; therefore, the recommendations for treatment are derived from those developed for the more common lower respiratory syndromes.
ENDPOINTS FOR MONITORING THERAPY
C. pneumoniae infections are, in general, diagnosed retrospectively after testing of acute and convalescent sera specimens. In addition, as previously described, persistence of the organism, as documented by detection by isolation or PCR of nasopharyngeal swab specimens, has been documented after treatment despite clinical improvement. Therefore, the clinical condition should be used as the primary endpoint for monitoring of therapy directed against acute C. pneumoniae infections.
VACCINES
There are no effective vaccines available for C. pneumoniae. An interesting review on the issue of innate immunity and vaccines in C. pneumoniae infection has been recently published (69).
PREVENTION
Since most infections are asymptomatic, there are no preventive measures.
REFERENCES
1. Airenne S, Surcel HM, Tuukkanen J, Leinonen M, Saikku P. Chlamydia pneumoniae inhibits apoptosis in human epithelial and monocyte cell lines. Scand J Immunol 2002;55:390–398. [PubMed]
2. Anderson JL, Muhlestein JB, Carlquist J, Allen A, Trehan S, Nielson C, Hall S, Brady J, Egger M, Home B, Lim T. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease and serological evidence for Chlamydia pneumoniae infection: The Azithromycin in Coronary Artery Disease: Elimination of Myocardial Infection with Chlamydia (ACADEMIC) study. Circulation 1999; 99:1540-7. [PubMed]
3. Apfalter P, Reischl U, Hammerschlag MR. In-house nucleic acid amplification assays in research: how much quality control is needed before one can rely upon the results? J Clin Microbiol 2005;43:5835–5841. [PubMed]
4. Balin BJ, Gerard HC, Arking EJ, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol 1998; 187: 23–42. [PubMed]
5. Balin BJ, Scott Little C, Hammond CJ, Appelet DM, Whittum-Hudson JA, Gerard HC, Hudson AP. Chlamydophila pneumoniae and the etiology of late-onset Alzheimer’s disease. J Alzheimer Dis 2008;13:371-380. [PubMed]
6. Black PN, Blasi F, Jenkins CR, et al. Trial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniae. Am J Respir Crit Care Med 2001; 164: 536–541. [PubMed]
7. Blasi F, Legnani D, Lombardo VM, et al. Chlamydia pneumoniae infection in acute exacerbations of COPD. Eur Respir J 1993;6:19–22. [PubMed]
8. Blasi F, Damato S, Cosentini R, et al. Chlamydia pneumoniae and chronic bronchitis: association with severity and bacterial clearance following treatment. Thorax 2002;57:672–676. [PubMed]
9. Blasi F, Aliberti S, Allegra L, et al. Chlamydophila pneumoniae induces a sustained airway hyperresponsiveness and inflammation in mice. Respir Res 2007; 8: 83. [PubMed]
10. Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J 1995;14:471-477. [PubMed]
11. Boelen E, Steinbusch HW, van der Ven AJ, Graulus G, Bruggeman CA, Strassen FR. Chlamydia pneumoniae infection of brain cells: an in vitro study. Neurobiol Aging 2007; 28: 524–532. [PubMed]
12. Boman J, Roblin PM, Sundstrom P et al. Failure to detect Chlamydia pneumoniae in central nervous system of patients with MS. Neurology 2000; 54: 265. [PubMed]
13. Cooper MA, Baldwin D, Matthews RS, Andrews JM, Wise R. In-vitro susceptibility of Chlamydia pneumoniae (TWAR) to seven antibiotics. J Antimicrob Chemother 1991;28:407 -413. [PubMed]
14. Cosentini R, Tarsia P, Canetta C, et al. Severe asthma exacerbation: role of acute Chlamydophila pneumonia and Mycoplasma pneumoniae infection. Resp Res 2008 May 30;9:48. [PubMed]
15. Dowell SF, Peeling RW, Boman J et al. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin Infect Dis 2001; 33: 492–503. [PubMed]
16. Dunne MW. Rationale and design of a secondary prevention trial of antibiotic use in patients after myocardial infarction: the WIZARD (weekly intervention with zithromax. J Infect Dis 2000;181 Suppl 3:S572-8. [PubMed]
17. Esposito S, Blasi F, Arosio C, et al. Importance of acute Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur Respir J 2000; 16: 1142–1146. [PubMed]
18. Esposito S, Bosis S, Faelli N, et al. Role of atypical bacteria and azithromycin therapy for children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2005; 24: 438-44. [PubMed]
19. Everett KD, Bush RM, Andersen AA. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. 1999 Apr;49 Pt 2:415-40. [PubMed]
20. Fan T, Lu H, Hu H et al. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 1998; 187: 487–496. [PubMed]
21. Fong IW, Chiu B, Viira E, Jang D, Fong MW, Peeling R, Mahony JB. Can an antibiotic (macrolide) prevent Chlamydia pneumoniae-induced atherosclerosis in a rabbit model? Clin Diagn Lab Immunol 1999;6:891-4. [PubMed]
22. Fong IW. Antibiotic effects in a rabbit model of Chlamydia pneumonia induced atherosclerosis. J Infect Dis 2000; 181 (suppl 3): S514–S518. [PubMed]
23. Gerard HC, Dreses-Werringloer U, Wildt KS et al. Chlamydophila (Chlamydia) pneumoniae in the Alzheimer’s brain. FEMS Immunol Med Microbiol 2006; 48: 355–366. [PubMed]
24. Gieffers J, Reusche E, Solbach W et al. Failure to detect Chlamydia pneumoniae in brain sections of Alzheimer’s disease patients. J Clin Microbiol 2000; 38: 881–882. [PubMed]
25. Gieffers J, van Zandbergen G, Rupp J, Sayk F, Kruger S, Ehlers S, Solbach W, Maas M. Phagocytes transmit Chlamydia pneumoniae from the lung to the vasculature. Eur Respir J 2004 ;23 :506-510. [PubMed]
26. Grayston JT, Kuo CC, Wang SP, Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infection. N Engl J Med 1986;315:161- 168. [PubMed]
27. Grayston JT. Chlamydia pneumoniae strain TWAR. Chest 1989; 95: 664–669. [PubMed]
28. Grayston JT, Campbell LA, Kuo CC, et al. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis 1990; 161: 618–625. [PubMed]
29. Grayston JT, Kronmal RA, Jackson LA et al. Azithromycin for the secondary prevention of coronary events. N Engl J Med 2005; 352:1637–1645. [PubMed]
30. Grimaldi LM, Pincherle A, Martinelli-Boneschi F et al. An MRI study of Chlamydia pneumoniae infection in Italian multiple sclerosis patients. Mult Scler 2003; 9: 467–471. [PubMed]
31. Gupta S, Leatham EW, Carrington D, Mendall MA, Kaski JC, Camm AJ. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation 1997;96:404-7. [PubMed]
32. Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA 1991; 266: 225–230. [PubMed]
33. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before–after trial. J Fam Pract 1995; 41: 345–351. [PubMed]
34. Hammerschlag MR. Activity of gemifloxacin and other new quinolones against Chlamydia pneumoniae: a review. J Antimicrobial Chemotherapy 2000;45 Suppl S1:35-39. [PubMed]
35. Hammerschlag MR. Antimicrobial susceptibility and therapy of infections caused by Chlamydia pneumoniae. Antimicrob Agents Chemother 1994;38: 1873-1878. [PubMed]
36. Hammerschlag MR, Chirgwin K, Roblin PM, Gelling M, Dumornay W, Mandel L, Smith P, Schachter J. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin Infect Dis 1992;14:178-182. [PubMed]
37. Hammerschlag MR, Qumei KK, Roblin PM. In vitro activities of azithromycin, clarithromycin, I-ofloxacin, and other antibiotics against Chlamydia pneumoniae. Antimicrob Agents Chemother 1992;36:1573-1574. [PubMed]
38. Hammerschlag MR, Roblin PM. Microbiologic efficacy of moxifloxacin for the treatment of community-acquired pneumonia due to Chlamydia pneumoniae. Int J Antimicrob Agents. 2000;15:149-52. [PubMed]
39. Hammerschlag MR. Chlamydia pneumoniae and the lung. Eur Respir J 2000; 16:1001–7. [PubMed]
40. Huovinen P, Lahtonen R, Ziegler T, Meurman O, Hakkarainen K, Miettinen A, Arstila P, Eskola J, Saikku P. Pharyngitis in adults: the presence and coexistence of J viruses and bacterial organisms. Ann Intern Med 1989;110:612-616. [PubMed]
41. Jackson LA. Description and status of the azithromycin and coronary events study (ACES). J Infect Dis 2000;181 Suppl 3:S579-81. [PubMed]
42. Johnston SL, Blasi F, Black PN, et al. The Effect of Telithromycin in Acute Exacerbations of Asthma: the TELICAST Study. New Engl J Med 2006;354:1589-600. [PubMed]
43. Jones RB, Van Der Pol B, Martin DH, Shepard MK. Partial characterization of Chlamydia trachomatis isolates resistant to multiple antibiotics. J Infect Dis 1990;162:1309-1315. [PubMed]
44. Kaufman M, Gaydos CA, Sriram S et al. Is Chlamydia pneumoniae found in spinal fluid samples from multiple sclerosis patients? Conflicting results Mult Scler 2002; 8: 289–294. [PubMed]
45. Ke Z, Lu F, Roblin P et al. Lack of detectable Chlamydia pneumoniae in brain lesions of patients with multiple sclerosis. Ann Neurol 2000;48: 400. [PubMed]
46. Kern JM, Maas V, Maas M. Chlamydia pneumoniae-induced pathological signaling in the vasculature. FEMS Immunol Med Microbiol 2009;55:131-139. [PubMed]
47. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest 2002; 121: 1782–1788. [PubMed]
48. Kumar S, Hammerschlag MR. Acute respiratory infection due to Chlamydia pneumoniae: current status of diagnostic methods. Clin Infect Dis 2007; 44: 568–576. [PubMed]
49. Kuo CC, Grayston JT. In vitro drug susceptibility of Chlamydia sp. strain TWAR. Antimicrob Agents Chemother 1988:32:257-258. [PubMed]
50. Kuo CC, Jackson LA, Lee A, Grayston JT. In vitro Activities of Azithromycin, Clarithromycin, and Other Antibiotics Against C. pneumoniae. Antimicrobial Agents Chemother 1996;40:2669-70. [PubMed]
51. Layh-Schmitt G, Bendl C, Hildt U et al. Evidence for infection with Chlamydia pneumoniae in a subgroup of patients with multiple sclerosis. Ann Neurol 2000; 47: 652–655. [PubMed]
52. Lieberman D, Lieberman D, Printz S, et al. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med 2003;167:406–410. [PubMed]
53. Lipsky BA, Tack KJ, Wang SP, Kuo CC, Grayston JT. Ofloxacin treatment of Chlamydia pneumoniae (strain TWAR) lower respiratory tract infections. Am J Med 1990;89:722-724. [PubMed]
54. Little CS, Hammond CJ, MacIntyre A, Balin BJ, Appelt DM. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice, Neurobiol Aging 2004;25: 419–429. [PubMed]
55. Loeb MB, Molloy DW, Smieja M et al. A randomized, control trial of doxycycline and rifampin for patients with Alzheimer’s disease. J Am Geriatr Soc 2004;52: 381–387. [PubMed]
56. Marrie TJ, Harczy M, Mann OE, Landymore RW, Raza A, Wang SP, Grayston JT. Culture-negative endocarditis probably due to Chlamydia pneumoniae. J Infect Dis 1990;161:127 -129. [PubMed]
57. Marrie TJ, Peeling RW, Reid T, et al. Chlamydia species as a cause of community-acquired pneumonia in Canada. Eur Respir J 2003; 21: 779–784. [PubMed]
58. Michel D, Antoine JC, Pozzetto B, Gaudin OG, Lucht F. Lumbosacral meningoradiculitis associated with Chlamydia pneumoniae infection. J Neurol Neurosurg Psychiatry 1992;55:511. [PubMed]
59. Miyashita N, Ouchi K, Kishi F et al. Rapid and simple diagnosis of Chlamydophila pneumoniae pneumonia by an immunochromatographic test for detection of immunoglobulin M antibodies. Clin Vaccine Immunol 2008; 15: 1128–1131. [PubMed]
60. Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, Saikku P, Juvonen T. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg 2001;34:606-10. [PubMed]
61. Mourad A, Sweet RL, Sugg N, Schachter J. Relative resistance to erythromycin in Chlamydia trachomatis. Antimicrob Agents Chemother 1980;18:696-698. [PubMed]
62. Muhlestein JB, Anderson JL, Hammond EH, Zhao L, Trehan S, Schwobe EP, Carlquist JF. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation 1998;97:633-6. [PubMed]
63. Mussa FF, Hong Chai H, Wang X et al. Chlamydia pneumoniae and vascular disease: an update. J Vasc Surg 2006; 43: 1301–1307. [PubMed]
64. Nagy A, Kozma GT, Keszei M et al. The development of asthma in children infected with Chlamydia pneumoniae is dependent on the modifying effect of mannose-binding lectin. J Allergy Clin Immunol 2003;112: 729–734. [PubMed]
65. Neumann F, Kastrati A, Miethke T, Pogatsa-Murray G, Mehilli J, Valina C, Jogethaei N, de Costa C. pneumoniae, Wagner H, Schomig A. Treatment of Chlamydia pneumoniae infection with roxithromycin and effect on neointima proliferation after coronary stent placement (ISAR-3): a randomised, double-blind, placebo-controlled trial. Lancet 2001;357:2085-9. [PubMed]
66. Nochlin D, Shaw CM, Campbell LA et al. Failure to detect Chlamydia pneumoniae in brain tissues of Alzheimer’s disease. Neurology 1999;53: 1888. [PubMed]
67. O’Connor CM, Dunne MW, Pfeffer MA,. et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA 2003; 290:1459–1466. [PubMed]
68. Ogawa H, Hashiguchi K, Kazuyama Y. Recovery of Chlamydia pneumoniae in six patients with otitis media and effusion. J Laryngol Otol 1992;106:490-492. [PubMed]
69. Puolakkainen M. Innate immunity and vaccines in chlamydial infection with special emphasis on Chlamydia pneumoniae. FEMS Immunol Med Microbiol 2009;55:167-177. [PubMed]
70. Rajalingam K, Al-Younes H, Müller A, Meyer TF, Szczepek AJ, Rudel T. Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect Immun 2001; 69: 7880–7888. [PubMed]
71. Richeldi L, Ferrara G, Fabbri LM, et al. Macrolides for chronic asthma (Cochrane Review). In: The Cochrane Library, Issue 1. Oxford: Update Software; 2003. [PubMed]
72. Roblin PM, Hammerschlag MR. Microbiologic efficacy of azithromycin and susceptibilities to azithromycin of isolates of Chlamydia pneumoniae from adults and children with community-acquired pneumonia. Antimicrob Agents Chemother. 1998;42:194-6. [PubMed]
73. Roblin PM, Kutlin A, Reznik T, Hammerschlag MR. Activity of grepafloxacin and other fluoroquinolones and new macrolides against recent clinical isolates of Chlamydia pneumoniae. Int J Antimicrob Agents 1999;12:181-4. [PubMed]
74. Roblin PM, Montalban G, Hammerschlag MR. Susceptibilities to clarithromycin and erythromycin of isolates of Chlamydia pneumoniae from children with pneumonia. Antimicrob Agents Chemother 1994;38:1588-9. [PubMed]
75. Saikku P, Mattila K, Nieminen MS, Makela PH, Huttunen JK, Valtonen V. Serological evidence of an association of a novel chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 1988;2:983-6. [PubMed]
76. Sriram S, Stratton CW, Yao S et al. Chlamydia pneumoniae infection of the central nervous system and multiple sclerosis. Ann Neurol 1999; 46: 6–14. [PubMed]
77. Stallings TL. Association of Alzheimer’s disease and Chlamydophila pneumoniae. J Infect 2008;56:423-431. [PubMed]
78. Stratton CW, Wheldon DB. Multiple sclerosis: an infectious syndrome involving Chlamydophila pneumoniae. Trends Microbiol 2006; 14:474–479. [PubMed]
79. Tang YW, Sriram S, Li H, Yao S, Meng S, Mitchell WM, Stratton CW. Qualitative and quantitative detection of Chlamydophila pneumonia DNA in cerebrospinal fluid from multiple sclerosis patients and controls. PLoS one 2009;4:e5200. [PubMed]
80. Thom DH, Grayston JT, Siscovick DS, Wang SP, Weiss NS, Daling JR. Association of prior infection with Chlamydia pneumoniae and angiographically demonstrated coronary artery disease. JAMA 1992;268:68-72. [PubMed]
81. Theegarten D, Mogilevski G, Anhem O, et al. The role of Chlamydia in the pathogenesis of pulmonary emphysema. Virchows Arch 2000;437:190–193. [PubMed]
82. Vammen S, Lindholt JS, Ostergaard L, Fasting H, Henneberg EW. Randomized double-blind controlled trial of roxithromycin for prevention of abdominal aortic aneurysm expansion. Br J Surg 2001;88:1066-72. [PubMed]
83. von Hertzen L, Alakärppä H, Koskinen R, et al. Chlamydia pneumoniae infection in patients with chronic obstructive pulmonary disease. Epidemiol Infect 1997;118:155–64. [PubMed]
84. von Hertzen LC. Role of persistent infection in the control and severity of asthma: focus on Chlamydia pneumoniae. Eur Respir J 2002; 19: 546–56. [PubMed]
85. Watson C, Alp NJ. Role of Chlamydia pneumoniae in atherosclerosis. Clin Sci 2008; 114: 509–531. [PubMed]
86. Welsh L, Gaydos C, Quinn TC. In vitro activities of azithromycin, clarithromycin, erythromycin, and tetracycline against 13 strains of Chlamydia pneumoniae. Antimicrob Agents Chemother 1996;40:212-214. [PubMed]
87. Welti M, Jaton K, Altwegg M, Sahli R, Wenger A, Bille J. Development of a multiplex real-time quantitative PCR assay to detect Chlamydia pneumoniae, Legionella pneumophila and Mycoplasma pneumoniae in respiratory tract secretions. Diagn Microbiol Infect Dis 2003; 45:85–95. [PubMed]
88. Wong YK, Gallagher PJ, Ward ME. Chlamydia pneumoniae and atherosclerosis. Heart 1999;81:232-8. [PubMed]
89. Wu L, Skinner SJM, Lambie N, et al. Immunohistochemical staining for Chlamydia pneumoniae is increased in lung tissue from subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:1148–51.[PubMed]
Tables
Table 1. Summary of The Results of In Vitro Susceptibility Testing of C. Pneumoniae.
| Antibiotic | MIC on first passage (µg/mL) | MIC on second passage (µg/mL) |
|---|---|---|
| Tetracycline | 0.05 | 0.05-4 |
| Doxycycline | 0.05-0.5 | 0.125-0.5 |
| Minocycline | 0.015 | NA |
| Erythromycin | 0.01-0.25 | 0.01-4.0 |
| Azithromycin | 0.06-1.0 | 0.125-2.0 |
| Clarithromycin | 0.004-0.25 | 0.004-2.0 |
| Roxithromycin | 0.125-0.25 | 0.125 |
| Ciprofloxacin | 0.25-4.0 | 0.25-8.0 |
| Ofloxacin | 0.5-2.0 | 0.5-2.0 |
| Levofloxacin | 0.25-1.0 | 0.25-1.0 |
| Fleroxacin | 2.0-8.0 | 2.0-8.0 |
| Temafloxacin | 0.125-4.0 | 0.125-40 |
| Gatifloxacin | 0.06-0.25 | 0.06-0.25 |
| Gemifloxacin | 0.06-0.25 | 0.125-0.25 |
| Grepafloxacin | 0.06-0.5 | 0.03-0.5 |
| Sparfloxacin | 0.06-0.5 | 0.06-0.25 |
| Trovafloxacin | 0.5-1.0 | 0.5-1.0 |
| Ampicillin | >100 | 0.8-1.6 |
| Penicillin | >100 | 0.1-0.2 |
| Sulfisoxazole | >400 | > 400 |
| Sulfamethoxazole | >500 | NA |
NA = not available.
Figure 1: C. pneumoniae Interaction with Different Human Cell Types
What's New
West, SK et al. Detection of Circulating Chlamydophila pneumoniae in Patients with Coronary Artery Disease and Healthy Control Subjects.Clin Infect Dis. 2009 Mar 1;48(5):560-7.

