Table 1: Medically significant Candida spp.
|
Common Species |
Less common Species |
|
C. albicans C. tropicalis C. glabrata (T. glabrata) C. parapsilosis C. krusei C. lusitaniae |
C. dubliniensis C. famata C. guilliermondii C. haemulonii C. kefyr (C. pseudotropicalis) C. norvegensis C. rugosa C. viswanathii |
While many other species of Candida have been isolated from clinical
specimens (380), their significance is unclear. See Eggiman et al. (185)
or Pfaller & Diekema (562) for discussions of rarer species.
See Sullivan et al. (734) for a specific discussion of C. dubliniensis.
Table 2: Characteristics of the major Candida spp.
|
Species |
Frequency |
Virulence |
Clinical Associations |
|
C. albicans |
42%-65% |
High |
Most common in all settings |
|
C. tropicalis |
11%-25% |
High |
Cancer |
|
C. glabrata |
7%-15% |
Low |
Cancer |
|
C. parapsilosis |
7%-18% |
Variable |
Plastic devices, hyperalimentation |
|
C. krusei |
1%-4% |
Low |
Cancer |
|
C. lusitaniae |
1%-2% |
Low |
Cancer |
Show are frequency estimates for the species causing invasive disease from (4, 223, 497, 609, 614, 817).
Virulence and special clinical associations are discussed and cited in the text.
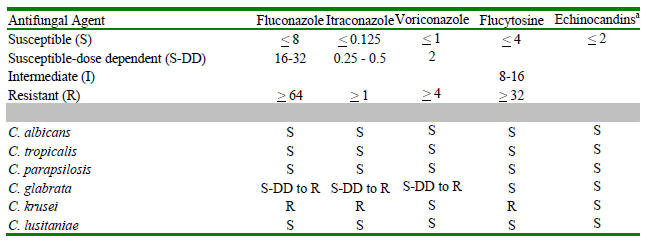
Table 3: Susceptibility of Candida spp. to antifungal agents
|
MIC50 |
AmB |
FLU |
ITRA |
VOR |
5FC |
ANID |
CAS |
MIC |
|
C. albicans |
0.5 |
0.5 |
0.12 |
0.03 |
< 0.25 |
0.03 |
0.03 |
0.015 |
|
C. tropicalis |
0.25 |
1 |
0.06 |
0.06 |
< 0.25 |
0.03 |
0.03 |
0.03 |
|
C. glabrata |
0.5 |
16 |
0.25 |
0.25 |
< 0.25 |
0.06 |
0.03 |
0.015 |
|
C. parapsilosis |
0.25 |
1 |
0.12 |
0.03 |
< 0.25 |
2 |
0.5 |
1 |
|
C. krusei |
0.25 |
64 |
0.5 |
0.5 |
16 |
0.03 |
0.06 |
0.06 |
|
C. lusitaniae |
> 1 |
2 |
0.25 |
0.03 |
< 0.25 |
0.06 |
0.12 |
0.12 |
Shown are typical species-specific MIC50s (μg/mL) adapted from reports describing collections of clinical isolates
(432, 520, 556, 559, 571, 562, 575, 616, 619). MICs were obtained by the NCCLS M27 methodology (488) for all
drugs but amphotericin B. As this method fails to detect amphotericin B-resistant Candida (616), the reported
amphotericin B MICs were obtained by a more sensitive method based on use of Antibiotic Medium 3 in a agar-based
testing format (798).
Table 4: Interpretive breakpoints for antifungal susceptibility testing

Shown are the breakpoints in μg/mL for Candida isolates against the indicated antifungal agents (130).
A disk diffusion-based testing methodology is also available and provides comparable interpretive categories (490).
See text for a discussion of the S-DD and I categories. Below this is shown the most common interpretive categories
for isolates of the most common species of Candida. Agreed interpretive breakpoints are not available for any of the
echinocandins. However, the available data obtained using the above described microdilution methods read at 24h at a
partial inhibition endpoint (509) would suggest that wild-type susceptibles isolates of all species have an MIC < 2 micrograms/ml.
Essentially all (>99%) current isolates appear susceptible using this interpretive principle. By contrast, isolates from cases of clinical
failure have often had MICs > 8 micrograms/ml.
Table 5. Summary of key points for selection of therapy
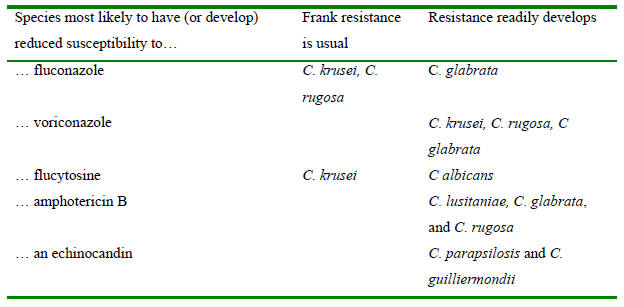
Combinations in the column labeled “Frank resistance is usual” should be avoided. For the other combinations,
successful therapy has been seen but frank resistance has also been shown to develop. Success against isolates
with elevated MICs may require use of maximal safe doses. This concept is best document for fluconazole and
C. glabrata (see (525, 814) for a good illustrations). It is speculative for other organism-drug
combinations but plausible based on best evidence. See text for additional literature support.
Table 6: Therapy for Vaginal Candidiasis: Topical Agents
|
Drug |
Formulation |
Dosage regimen |
|
*Butoconazole |
2% cream |
5 g/d x 3 d |
|
|
2% cream, slow-release |
5g/d x 1d |
|
*Clotrimazole |
1% cream |
5 g/d x 7-14 d |
|
|
100 mg vaginal tablet |
1 tablet/d x 7 d |
|
|
100 mg vaginal tablet |
2 tablet/d x 3 d |
|
|
500 mg vg. tab |
1 tablet/d x 1 d |
|
*Miconazole |
2% cream |
5 g/d x 7 d |
|
|
100 mg vaginal suppository |
1 suppository x 7 d |
|
|
200 mg vaginal suppository |
1 suppository/d x 3 d |
|
|
1200 mg vaginal suppository |
1 suppository/d x 1 d |
|
Econazole |
150 mg vaginal tablet |
1 tablet/d x 3 d |
|
Fenticonazole |
2% cream |
5 g/d x 7 d |
|
*Tioconazole |
2% cream |
5 g/d x 3 d |
|
|
6.5% cream |
5 g/d x 1 d |
|
Terconazole |
0.4% cream |
5 g/d x 7 d |
|
|
0.8% cream |
5 g/d x 3 d |
|
|
80 mg vaginal suppository |
80 mg/d x 3 d |
|
Nystatin |
100,000 U vaginal tablet |
1 tablet/d x 14 d |
Table 7: Therapies of choice for Invasive Candidiasisa
C. albicans: Fluconazole
C. tropicalis: Fluconazole
C. parapsilosis: Fluconazole
C. glabrata: An echinocandinb
C. krusei: An echinocandinb (IV); voriconazole (PO)
C. lusitaniae: Fluconazole
Species unknown: An echinocandinb
Neutropenia: An amphotericin B preparation or caspofunginc until neutrophil recovery, then as above.
Febrile neutropenia without proven candidiasis: Caspofunginc
Meningitis: Echinocandins should probably be avoided—see text
Dosages
Amphotericins
1. Amphotericin B deoxycholate (Fungizone): 0.6–0.8 mg/kg/d IV
2. Liposomal amphotericin B (Ambisome): 3 mg/kg/d IV
3. Amphotericin B lipid complex (ABLC, Abelcet): 5 mg/kg/d IV
4. Amphotericin B colloidal dispersion (ABCD, Amphotec): 3-4 mg/kg/d IV (but not recommended due to inferior toxicity profile)
Azoles
5. Fluconazole: Initiate therapy with 800 mg loading dose then 400 mg/day IV. Followup at 400 mg/day PO.
6. Voriconazole: Initiate IV therapy with 6 mg/kg q12h x two doses then 3 mg/kg q12h. Followup therapy is 200 mg q12h PO.
Echinocandins
7. Anidulafungin: 200 mg IV load then 100 mg/day IV
8. Caspofungin: 70 mg IV load then 50 mg/d day IV
9. Micafungin: 100 mg/day IV
aThese guidelines apply principally to candidemia but can in general be extrapolated to renal, urinary, ocular, cardiac, pericardial, suppurative vascular (phlebitis),
peritoneal, gallbladder, pancreatic, skeletal (osteomyelitis), joint (arthritis), meningeal, and pulmonary candidiasis. Unfortunately, the amount of data in these other
settings is limited and there is usually no obvious best choice of therapy. The only other general guidelines are (a) durable cure of infected devices is very difficult to
achieve without device removal (although infection can often be suppressed—see for example the section entitled “Candida Endocarditis, Pericarditis, and Suppurative Phlebitis”)
and (b) an agent that penetrates well is needed for protected spaces (the eye and brain—see those sections for details). Helpful anecdotes are found in the chapter sections
that discuss individual disease forms.
bThe echinocandins are caspofungin, anidulafungin, and micafungin. These agents appear similar in their efficacy for invasive candidiasis.
cCaspofungin has the most data in neutropenic patients. In particular, it is the only echinocandin as yet studied in febrile neutropenia.
When following these general guidelines, remember also that (a) Candida is not the only yeast to cause invasive fungal infections (be especially alert for cryptococcosis and histoplasmosis),
(b) there can be mechanical and pharmacological factors that drive failure or breakthrough (see the section entitled “Persistent and Breakthrough Disease”), and
(c) catheters and other potentially infected prosthetic devices usually need to be removed (see sections entitled “Management of Catheters” and “Candida Endocarditis, Pericarditis, and Suppurative Phlebitis”).