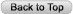|
Rhodotorula species
Previous Authors:
Gil Redelman-Sidi, MD,
Arthur E. Brown, MD,
Susan K. Seo, MD
Microbiology
Rhodotorula
species are pigmented basidiomycetous yeasts in the family Sporidiobolaceae
(21). The genus
contains 37 species, of which only three, including R. mucilaginosa
(formerly R. rubra), R. minuta, and R. glutinis, have
been reported as causes of infection in humans (7).
Three novel species, which are non-pathogenic to humans, have recently been
described: R. rosulata,
R. silvestris and
R. straminea (30).
Most Rhodotorula
species produce colonies that are pink to coral in color but can also be
orange to red on Sabouraud agar due to the presence of carotenoid pigments (Figure
1). Colony morphology
has been described as soft, smooth, moist, and sometimes mucoid.
Rhodotorula species are nutritionally non-fastidious, grow easily on
most media, and are characterized by a rapid growth rate. They appear as
round or oval budding cells under microscopy, and pseudohyphae are rarely
present. A faint capsule is sometimes formed. Rhodotorula species
produce the enzyme urease and do not ferment carbohydrates. They can be
differentiated from Cryptococcus species by their inability to
assimilate
inositol and
from Candida species by production of pigmented colonies and the lack
of pseudohyphae (47).
Epidemiology Rhodotorula
species are widespread in nature and can be isolated from a variety of
sources including air, soil, seawater, plants, dairy products, and the
household environment (e.g., shower curtains, bathtub grout) (1,
45, 85). It is also possible for
laboratory specimens to become contaminated with this organism (35).
In humans, Rhodotorula species have been recovered from cultures of
skin, nails, and respiratory, gastrointestinal, and urinary tracts and are
generally thought to be commensals. Rhodotorula
species were initially thought to be non-pathogenic until 1960 when the
first case of fatal endocarditis in a 47-year-old woman with rheumatic heart
disease was published (50). Since
then, ~215 cases of
Rhodotorula species infection in humans have been reported in the
literature, primarily over the last two
decades (2,
5, 12,
20, 26, 51,
58, 62, 65,
71, 76, 81,
84).
R. mucilaginosa is the most common species involved in human
infections, causing 72%
of the cases, followed by R. glutinis (8%) and R. minuta (3%);
speciation is not reported for the remaining
17% of cases in the literature (Table
1).
Rhodotorula Infection appears to
be more common in tropical countries than in Northern regions (3).
There has been at least one
documented R. mucilaginosa outbreak in a neonatal intensive care unit
where four premature infants with indwelling catheters developed fungemia
over a period of 19 days and fully recovered with intravenous (IV) liposomal
amphotericin B therapy. Attempts to identify the environmental source were
unsuccessful (65). Nearly
90% of patients with Rhodotorula species infection have underlying
solid or hematologic malignancy, organ/bone marrow transplant, or
immunosuppression due to corticosteroid use, neutropenia, Acquired
Immunodeficiency Syndrome (AIDS), or malnutrition (9,
81). The most common
risk factor is the presence of a central venous catheter (CVC) (12,
42, 51,
82, 88). One third of the cases are
receiving parenteral nutrition when Rhodotorula infection appears (8, 85,
86).
The
average length of time that an indwelling catheter is in place prior to
diagnosis of fungemia can be short, as in one series by De Almeida and
colleagues (86.5 days, range: 4-261 days) (12),
or long, as reported by Kiehn et al. (9.3 months, range: 1-22 months) (42).
Although the precise incidence is unknown, infection due to Rhodotorula
species is much less common than infection with other yeast species such
as Candida and Cryptococcus.
Nevertheless, the number of infections has
clearly increased during the last few years (86).
In a retrospective study at
a teaching hospital in Brazil over a 9-year period, only 2.3% of fungal
blood isolates were Rhodotorula species, compared to 83.4% and 6.6%
for Candida and Cryptococcus species, respectively (12).
Clinical
Manifestations Rhodotorula
species have a clear tendency to produce central nervous system (CNS)
infection, of which twenty-three cases have been reported to date (8). These
included 20 cases of meningitis
and three cases of ventriculitis (due to CSF drainage catheter or shunt).
Although a case of meningitis has been described in an immunocompetent adult
(46), patients with Rhodotorula species meningitis generally have a
predisposing condition, such as AIDS, malignancy, or autoimmune disease
(e.g., systemic lupus erythematosus) (5, 33,
62, 68, 76,
79, 80).
In the case of
ventriculitis, the underlying factor was the use of an intraventricular
catheter, which was removed as an adjunct to antifungal treatment (18).
Presentation may be acute (55, 46), subacute (79,
80) or chronic (33).
Symptoms include fever, headache, altered sensorium,
and nuchal rigidity.
The cerebrospinal fluid usually shows lymphocytic pleocytosis with decrease
of glucose and increased protein (80).
MRI imaging may
show foci of increased signal throughout the brain and brain-stem without
hemorrhage (80).
Although Rhodotorula is a rapid growing yeast, prolonged periods of
incubation have been observed prior to the appearance of symptoms. This
entails either low fungal load or quiescent infection (61,
80). The
identification of microcalcifications in the brain biopsy may be indicative
of a subjacent indolent course (80). On the other hand, the
extensive brain edema observed in some cases may suggest immune
reconstitution inflammatory syndrome (4).
Although the organism can generally be
recovered from the cerebrospinal fluid (CSF) culture, it may initially be
considered to be a laboratory contaminant resulting in the institution of
antifungal therapy being delayed (5,
46).
Unlike fungemia, localized infections, including skin, ocular, meningeal,
prosthetic
joint and peritoneal infections, are not necessarily related to
immunosuppression or to
the use of
CVCs.
Thirteen cases of
ocular infection due to Rhodotorula species have been reported in the
literature and include keratitis and endophthalmitis (81). A predisposing
cause can often be elicited. Keratitis may occur after trauma, keratoplasty
and corneal grafting (6, 27,
48, 70, 72) and endophthalmitis has been
described in patients with a history of injected drug use (54,
67).
In comparison to keratitis, endophthalmitis is associated with a
worse prognosis with some patients requiring either a vitrectomy or
enucleation (32, 54, 67).
Anecdotal presentations of Rhodotorula species causing infection have
included orthopedic prosthesis infections (11,
74),
hydrosalpingitis (28),
lymphadenitis (23),
skin infection (10, 39, 53), onychomycosis (13,
83), infected oral ulcers
(14, 40) and disseminated infection with bone marrow isolation of the
organism (13, 56, 83,
86). The two reported cases of orthopedic prosthesis
infection required hardware removal to cure the infection (11,
74). The overall crude mortality of infections caused by Rhodotorula species is 12.6% (81). However, it is difficult to estimate how much of this mortality is directly attributable. In fact, some cases of fungemia have evolved favorably without antifungal treatment (66).
Laboratory
Diagnosis
Isolation
of Rhodotorula species from non-sterile sites such as skin, sputum,
or stool is more likely to be due to colonization or contamination, and
treatment in such cases should only be started in the presence of symptoms
strongly suggestive of infection and after other causes have been excluded.
The clinician should also be aware that
pseudo-outbreaks have occurred due to
specimen contamination (35).
One communicated pseudo-outbreak was due to fungal contamination of brushes
used to clean bronchoscopes (37). The
recovery of Rhodotorula species from a sterile site, such as blood,
peritoneal fluid or CSF, is usually indicative of infection. The clinician
should maintain a high degree of suspicion in such cases, especially if the
patient has no suggestive symptoms of infection. Morphological and
biochemical confirmation of the diagnosis as described in the preceding
‘Microbiology’ section should be sought since yeast cells can usually be
seen on microscopic examination (47). In
cases of meningitis, the CSF typically shows lymphocytic pleocytosis, and
India ink stain can reveal encapsulated budding yeast cells (5,
76, 79). It
may be difficult to morphologically differentiate Rhodotorula species
meningitis from cryptococcal meningitis, and an instance of false-positive
latex agglutination test (LAT) for cryptococcal antigen has been reported (79).
Characteristic pigmentation of the colonies, confirmatory biochemical tests
(e.g., absence of carbohydrate fermentation, production of urease), and
absence of ballistospore formation should lead to a specific microbial
identification (5,
79).
The mainstay in the diagnosis of invasive
Rhodotorula spp. infection is
blood culture as 78% of the systemic infections present as fungemia (81). It
should be noted that that isolates of
Rhodotorula have been found to cross react with the
Candida glabrata/ Candida krusei
probe in the commercially available fluorescence in situ hybridization
(FISH) test for species identification in positive blood cultures, which
could lead to inappropriate echinocandin treatment (36).
Panfungal PCR allows for
highly sensitive, specific detection and identification of a wide spectrum
of fungal pathogens in blood samples, including
Rhodotorula (40,
78), however it
is difficult to predict when these techniques will be incorporated into
conventional clinical practice (40).
Since the CVC is often involved, the catheter tip should be cultured when
removed so as to capture cases where blood cultures are falsely negative
(86). Rhodotorula isolates are easily recognizable in the laboratory due
to their distinctive orange to salmon-colored colonies, morphology,
formation of rudimentary hyphae and urease production.
Antigen detection has not been reported in clinical cases of systemic
Rhodotorula infections.
ß1-3-D-glucan has been detected in the supernatant from isolates of
R. mucilaginosa at an average
concentration of two-thirds of that of
Candida spp (60). Whether the ß 1-3-D-glucan test would be useful as a
surrogate marker for invasive
Rhodotorula infection remains to be investigated (60). As could be
expected, Cryptococcal antigen, Aspergillus galactomannan and Candida mannan
are usually negative in biologic fluids (80).
Pathogenesis
The pathogenesis of
infection due to Rhodotorula species has not been studied. As
mentioned previously, in almost all cases there is underlying
immunosuppression and/or the presence of a foreign body.
The low pathogenicity of
Rhodotorula spp. is probably
related to its reduced ability to grow at 37°C. Immunocompromised
individuals form few epithelioid cells or multinucleated giant cells thereby
promoting yeast growth (87). It has been demonstrated that
Rhodotorula species are able to
form biofilms which could play a role in the pathogenesis of infections
caused by these species (73). According to one study,
R. minuta and
R. mucilaginosa are able to
produce more biofilm than R. glutinis
(59). It can be speculated that
invasive infections may occur as a result of environmental contamination of
an inserted prosthetic device (16, 18,
19), but it seems more
likely that the organism is an opportunist that takes advantage of
immunocompromising conditions, indwelling devices, and exposure to
broad-spectrum antibiotics to colonize and infect at-risk patients (12,
42,
52). Antibiotics
and exposure to cytotoxic agents may increase gastrointestinal colonization
and intestinal mucosa damage. Few studies have investigated the role of the
digestive tract as a source of Rhodotorula spp (82).
SUSCEPTIBILITY
IN VITRO AND IN VIVO There
are few publications reporting on in vitro susceptibility testing of
Rhodotorula species with Clinical and Laboratory Standard Institute
(CLSI) methodology. In general, flucytosine and amphotericin B appear to be
the most active agents in vitro against R. mucilaginosa, R.
glutinis, and other Rhodotorula species.
Table 2
summarizes the susceptibilities of Rhodotorula species to antifungals
as assessed in four studies (17, 24,
31, 88). Zaas and colleagues determined
antifungal susceptibilities for eight R. mucilaginosa and two R.
glutinis blood isolates identified over a 9-year period at their
institution. Flucytosine MICs ranged from 0.125-0.25 µg/mL, and amphotericin
B MICs ranged from 0.25-1 µg/mL (88). Diekema et al. studied 64 isolates
between 1987 and 2003 (17).
All isolates were inhibited by flucytosine at <0.5 µg/mL. Amphotericin B
MICs by broth dilution were < 1µg/mL, but interestingly, Etest detected 8
isolates with MICs >1 µg/mL, raising the possibility that some
Rhodotorula isolates may be less susceptible in vitro or in
vivo. One Spanish study utilized a somewhat modified European Committee
on Antibiotic Susceptibility Testing (EUCAST) protocol for yeast and found a
wider MIC range for flucytosine (0.06 to >64 µg/mL) and amphotericin B (0.06
to 8 µg/mL) in their collection of 29 Rhodotorula species isolates
(25 R. mucilaginosa, 4 R. glutinis) (31).
However, the authors concluded that both drugs showed good in vitro
activity against Rhodotorula species. Rhodotorula
species appear to be resistant to the echinocandins with high MICs. Reported
MICs ranged from 8 to 16 µg/mL in one study (17)
and were >16 µg/mL in another (88).
MICs >64 µg/mL have been reported for micafungin (88). Fluconazole can also
be predicted to be ineffective against Rhodotorula species since the
majority of isolates exhibit MICs >64 µg/mL (17, 31, 88). The
resistance mechanism is unknown, but the
repeated pattern of high MICs suggests intrinsic resistance (81).
The newer triazoles show variable degrees of potency. In the largest study
of 64 Rhodotorula species isolates, 17% of isolates had MICs >4 µg/mL
when tested against posaconazole in contrast to itraconazole (33%) and
voriconazole (31%) (17).
Ravuconazole, an investigational agent, appeared to be the most active with
MICs ranging from <0.06 to 2 µg/mL. No
significant differences in the activities of any of the tested antifungal
agents according to Rhodotorula species have been noted in the cited
studies (17, 31, 88).
The majority of the patients presented were receiving antifungal
prophylaxis, mainly fluconazole, when fungemia was noticed (55,
86). This
fact could be related to the intrinsic resistance of
Rhodotorula to fluconazole (and
echinocandins) (17,
31,
51). Some strains also show resistance to voriconazole and posaconazole, although these drugs may be effective.
According to laboratory results and clinical experience
in HSCT recipients,
amphotericin B appears to be the drug of choice for
Rhodotorula infection (22, 25,
55).
One study
tested 35 different strains of Rhodotorula species utilizing the
Sensititre YeastOne MIC Susceptibility Test (AccuMed International Ltd, UK),
a commercial broth microdilution test that correlates highly with reference
procedures (24).
All strains were considered to be susceptible to amphotericin B (MIC range:
0.125 to 0.5 µg/mL), flucytosine (MIC range: 0.064 to 0.25 µg/mL),
ketoconazole (MIC range: 0.125 to 0.25 µg/mL), and itraconazole (MIC range:
0.25 to 1 µg/mL), and resistant to fluconazole (MICs >32 µg/mL). It is worth
noting that MIC results for ketoconazole and itraconazole are lower than
what has been reported with the CLSI and EUCAST protocols. Gomez-Lopez and
colleagues found similar results when reviewing MIC data between reference
and Sensititre YeastOne methods (31).
Further studies are needed to clarify the role of extended-spectrum
triazoles.
There are
scant data on the susceptibility of Rhodotorula species to
combinations of antifungals. A study by Serena et al. evaluated the in
vitro activity of combinations of micafungin with amphotericin B or
triazole agents against basidiomycetous yeasts including 10 isolates of
R. glutinis (75).
Drug interactions were assessed by the checkerboard technique using the CLSI
microdilution method (M27-A2). The fractional inhibitory concentration index
(FICI) was used to classify drug interactions as synergistic (FICI <0.5),
null (FICI >0.5 and <4.0), or antagonistic (FICI >4.0). For R. glutinis,
the combination of micafungin and fluconazole showed only 20% synergism.
Micafungin combined with ravuconazole showed the highest percentage of
synergistic interactions (80%), followed by micafungin/amphotericin B (70%),
micafungin/itraconazole (60%), and micafungin/voriconazole (60%).
Limitations include the small number of isolates available for testing, lack
of data for other Rhodotorula species, and lack of clinical data to
support these in vitro results.
While there
is limited clinical experience using amphotericin B and flucytosine together
(81),
this combination has been suggested due to good in vitro activity of
flucytosine (17).
ANTIMICROBIAL
THERAPY
Drug
of Choice
There are no
randomized controlled trials that have evaluated treatment for
Rhodotorula species infections. Based on the available in vitro
susceptibility data, amphotericin B or one of its lipid formulations appears
to be the drug of choice (81).
Recommended antifungals for treatment of Rhodotorula species
infections are listed in
Table 3.
Special
Infections
Clinical
experience in treatment of bloodstream infections has by and large been with
conventional amphotericin B administered intravenously at dosages from 0.7
to 1 mg/kg daily (12, 42,
51) or one of its lipid formulations at a dose of
3 to 5 mg/kg daily (26, 65). On occasion, combination therapy with flucytosine has been reported (51,
81). One patient, who was a recipient of
simultaneous liver-kidney transplant, received combination treatment using voriconazole (200 mg intravenously twice daily) and micafungin (100 mg
intravenously once daily) for catheter-associated C. glabrata and
R. glutinis fungemia (71). Treatment was selected on the basis of
antifungal susceptibility testing and in consideration of the patient’s
underlying renal and hepatic insufficiency. Length of treatment is variable
in the literature, ranging from 14 to 41 days (81). One report has suggested
two weeks’ duration if the CVC is not removed and one week with CVC removal
(42). There
are no data regarding the optimal treatment and duration for endocarditis.
One 7-year-old boy with presumptive rheumatic fever and Rhodotorula
species endocarditis was treated with 3 months of oral flucytosine 100 mg/kg
daily divided into 4 doses. He defervesced within 3 days of starting
effective therapy and had normalization of erythrocyte sedimentation rate
(ESR) in 2 months (57).
In another report, a 53-year-old man with R. mucilaginosa homograft
endocarditis was managed surgically in combination with amphotericin B (a
total of 2 grams administered over 28 days), followed by oral itraconazole
(200 mg twice daily for one month) (52).
Two of the reported
cases (one with concomitant meningitis) were managed without surgery
(49, 57), nevertheless, surgical treatment is strongly indicated in all cases
of fungal endocarditis (34). CNS
infections have been treated with conventional amphotericin B (0.7 to 1
mg/kg IV daily) (5, 46,
76),
flucytosine monotherapy (25 mg/kg orally every 6 hours) (33),
or combination therapy using amphotericin B and flucytosine (18,
79).
Treatment is generally for 2 weeks, but it must be taken into account that
relapses are possible (33)
and mortality may be over 40% despite antifungal treatment (80). For
patients with AIDS, consideration should be given to starting antiretroviral
therapy as an adjunct (5,
79). For
ocular infections with Rhodotorula, topical or intravitreal
amphotericin has commonly been used with or without the addition of systemic
antifungals (6, 32, 48,
54, 67).
Keratitis generally responds to topical treatment alone, whereas more
serious infections like endophthalmitis typically require systemic
antifungal administration and surgical drainage as part of management (72,
81).
Similarly, intraperitoneal amphotericin B, in combination with removal of
the peritoneal catheter, has been used successfully in the treatment of
peritonitis due to Rhodotorula species (19,
77).
Systemic antifungal therapy with or without catheter removal has also been
reported for the treatment of peritonitis. One patient in whom the
peritoneal catheter was not removed was successfully treated with one month
of oral ketoconazole (16),
whereas another patient, who was a liver transplant recipient, was treated
initially with catheter removal and concomitant amphotericin B deoxycholate,
then liposomal amphotericin B for a total of 10 days (2).
Alternative
Therapy
Although fluconazole has been prescribed in some cases with sporadic success
(44, 81),
it should probably not be used given the high level of
in vitro resistance unless
susceptibility testing suggests otherwise. There has been one report of
successful itraconazole treatment of R. mucilaginosa lymphadenitis in
a patient with HIV (200 mg orally once daily
for 8 months) (23).
Half of the reported cases of onychomycosis evolved favorably with
itraconazole (those strains showed MIC <= 0.125
μg/mL) (83). Itraconazole has also successfully employed in one case of an infected oral
ulcer (40).
Echinocandins
alone should not be used for the treatment of Rhodotorula species
infections because of the high level of in vitro resistance. While
there is some evidence that micafungin has in vitro synergy when used
in combination with several antifungals (see ‘Combination Drugs’), there is
limited clinical evidence suggesting that it is of benefit. It has been used
successfully in combination with voriconazole in at least one case of R.
glutinis fungemia (71). For patients unable to tolerate amphotericin B or
flucytosine, susceptibility testing of extended-spectrum triazole agents may
help to guide appropriate therapy. In such cases, special care should be
taken to remove any foreign bodies associated with the infection (see
‘Adjunctive Therapy’) in order to maximize the chance of a favorable
outcome.
ADJUNCTIVE
THERAPY
As in
candidemia (63),
CVC removal should be strongly considered as part of treatment for
Rhodotorula species fungemia as it may hasten blood clearance (58,
82).
There have even been cases when CVC-related fungemia due to Rhodotorula
species was treated successfully with CVC removal alone (42,
51).
However, there do not seem to be clear guidelines as to which patients would
warrant CVC removal only. Therefore it seems prudent to consider CVC removal
in light of concomitant systemic antifungal
therapy. For peritonitis,
the literature supports the removal of the peritoneal dialysis catheter as
part of management (19, 64,
77).
CNS infections, particularly ventriculitis, may also require removal of the
foreign body (18).
ENDPOINTS
FOR MONITORING THERAPY Duration of antifungal therapy should be guided by the
patient’s clinical presentation, underlying disease, and degree of
immunosuppression.
VACCINES There are no available vaccines for prevention of
Rhodotorula species infection.
PREVENTION
OR INFECTION CONTROL MEASURES As with other opportunistic infections, an important
aspect of prevention of Rhodotorula species infections is to minimize
associated risk factors. CVCs should be used judiciously and should be
removed as soon as they are no longer needed. Great care should be taken to
maintain sterility when inserting intravascular and intraperitoneal
catheters, and sterility should be maintained during the long-term
maintenance of these and other devices. There are
no studies to date evaluating antifungal prophylaxis for prevention of
Rhodotorula species infections. Given the rarity of these
infections, specific prophylaxis is not recommended. There are no data to
suggest that antifungal agents used for prophylaxis in other situations
(e.g., after bone marrow transplantation) are effective in the prevention of
Rhodotorula species infection. Breakthrough infections have been
reported in patients receiving azoles or echinocandin prophylaxis (8,
22,
25, 26, 29,
51, 55, 65,
69).
Infection Control
Patients infected with Rhodotorula species do not require any
specific infection control precautions. The organisms are common in the
environment and are likely acquired through colonization with environmental
strains. There is no evidence of human-to-human transmission of
Rhodotorula species. However, health care workers’ hands (as well as any
rings) can be contaminated with
Rhodotorula spp (41). In the
absence of specific data, standard infection control precautions, including
hand-washing and proper skin cleansing and preparation prior to invasive
procedures, should be emphasized.
Figure 1: Colonies of Rhodotorula glutinis
*Onychomycosis (n=4), Skin infection (n=3), oral ulcer (n=1) Orthopedic
prosthesis infections (n=2), hydrosalpingitis (n=1), lymphadenitis (n=1),
disseminated infection (n=1)
**Concomitant endocarditis and meningitis in one patient
Table 2: Summary of Five Reports on Susceptibility Data
of Rhodotorula Species
Table
3: Recommended Antifungals for Rhodotorula Species
REFERENCES 1.
Ahearn DG, Roth FJ, Jr., Meyers SP. A comparative study of marine and
terrestrial strains of Rhodotorula. Can J Microbiol. 1962;8:121-32.
[PubMed]
2. Alothman
A. Rhodotorula species peritonitis in a liver transplant recipient: a
case report. Saudi J Kidney Dis Transpl. 2006;17:47-9.
[PubMed] 3. Arendrup MC, Boekhout T, Akova M,
Meis JF, Cornely OA, Lortholary O.
ESCMID and ECMM joint clinical guidelines for the diagnosis and
management of rare invasive yeast infections. Clin Microbiol Infect.
2014;20:76-98. 4. Bahr N, Boulware DR, Marais S, Scriven
J, Wilkinson RJ, Meintjes G.
Central nervous system immune reconstitution inflammatory syndrome. Curr
Infect Dis Rep. 2013;15:583-93 5. Baradkar VP, Kumar S. Meningitis
caused by Rhodotorula mucilaginosa in human immunodeficiency virus
seropositive patient. Ann Indian Acad Neurol. 2008;11:245-7.
6. Bawazeer AM, Hodge WG. Rhodotorula infection in a corneal
graft following penetrating keratoplasty. Can J Ophthalmol. 2003;38:225-7.
[PubMed]
7. Biswas SK, Yokoyama K, Nishimura K, Miyaji M. Molecular phylogenetics
of the genus Rhodotorula and related basidiomycetous yeasts inferred
from the mitochondrial cytochrome b gene. Int J Syst Evol Microbiol.
2001;51(Pt 3):1191-1199.
[PubMed] 8. Capoor MR, Aggarwal S, Raghvan C,
Gupta DK, Jain AK, Chaudhary R. Clinical and microbiological characteristics
of Rhodotorula mucilaginosa infections in a tertiary-care facility. Indian J
Med Microbiol. 2014;32:304-9 9. Chitasombat MN, Kofteridis DP,
Jiang Y, Tarrand J, Lewis RE, Kontoyiannis DP. Rare opportunistic
(non-Candida, non-Cryptococcus) yeast bloodstream infections in patients
with cancer. J Infect 2012; 64: 68–75. 10. Coppola R, Zanframundo S, Rinati
MV, Carbotti M, Graziano A, Galati G, De Florio L, Panasiti V. Rhodotorula
mucilaginosa skin infection in a patient treated with sorafenib. J Eur Acad
Dermatol Venereol. March 2014. [Epub ahead of print]
[PubMed]
11.
Cutrona AF, Shah M, Himes MS, Miladore MA. Rhodotorula minuta: an
unusual fungal infection in hip-joint prosthesis. Am J Orthop (Belle Mead
NJ). 2002;31:137-40.[PubMed]
12.
De Almeida GM, Costa SF, Melhem M, Motta AL, Szeszs MW, Miyashita F,
Pierrotti LC, Rossi F, Burattini MN. Rhodotorula spp. isolated from
blood cultures: clinical and microbiological aspects. Med Mycol.
2008;46:547-56.
[PubMed] 13. da Cunha MM, dos Santos LP,
Dornelas-Ribeiro M, Vermelho AB, Rozental S. Identification, antifungal
susceptibility and scanning electron microscopy of a keratinolytic strain of
Rhodotorula mucilaginosa: a primary causative agent of onychomycosis. FEMS
Immunol Med Microbiol. 2009;55:396-403
15. Diktas H, Gulec B, Baylan O, Oncul O, Turhan V, Acar
A, Gorenek L. Intraabdominal abscess related fungaemia caused by Rhodotorula
glutinis in a non-neutropenic cancer patient. Acta Clin Belg. 2013;68:62-4.
16. de Zoysa JR, Searle M, Lynn KL, Robson RA. Successful
treatment of CAPD peritonitis caused by rhodotorula mucilaginosa.
Perit Dial Int. 2001;21:627-8.
[PubMed] 17.
Diekema DJ, Petroelje B, Messer SA, Hollis RJ, Pfaller MA. Activities of
available and investigational antifungal agents against rhodotorula
species. J Clin Microbiol. 2005;43:476-8.
[PubMed]
18.
Donald FE, Sharp JF, Firth JL, Crowley JL, Ispahani P. Rhodotorula rubra
ventriculitis. J Infect. 1988;16:187-91.
[PubMed]
19.
Eisenberg ES, Alpert BE, Weiss RA, Mittman N, Soeiro R. Rhodotorula rubra
peritonitis in patients undergoing continuous ambulatory peritoneal
dialysis. Am J Med. 1983;75:349-52.
[PubMed]
20.
Elias ML, Soliman AK, Mahoney FJ, Karam El-Din AZ, El-Kebbi RA, Ismail TF,
Wasfy MM, Mansour AM, Sultan YA, Pimentel G, Earhart KC. Isolation of
cryptococcus,Candida, Aspergillus, Rhodotorula and nocardia from
meningitis patients in egypt. J Egypt Public Health Assoc.
2009;84(1-2):169-81.
[PubMed]
21.
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A.
Biodiversity and systematics of basidiomycetous yeasts as determined by
large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Microbiol.
2000;50 Pt 3:1351-71.
[PubMed] 22. Forés R, Ramos A, Orden B, de
Laiglesia A, Bautista G, Cabero M, Muńez E, Sánchez-Romero I, Navarro B,
Bravo J, Cabrera R. Rhodotorula species fungaemia causes low mortality in
haematopoietic stem-cell transplantation. A case report and review. Mycoses.
2012;55:e158-62.
23.
Fung HB, Martyn CA, Shahidi A, Brown ST. Rhodotorula mucilaginosa
lymphadenitis in an HIV-infected patient. Int J Infect Dis.
2009;13(1):e27-9.
[PubMed]
24. Galan-Sanchez F, Garcia-Martos P, Rodriguez-Ramos C,
Marin-Casanova P, Mira-Gutierrez J. Microbiological characteristics and
susceptibility patterns of strains of Rhodotorula isolated from
clinical samples. Mycopathologia. 1999;145:109-12.
[PubMed] 25. García-Suárez J1, Gómez-Herruz P,
Cuadros JA, Burgaleta C. Epidemiology and outcome of Rhodotorula infection
in haematological patients. Mycoses. 2011;54:318-24. 26. Garcia-Suarez J, Gomez-Herruz P, Cuadros JA, Guillen H, Burgaleta C. Rhodotorula mucilaginosa catheter-related fungaemia in a patient with multiple myeloma. Mycoses. 2009 Dec 23. 27. Giovannini J, Lee R, Zhang SX,
Jun AS, Bower KS. Rhodotorula keratitis: a rarely encountered ocular
pathogen. Case Rep Ophthalmol. 2014;5:302-10
28.
Gogate A, Deodhar L, Gogate S. Hydrosalpinx due to Rodotorula glutinis
(a case report). J Postgrad Med. 1987;33:34.
[PubMed]
29.
Goldani LZ, Craven DE, Sugar AM. Central venous catheter infection withRhodotorula
minuta in a patient with AIDS taking suppressive doses of fluconazole. J
Med Vet Mycol. 1995;33:267-70.
[PubMed] 30. Golubev WI, Scorzetti G.
Rhodotorula rosulata sp. nov., Rhodotorula silvestris sp. nov. and
Rhodotorula straminea sp. nov., novel myo-inositol-assimilating yeast
species in the Microbotryomycetes. Int J Syst Evol Microbiol. 2010; 60(Pt
10):2501-6.
31.
Gomez-Lopez A, Mellado E, Rodriguez-Tudela JL, Cuenca-Estrella M.
Susceptibility profile of 29 clinical isolates of Rhodotorula spp.
and literature review. J Antimicrob Chemother. 2005;55:312-6.
[PubMed]
32.
Gregory JK, Haller JA. Chronic postoperative Rhodotorula
endophthalmitis. Arch Ophthalmol. 1992;110:1686-7.
[PubMed]
33.
Gyaurgieva OH, Bogomolova TS, Gorshkova GI. Meningitis caused by
Rhodotorula rubra in an HIV-infected patient. J Med Vet Mycol.
1996;34(5):357-9. [PubMed] 34. Habib G, Hoen B, Tornos P, Thuny
F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U,
Lekakis J, Lengyel M, Müller L, Naber CK, Nihoyannopoulos P, Moritz A,
Zamorano JL. Guidelines on the prevention, diagnosis, and treatment of
infective endocarditis (new version 2009).
Eur Heart J. 2009;19:2369-413.
[PubMed] 35.
Hagan ME, Klotz SA, Bartholomew W, Potter L, Nelson M. A pseudoepidemic of
Rhodotorula rubra: a marker for microbial contamination of the
bronchoscope. Infect Control Hosp Epidemiol. 1995;16:727-8.
[PubMed] 36. Hall L, Le Febre KM, Deml SM,
Wohlfiel SL, Wengenack NL. Evaluation of the Yeast Traffic Light PNA FISH
probes for identification of Candida species from positive blood cultures. .
J Clin Microbiol. 2012;50:1446-8 37. Hoffmann KK, Weber DJ, Rutala WA.
Pseudoepidemic of Rhodotorula rubra in patients undergoing fiberoptic
bronchoscopy. Infect Control Hosp Epidemiol. 1989;10:511-4. 38. Hsiue HC, Huang YT, Kuo YL, Liao
CH, Chang TC, Hsueh PR. Rapid identification of fungal pathogens in positive
blood cultures using oligonucleotide array hybridization. Clin Microbiol
Infect. 2010;16:493-500. 39. Jaeger T, Andres C, Ring J,
Anliker MD. Rhodotorula mucilaginosa infection in Li-Fraumeni-like
syndrome: a new pathogen in folliculitis. Br J Dermatol. 2011;164(5):1120-2
40. Kaur R, Wadhwa A, Agarwal SK.
Rhodotorula mucilaginosa: an unusual cause of oral ulcers in AIDS patients.
AIDS. 2007;21:1068-9. 41. Khodavaisy S, Nabili M, Davari B, Vahedi M.
Evaluation of bacterial and fungal contamination in the health care workers'
hands and rings in the intensive care unit. J Prev Med Hyg.
2011;52:215-8.
42. Kiehn TE,
Gorey E, Brown AE, Edwards FF, Armstrong D. Sepsis due to Rhodotorularelated
to use of indwelling central venous catheters. Clin Infect Dis.
1992;14:841-6.[PubMed] 43. Kim HA, Hyun M, Ryu SY. Catheter-Associated
Rhodotorula mucilaginosa Fungemia in an immunocompetent host. Infect
Chemother. 2013 ;45:339-42.
44.
Kofteridis D, Mantadakis E, Christidou A, Samonis G. Rhodotorula glutinis
fungemia successfully treated with fluconazole: report of two cases. Int J
Infect Dis. 2007;11:179-80.
[PubMed]
45. Kutty SN,
Philip R. Marine yeasts-a review. Yeast. 2008;25:465-83.
[PubMed] 46.
Lanzafame M, De Checchi G, Parinello A, Trevenzoli M, Cattelan AM.
Rhodotorula glutinis-related meningitis. J Clin Microbiol.
2001;39:410.
[PubMed] 47.
Larone DH. Medically important fungi. 4th ed. Washington, D.C.: ASM Press;
2002. 48.
Lifshitz T, Levy J. Rhodotorula rubra keratitis and melting after
repeated penetrating keratoplasty. Eur J Ophthalmol. 2005;15:135-7.
[PubMed] 49. Loss SH, Antonio AC, Roehrig C, Castro PS, Maccari
JG. Meningitis and infective endocarditis caused by Rhodotorula mucilaginosa
in an immunocompetent patient. Rev Bras Ter Intensiva. 2011;23:507-9. 50.
Louria
DB, Greenberg SM, Molander DW. Fungemia caused by certain nonpathogenic
strains of the family Cryptococcaceae. Report of two cases due to
Rhodotorula andTorulopsis glabrata. N Engl J Med. 1960;263:1281-4.
[PubMed]
51. Lunardi
LW, Aquino VR, Zimerman RA, Goldani LZ. Epidemiology and outcome ofRhodotorula
fungemia in a tertiary care hospital. Clin Infect Dis. 2006;43:e60-3.[PubMed]
52. Maeder M,
Vogt PR, Schaer G, von Graevenitz A, Gunthard HF. Aortic homograft
endocarditis caused by Rhodotorula mucilaginosa. Infection.
2003;31:181-3.[PubMed] 53. Means AD, Sisto K, Lichon V, Monaghan D, O'Keefe P,
Tung R. Cutaneous Rhodotorula treated with photodynamic therapy. Dermatol
Surg. 2012; 38(7 Pt 1):1100-3
54. Merkur
AB, Hodge WG. Rhodotorula rubra endophthalmitis in an HIV positive
patient. Br J Ophthalmol. 2002;86:1444-5.
[PubMed] 55. Mori T, Nakamura Y, Kato J, Sugita K, Murata M,
Kamei K, Okamoto S. Fungemia due
to Rhodotorula mucilaginosa after allogeneic hematopoietic stem cell
transplantation. Transpl Infect Dis. 2012;14:91-4.
56. Navarro
JT, Lauzurica R, Gimenez M. Rhodotorula rubra infection in a kidney
transplant patient with pancytopenia. Haematologica. 2001;86:111.
[PubMed]
57. Naveh Y,
Friedman A, Merzbach D, Hashman N. Endocarditis caused by Rhodotorula
successfully treated with 5-fluorocytosine. Br Heart J. 1975;37:101-4.
[PubMed]
58. Neofytos
D, Horn D, De Simone JA, Jr. Rhodotorula mucilaginosa
catheter-related fungemia in a patient with sickle cell disease: case
presentation and literature review. South Med J. 2007;100:198-200.
[PubMed] 59. Nunes JM, Bizerra FC, Ferreira RC, Colombo AL.
Molecular identification, antifungal susceptibility profile, and biofilm
formation of clinical and environmental Rhodotorula species isolates.
Antimicrob Agents Chemother. 2013;57:382-9. 60. Odabasi Z, Paetznick VL, Rodriguez JR, Chen E,
McGinnis MR, Ostrosky-Zeichner L. Differences in b-glucan levels in culture
supernatants of a variety of fungi. Med Mycol 2006;44:267–272. 61. O'Meara TR, Alspaugh JA. The Cryptococcus
neoformans capsule: a sword and a shield. Clin Microbiol
Rev. 2012;25:387-408.
62.
Pamidimukkala U, Challa S, Lakshmi V, Tandon A, Kulkarni S, Raju SY. Sepsis
and meningoencephalitis due to Rhodotorula glutinis in a patient with
systemic lupus erythematosus, diagnosed at autopsy. Neurol India.
2007;55:304-7.
[PubMed]
63. Pappas
PG, Kauffman CA, Andes D, Benjamin DK, Jr., Calandra TF, Edwards JE, Jr.,
Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH,
Walsh TJ, Sobel JD. Clinical practice guidelines for the management of
candidiasis: 2009 update by the Infectious Diseases Society of America. Clin
Infect Dis. 2009;48:503-35.
[PubMed]
64.
Pennington JC, 3rd, Hauer K, Miller W. Rhodotorula Rubra peritonitis
in an HIV+ patient on CAPD. Del Med J. 1995;67:184.
[PubMed]
65. Perniola
R, Faneschi ML, Manso E, Pizzolante M, Rizzo A, Sticchi Damiani A, Longo R.
Rhodotorula mucilaginosa outbreak in neonatal intensive care unit:
microbiological features, clinical presentation, and analysis of related
variables. Eur J Clin Microbiol Infect Dis. 2006;25:193-6.
[PubMed] 66. Pien FD, Thompson RL, Deye D, Roberts GD.
Rhodotorula septicemia: two cases and a review of the literature. Mayo Clin
Proc. 1980;55:258-60.
67. Pinna A,
Carta F, Zanetti S, Sanna S, Sechi LA. Endogenous Rhodotorula minuta
andCandida albicans endophthalmitis in an injecting drug user. Br J
Ophthalmol. 2001;85:759.
[PubMed]
68. Pore RS,
Chen J. Meningitis caused by Rhodotorula. Sabouraudia.
1976;14:331-5.
[PubMed] 69. Pulvirenti F, Pasqua P, Falzone E, Maffeo F,
Gugliara C, Guarneri L. Sepsi da Rhodotorula glutinis. Descrizione di um caso
clínico. Infez Med.
2010;18:124-6. 70. Rajmane VS, Rajmane ST, Ghatole MP. Rhodotorula
species infection in traumatic keratitis--a case report. Diagn Microbiol
Infect Dis. 2011;71:428-9. 71.
Riedel DJ, Johnson JK, Forrest GN. Rhodotorula glutinis fungemia in a
liver-kidney transplant patient. Transpl Infect Dis. 2008;10:197-200.
[PubMed] 72. Saha S, Sengupta J, Chatterjee D, Banerjee D.
Rhodotorula mucilaginosa Keratitis: a rare fungus from Eastern India. Indian
J Ophthalmol. 2014;62:341-4. 73. Sardi Jde C, Pitangui Nde S, Rodríguez-Arellanes
G, Taylor ML, Fusco-Almeida AM, Mendes-Giannini MJ. Highlights in pathogenic
fungal biofilms. Rev Iberoam Micol. 2014;31:22-9
74. Savini V,
Sozio F, Catavitello C, Talia M, Manna A, Febbo F, Balbinot A, Di
Bonaventura G, Piccolomini R, Parruti G, D'Antonio D. Femoral prosthesis
infection by Rhodotorula mucilaginosa. J Clin Microbiol.
2008;46:3544-5.
[PubMed]
75. Serena C,
Marine M, Pastor FJ, Nolard N, Guarro J. In vitro interaction of
micafungin with conventional and new antifungals against clinical isolates
of Trichosporon, Sporobolomyces and Rhodotorula. J Antimicrob
Chemother. 2005;55:1020-3.
[PubMed]
76. Shinde
RS, Mantur BG, Patil G, Parande MV, Parande AM. Meningitis due toRhodotorula
glutinis in an HIV infected patient. Indian J Med Microbiol.
2008;26:375-7.
[PubMed]
77. Soylu A,
Demircioglu F, Turkmen M, Yucesoy M, Kavukcu S. Unusual cause of peritonitis
during peritoneal dialysis. Rhodotorula rubra and amphotericin B.
Pediatr Nephrol. 2004;19:1426-8.
[PubMed] 78. Sugawara Y, Nakase K, Nakamura A, Ohishi K,
Sugimoto Y, Fujieda A, Monma F, Suzuki K, Masuya M, Matsushima Y, Wada H,
Nobori T, Katayama N. Clinical utility of a panfungal polymerase chain
reaction assay for invasive fungal diseases in patients with haematologic
disorders. Eur J Haematol. 2013;90:331-9.
79. Thakur K,
Singh G, Agarwal S, Rani L. Meningitis caused by Rhodotorula rubra in
a human immunodeficiency virus infected patient. Indian J Med Microbiol.
2007;25:166-8.
[PubMed] 80. Tsiodras S, Papageorgiou S, Meletiadis J, Tofas P, Pappa
V, Panayiotides J, Karakitsos P, Armaganidis A, Petrikkos G. Rhodotorula
mucilaginosa associacted meningitis: A subacute entity with high mortality.
Case report and review. Med Mycol Case Rep. 2014;6:46-50.
81. Tuon FF,
Costa SF. Rhodotorula infection. A systematic review of 128 cases
from literature. Rev Iberoam Micol. 2008;25:135-40.
[PubMed]
82. Tuon FF,
de Almeida GM, Costa SF. Central venous catheter-associated fungemia due to
Rhodotorula spp. --a systematic review. Med Mycol. 2007;45:441-7.
[PubMed] 83. Uludag Altun H, Meral T, Turk Aribas E, Gorpelioglu
C, Karabicak N.A Case of Onychomycosis Caused by Rhodotorula glutinis.
Case Rep Dermatol Med. 2014;2014:563261.
84. Unal A,
Koc AN, Sipahioglu MH, Kavuncuoglu F, Tokgoz B, Buldu HM, Oymak O, Utas C.
CAPD-related peritonitis caused by Rhodotorula mucilaginosa. Perit Dial Int.
2009;29:581-2.
[PubMed]
85. Vishniac
HS, Takashima M. Rhodotorula arctica sp. nov., a basidiomycetous
yeast from Arctic soil.
Int J Syst Evol Microbiol. 2010;60(Pt 5):1215-8.
[PubMed] 86. Wirth F, Goldani LZ. Epidemiology of Rhodotorula:
an emerging pathogen. Interdiscip Perspect Infect Dis. 2012;2012:465717.
87. Wirth F, Goldani LZ. Experimental Rhodotorulosis
infection in rats. APMIS. 2012;120:231-5 88. Zaas AK, Boyce M, Schell W, Lodge BA, Miller JL, Perfect JR. Risk of fungemia due to Rhodotorula and antifungal susceptibility testing of Rhodotorula isolates. J Clin Microbiol. 2003;41:5233-5. [PubMed] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||