Vaccinations in Adults
Authors: Steven D. Burdette
Vaccines are recommended for adults on the basis of age, prior vaccinations, health conditions, lifestyle, occupation, and travel. Current vaccination rates among adults are well below the desired levels as established by the CDC. Health-care providers should be aware of the importance of routinely assessing patients’ vaccination histories and recommending and providing routinely recommended vaccines. A strong recommendation from a health-care provider is the best way to increase rates of vaccination.
The Advisory Committee on Immunization Practices (ACIP) annually reviews and updates the adult immunization schedule, which is designed to provide vaccine providers with a summary of existing ACIP recommendations regarding the routine use of vaccines for adults (Figures 1 and 2). In light of increasing antibiotics resistance among many bacterial infections, every effort should be made to minimize infections that may be preventable.
Live versus Inactivated Vaccines
Inactivated vaccines are generally safe for patients with compromised immune systems (organ transplantation, hematologic malignancies, patients receiving chemotherapy or TNF alpha inhibitors) while live-attenuated vaccines are not commonly administered to those with immune-compromising conditions. Varicella can cause a severe infection in immunosuppressed individuals, and vaccination is recommended prior to transplantation in children and adolescents. Vaccination for varicella has also been recommended for seronegative household contacts of immunocompromised patients by numerous organizations. MMR is also a live-attenuated vaccine, but its use in adults is rarely required, except in the prevention of rubella in young female patients. Rubella infection does not cause severe infection in solid-organ recipients, but the congenital rubella syndrome is a concern because of the increased number of pregnancies in young women of child-bearing age who survived transplantation.
Some vaccines are absolutely contraindicated in the immunosuppressed. These include oral polio vaccine, vaccinia, BCG, and live oral typhoid. Although case reports of yellow fever vaccination with human immunodeficiency virus infection have been published, no data is available in solid-organ recipients and should be considered contraindicated. Oral polio vaccine is also contraindicated in family members of transplant recipients because of possible contagion by shedding of the virus and close contact with excreta. Inactivated polio vaccine (given IM) is safe and effective in renal transplant recipients. For safety concerns, the killed parenteral Vi polysaccharide vaccine for typhoid fever should be used instead of the live attenuated oral Ty21a.
Specific Vaccines
BCG (Bacillus Calmette-Guerin)
Indications
- People who live in areas with high rates of TB and test negative for latent TB
- Infants may be vaccinated at birth prior to TB exposure
- Not routinely administered in the United States
Vaccine Side Effects
After the vaccination, a small erythematous lesion usually develops within 2-6 weeks. After a few weeks it becomes scaly, crusting and with slight bruising. This will eventually heal to form a round flat scar.
Comments
BCG, a live attenuated form of Mycobacterium bovis, is utilized in some countries to help prevent tuberculosis. Studies have demonstrated 70-80% effectiveness in preventing tuberculosis following a single dose. This vaccine is not routinely utilized in the United States due to the low prevalence of tuberculosis and should never be given to transplant recipients. BCG vaccination may lead to false positive PPD results, though a patient’s BCG vaccination history should not be taken into account when determining whether or not to treat for latent TB. Besides being utilized as a vaccine, BCG is also used as intra-vesicular therapy for bladder carcinomas.
Hepatitis A Vaccine
Indications
- Chronic liver disease
- Persons who receive clotting factor concentrates
- Men who have sex with men
- Illegal drug use
- Persons working with hepatitis A virus (such as infected primates or a research laboratory)
- Persons traveling to or working in countries with high or intermediate rates of hepatitis A
Vaccine Side Effects
Mild symptoms include injection site soreness, headache, loss of appetite, or tiredness. Severe problems such as allergic reactions typically occur within a few minutes to hours after the shot and are rare.
Comments
Antibody titers are lower in patients with ESLD and after renal and liver transplantation compared with antibody titers in healthy control subjects. Serologic response should be assessed 1–3 months after completion of the primary hepatitis A vaccine series and a single Hepatitis A vaccine booster dose should be administered to non-responders.
Hepatitis B Vaccine (HBV)
Indications
- End-stage renal disease, including hemodialysis
- HIV
- Chronic liver disease
- Health-care and public-safety workers exposed to blood or other infectious body fluids.
- Sexually active persons not in a long-term, mutually monogamous relationship
- Seeking evaluation for STD
- Current or past injection-drug users
- Men who have sex with men
- Household contacts and sex partners of persons with chronic HBV infection
- International travelers to countries with high or intermediate prevalence of HBV
Vaccine Side Effects
Mild problems include injection site soreness and low grade fever. Severe problems are extremely rare. Severe allergic reactions are believed to occur approximately once in 1.1 million doses.
Comments
Patients with ESRD on hemodialysis have a decreased serologic response to vaccination, thus higher‐dose vaccine formulations (2-4 times the standard dose) have been developed. Patients with ESLD also produce lower antibody titers in response to vaccination and may benefit from the higher-dose hepatitis B vaccine. Hepatitis B surface antibody titers should be assessed 1–3 months after completion of the primary HBV vaccine series.For patients who do not respond to the initial HBV series (i.e., who have antibody titers 10 IU/L), administration of an additional 3, or 4 dose series is recommended.
Human Papilloma Virus (HPV) Vaccine
Indications
Prevention of anal cancer caused by human papillomavirus (HPV) types 16 and 18 and for the prevention of anal intraepithelial neoplasia (AIN) grades 1, 2 and 3 (anal dysplasias and precancerous lesions) caused by HPV types 6, 11, 16 and 18, in males and females 9 through 26 years of age.
Side Effects
Mild problems include injection site pain, itching, redness or swelling, mild-to-moderate fever (100-102 degrees F). There are reports of headache and neurologic complications from the vaccine as well.
Comments
This vaccine utilizes the L1 major capsid protein of HPV for types 6, 11, 16 and 18, and is an vaccine. The recommendation for the vaccination of males is a recently approved indication. The vaccine appears to be effective for prevention of persistent infection and cervical cancer due to the vaccine strains. Ideally, vaccine should be administered before potential exposure to HPV through sexual activity; however, males and females who are sexually active should still be vaccinated consistent with age-based recommendations. The recommended gender and age may broaden when further data becomes available. History of genital warts, abnormal Papanicolaou test, or positive HPV DNA test is not evidence of prior infection with all vaccine HPV types thus vaccination is still recommended. Since it is an inactivated vaccine, it can be given to those who are immunosuppressed.
Influenza Vaccine Indications
- Chronic disorders of the cardiovascular or pulmonary systems
- Chronic metabolic diseases (DM, renal or hepatic dysfunction, hemoglobinopathies)
- Immunocompromising conditions including HIV
- Any condition that compromises respiratory function or the handling of respiratory secretions (e.g., cognitive dysfunction, spinal cord injury, seizure disorder or other neuromuscular disorder)
- Pregnancy during the influenza season
- Long-term care and assisted-living facilities employees -Caregivers of children less than 5 years old -Residents of long-term care and assisted-living facilities
- Persons likely to transmit influenza to persons at high risk
- Anyone who would like to decrease their risk of getting influenza
- FluMist Indications
- Healthy, nonpregnant adults aged less than 50 years without high-risk medical conditions who are not contacts of severely immunocompromised persons in special care units
- FluMist for H1N1 includes target groups listed above who meet these criteria
- Do NOT administer to immunocompromised patients
Vaccine Side Effects (Intramuscular)
Mild problems include soreness, redness, tenderness, or swelling at the injection site, fainting (mainly adolescents), headache, muscle aches, fever or nausea. They usually begin soon after the shot and last 1-2 days. Severe problems including life-threatening allergic reactions are very rare. If they do occur, it is usually within a few minutes to a few hours.
Vaccine Side Effects (FluMist)
Mild problems include runny nose, nasal congestion, cough, headache, muscle aches, fever, wheezing, abdominal pain, vomiting or diarrhea. Severe problems include life-threatening allergic reactions are very rare. If they do occur, it is usually within a few minutes to a few hours after the vaccination.
Comments
Seasonal influenza vaccine is approved for infants and children greater than 6 months of age. Pediatric transplant recipients do respond to influenza vaccines although their cellular responses may not be as vigorous as that found in healthy children therefore household and close contacts should also be vaccinated. Given that the risks of the trivalent inactivated vaccine are minimal, this vaccine should be administered annually to transplant candidates as well as recipients. In 2013 forms of the vaccine were made available that were not produced with eggs, thus can be safely given to patients with egg allergies.
Meningococcal Vaccine Indications
- Anatomic or functional asplenia, or terminal complement deficiencies
- First-year college students living in dormitories
- Microbiologists exposed to isolates of Neisseria meningitidis
- Military recruits
- Persons who travel to or live in countries in which meningococcal disease is hyperendemic or epidemic (e.g., the “meningitis belt” of sub-Saharan Africa during the dry season [December–June]), particularly if their contact with local populations will be prolonged
- Required by the government of Saudi Arabia for all travelers to Mecca during the annual Hajj
- Pretransplant candidate’s ages 11-18-year-old
Vaccine Side Effects
Mild problems include injection site redness or pain may occur in nearly half of those vaccinated, usually lasting for 1 or 2 days. A small percentage of people who receive the vaccine develop a fever. Severe problems include allergic reactions which occur within a few minutes to a few hours of the shot and are very rare. Guillain-Barre Syndrome has been reported among some people who received MCV4. This happens so rarely that it is currently not possible to tell if the vaccine might be a factor.
Comments
The meningococcal conjugate vaccine (MCV 4) confers protection against meningococcal types A, C, Y and W-135, but not type B and is recommended for ages 11-54.14 For high-risk individuals ages 2-10 or >55, MPSV 4 (quadrivalent polysaccharide vaccine) should be administered. Immunogenicity of polysaccharide vaccines is limited under the age of 2 years; consequently, conjugated vaccines have been developed. ACIP has recommended that all 11-12-year-olds be immunized with MCV 4 at their preadolescent visit. College students who will be living in dormitories are at higher risk and should be offered the vaccine. Revaccination with MCV after 5 years might be indicated for adults previously vaccinated with MPSV 4 who remain at increased risk for infection (e.g., persons residing in areas in which disease is epidemic).
Measles Mumps and Rubella (MMR)
Adults born before 1957 can be considered immune to measles. Adults born in or after 1957 should receive at least one dose of MMR unless they have a medical contraindication, documentation of at least one dose, history of measles based on health-care provider diagnosis, or laboratory evidence of immunity. A second dose of MMR is recommended for adults if:
- Exposed to measles or in an outbreak setting
- Previously vaccinated with killed measles vaccine
- Vaccinated with an unknown type of measles vaccine between 1963 and 1967
- Students in post-secondary educational institutions
- Work in health care facilities
- International travel
Adults born before 1957 can generally be considered immune to mumps. Adults born during or after 1957 should receive 1 dose of MMR unless they have a medical contraindication, a history of mumps based on health-care provider diagnosis, or laboratory evidence of immunity. A second dose of MMR is recommended for the following adults if:
- Age group is affected during a mumps outbreak in their community
- Students in post-secondary educational institutions
- International travel
- Work in health care facilities
- Unvaccinated health-care workers born before 1957 who do not have other evidence of mumps immunity (consider giving 1 dose on a routine basis and strongly consider giving a second dose during an outbreak)
One dose of MMR vaccine is recommended for women whose vaccination history is unreliable or who lack laboratory evidence of immunity against Rubella. For women of childbearing age, regardless of year of birth, rubella immunity should be determined. Women without evidence of immunity should receive MMR vaccine upon completion of pregnancy and before discharge from the health-care facility.
Vaccine Side Effects
Mild Problems include fever, rash, and glandular swelling in the cheeks or neck (rare). If these occur, it is usually within 7-12 days after vaccination. They occur less often after the second dose. Moderate problems include febrile seizures, joint pain and stiffness (mostly in teenage or adult women), temporary thrombocytopenia. Severe problems (rare) include allergic reaction, deafness, long-term seizure disorder, coma, or permanent brain damage. Whether these severe side effects are truly vaccine related is a matter of debate.
Comments
The measles, mumps, rubella vaccine is a live attenuated vaccine and should be administered, when indicated, prior to transplantation. The MMR vaccine has been proven to be safe in bone marrow transplant recipients and in patients with human immunodeficiency virus (HIV) infection and may eventually be proven safe after solid organ transplantation.
Pneumococcal Vaccine Indications
- Chronic lung disease (including asthma)
- Chronic cardiovascular diseases
- Diabetes mellitus
- Chronic liver diseases, cirrhosis; chronic alcoholism
- Chronic renal failure or nephrotic syndrome
- Functional or anatomic asplenia
- Immunocompromising conditions
- Cochlear implants and cerebrospinal fluid leaks
- Residents of nursing homes or other long-term care facilities and persons who smoke cigarettes.
- One-time revaccination after 5 years is recommended for:
- Chronic renal failure or nephrotic syndrome
- Functional or anatomic asplenia (e.g., sickle cell disease or splenectomy)
- Immunocompromising conditions
- For persons > age 65, one-time revaccination if they were vaccinated 5 or more years previously and were < 65 years at the time of primary vaccination.
- Additional doses should be avoided to prevent the possible development of immune tolerance
Side Effects
Approximately half have mild side effects, such as redness or pain. Less than 1 percent develops a fever, muscle aches, or more severe local reactions.
Comments
The traditional pneumococcal vaccine is a 23-valent polysaccharide vaccine (PPV23), which is not immunogenic in children under the age of 2. The 13-valent pneumococcal conjugate vaccine (PCV13) is licensed for infants 2 months and older, with a recommended primary dosing series of 2, 4, 6 and 12-15 months and is also approved for the adults with immunocomprosing conditions. Revaccination protocols continue to be adjusted over time and remain confusing for health care providers (Table 2). According to the ACIP recommendations published in the MMWR in October of 2012:
Pneumococcal vaccine naïve persons
ACIP recommends that adults aged ≥19 years with immunocompromising conditions, functional or anatomic asplenia, CSF leaks, or cochlear implants, and who have not previously received PCV13 or PPSV23, should receive a dose of PCV13 first, followed by a dose of PPSV23 at least 8 weeks later (Table). Subsequent doses of PPSV23 should follow current PPSV23 recommendations for adults at high risk. Specifically, a second PPSV23 dose is recommended 5 years after the first PPSV23 dose for persons aged 19–64 years with functional or anatomic asplenia and for persons with immunocompromising conditions. Additionally, those who received PPSV23 before age 65 years for any indication should receive another dose of the vaccine at age 65 years, or later if at least 5 years have elapsed since their previous PPSV23 dose.
Previous vaccination with PPSV23: Adults aged ≥19 years with immunocompromising conditions, functional or anatomic asplenia, CSF leaks, or cochlear implants, who previously have received ≥1 doses of PPSV23 should be given a PCV13 dose ≥1 year after the last PPSV23 dose was received. For those who require additional doses of PPSV23, the first such dose should be given no sooner than 8 weeks after PCV13 and at least 5 years after the most recent dose of PPSV23.”
Tetanus, Diphtheria and Pertussis (DTaP, Td and Tdap) Vaccine
- DTaP vaccination is one of the recommended childhood immunizations.
- DTaP vaccine can be safely given to infants
- Five DTaP injections are recommended and are usually given to children at ages 2 months, 4 months, 6 months, 15-18 months, and 4-6 years
- DTaP immunization is generally required before a child can start school
- Tdap vaccine is recommended for children around ages 11 – 12
- Adults ages 19 – 64 should receive one dose of TDaP as a substitute for the Td vaccine
- Td can be used for the 10 year booster once adults have received the TDaP booster
Vaccine Side Effects
Mild problems include fever, redness, swelling or injection site soreness. These problems occur more often after the 4th and 5th doses. Sometimes the 4th or 5th dose of vaccine is followed by swelling of the entire arm or leg in which the shot was given, for 1 to 7 days. Other mild problems include tiredness or poor appetite and vomiting. These problems generally occur 1 to 3 days after the vaccine. Other more significant problems include seizure and high fever.
Comments
Tetanus, diphtheria and pertussis immunization is part of the routine vaccination series for infants and young children, but until recently adolescents and adults received only booster doses of tetanus-diphtheria (Td) vaccine. However, pertussis immunity wanes 5-10 years after initial immunization. In 2005, the Tdap vaccine was licensed in the US for persons 11-64 years of age. Currently, the ACIP recommends a single dose of Tdap as a booster for adults whose last Td was >10 years ago, for health-care workers and for persons who are in close contact with infants <12 months of age. Tdap can be given as little as 2 years (or shorter intervals in special siuations) after Td vaccine in high-risk persons. Currently a single booster with Tdap is indicated, with future tetanus vaccinations being the Td vaccine.
Varicella Zoster Vaccine Indications
- All adults without evidence of immunity to varicella should receive 2 doses if not previously vaccinated or the second dose if they have received only one dose
- Special consideration should be given to those who:
- Close contact with persons at high risk for severe disease (e.g., health-care personnel and family contacts of persons with immunocompromising conditions)
- High risk for exposure or transmission (e.g., teachers; child care employees; residents and staff members of institutional settings, college students; military personnel; adolescents and adults living in households with children; non-pregnant women of childbearing age; and international travelers)
Vaccine Side Effects
Mild problems include injection site soreness or swelling, fever, mild rash (up to a month after vaccination). It is possible for these people to infect other members of their household, but this is extremely rare. More significant problems include seizures (often febrile) and pneumonia.
Comments
Greater than 95% of U.S. born adults is immune to VZV. Serologic testing may be performed as a cost-effective way to determine if vaccination is indicated. Evidence of immunity to varicella in adults includes any of the following:
-Documentation of 2 doses of varicella vaccine at least 4 weeks apart
-US born before 1980 (for health-care workers and pregnant women, birth before 1980 should not be considered evidence of immunity)
-A health-care provider diagnosis of varicella or verification of history of varicella (for patient reporting a history of or presenting with an atypical and/or mild case, health-care providers should seek either an epidemiological link with a typical varicella case or evidence of laboratory confirmation, if it was performed at the time of acute disease)
-History of herpes zoster based on health-care provider diagnosis or verification of zoster by a health-care provider
-Laboratory evidence of immunity or laboratory confirmation of disease
Varicella vaccine, a live-attenuated vaccine, was traditionally considered contraindicated in immunocompromised patients.
Zoster Vaccine (Herpes zoster Vaccine) Indications
All adults age 50 years and older should receive one dose of herpes zoster vaccine whether or not they report a prior episode of herpes zoster.
Side Effects
Mild problems include redness, swelling, or itching at the site of the injection and headache.
Comments
Herpes zoster (shingles) represents the reactivation of varicella-zoster virus (VZV) acquired during primary varicella infection earlier in life. This vaccine contains 19,400 PFU of the Oka/Merck strain of VZV, the same live virus strain used in varicella vaccine for children and adults but with14 time’s higher amount of antigen. A randomized trial of over 38,000 adults demonstrated a reduction in herpes zoster of >50%. In zoster victims who previously received the herpes zoster vaccine, the risk of post herpetic neuralgia (PHN) is reduced by 39% (overall reduction of PHN is 67% in all vaccine recipients compared with placebo). These benefits are thought to be a result of boosting the immunity to herpes zoster virus, leading to suppression of reactivation of VZV in the dorsal root (sensory) ganglia. The ACIP recommends the zoster vaccine specifically for “persons anticipating immunosuppression”. They suggest that the zoster vaccine be administered 14 days before immunosuppressive agents are initiated with the caveat that some experts advise a period of 1 month between administration of live vaccines and immunosuppression. The duration of protection against shingles in unknown but is at least 4 years. Clinical manifestations in transplant recipients vary from a painful, blistering, uni- or multi-dermatomal eruption to a severe, sometimes fatal form with cutaneous and/or visceral dissemination. Presentation with abdominal pain and without rash is a particularly challenging diagnostic dilemma. ACIP recommendations call for a single dose of zoster vaccine for individuals ages 60 and above, whether or not they report a prior episode of herpes zoster, unless a contraindication exists. According to licensing information, contraindications include immunodeficiency states and immunosuppressive therapy, as well as pregnancy and active tuberculosis.
References
1. ACIP Recommended Immunization schedule for adults aged 19 years and older – United States, 2013. MMWR 2013; 62: 10-18.
2. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocomprosing conditions: recommendations of the ACIP. MMWR 2012; 61: 816-819.
3. Immunizations of Health Care Personnel: recommendations of the ACIP. MMWR 2011; 60: 1-46.
4. ACIP provisional recommendations for the use of zoster vaccine. Available from: http://www.cdc.gov/nip/recs/provisional_recs/zoster-11-20-06.
5. Centers for Disease Control and Prevention National Immunization Program. Meningococcal disease and meningococcal vaccines. Available from: http://www.cdc.gov/nip/vaccine/mening/mening_fs.htm. Accessed 22 April, 2007.
6. Cohen JI. Strategies for herpes zoster vaccination of immunocompromised patients. J Infect Dis 2008; 197(Suppl 2):S237–41. [PubMed]
7. Denits-Pensy M, Forrest GN, Cross AS, Hise MK. The use of vaccines in adult patients with renal disease. Am J Kidney Dis 2005; 46:997–1011. [PubMed]
8. Denziber-Isakov L and Kumar D. Vaccination in solid organ transplantion. Am J Transplant 2013; 13: 311-317.
9. Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, Mijalski CM, Brown KH, Murphy TV; Centers for Disease Control and Prevention; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Preventing tetanus, diphtheria, and pertussis among adults: Use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55(RR-17): 1-37. [PubMed]
10. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56 (RR-2): 1-24. [PubMed]
11. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352: 2271-2284. [PubMed]
12. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Preventing pneumococcal disease among infants and young children. MMWR Recomm Rep 2000; 49(RR-9): 1-35. [PubMed]
Figure 1
http://www.cdc.gov/vaccines/schedules/downloads/adult/mmwr-adult-schedule.pdf
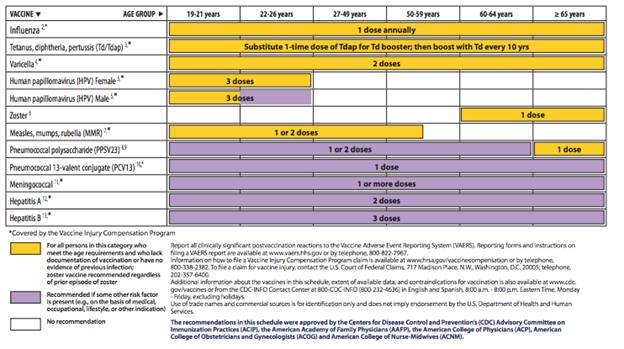
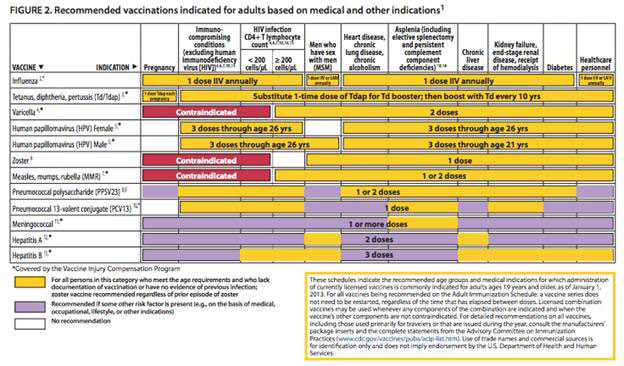
Figure 2: Recommended vaccinations indicated for adults basedon medical and other indications
Table 1. Vaccine Overview
Vaccine |
Indications |
Ages |
Primary Series |
Catch-up |
Live virus |
Comments |
|---|---|---|---|---|---|---|
| Haemophilus influenza B | All children | >6 weeks of age | 2m, 4m, 6m, 12-15m | Variable | No | Not routinely given once >5 years old |
| Hepatitis A | All children, travelers, high risk groups, post-expsure | >1 years old | 2 doses 6 months apart | 2nd dose can be given anytime after 6 months. Repeating entire series not indicated | No | Antibody titers recommended to ensure protection |
| Hepatitis B | All children, travelers, high risk groups | All | 3 doses at 0, 1 and 6 months. | Ok to administer 6 month dose at a later interval but need to confirm antibody response | No | Antibody titers recommended to ensure protection, a repeat 3 shot series often needed. No indication for more than 2 vaccination series |
| Herpes zoster | Adults age >60 | >60 | 1 dose | NA | Yes | |
| HPV | Females age 11-26 | 11-12 | 0,2, 6 months | Must be at least 24 weeks between doses #2 and #3 | No | |
| Influenza Flumist (seasonal) | All children 2-18 years and all high risk patients | >2 years of age and <50 | Yearly | If first flu vaccine, 2nd dose required 4 weeks later | Yes | |
| Influenza IM (seasonal) | All children 6mos -18 years and all high risk patients | >6 mos of age | Yearly | If first flu vaccine, 2nd dose required 4 weeks later | No | |
| Influenza Flumist (H1N1) | All children 2-18 years and all high risk patients | >2 years of age and <50 | NA | If first flu vaccine, 2nd dose required 4 weeks later | Yes | |
| Influenza IM (H1N1) | All children 6mos -18 years and all high risk patients | >6 mos of age | NA | If first flu vaccine, 2nd dose required 4 weeks later | No | |
| MMR | Everyone | >12 months | #1 12-15mos, #2 4-6 years | Must space 2 doses at least 4 weeks apart. | Yes | |
| Meningococcal (Menactra* or Menomune) | Menactra age 11 or later and to unvaccinated college students | Menomune if ages 2-10 or >55 | 1 dose | NA | No | |
| Pneumococcal | All pediatrics but only high risk adults | Conjugate (PCV) vaccine < 5 yo; polysaccharide (PPSV) vaccine >2 yo | PCV 2m, 4m, 6m, 12-15m; PPSV at any age >2, repeat dose 5 years later | PCV catch-up varies, 2nd dose of PPSV can be given at any interval after 5 years | No | |
| DTaP | Everyone | Ages 0-10 | 5 doses; 2m, 4m, 6m, 15-18m,4-6 yrs | No | ||
| Tdap | Used for DTap booster once >11 yo | Age >11 | Booster every 10 years | Use Td vaccine at weeks 0, 2, 24 then every 10 years. 1 dose of Tdap during series is acceptable | No | |
| Varicella | Age >12 months and no history of Varicella infection | >12 months | Dose #1 at 12 months, #2 after age of 4. | 2nd dose can be given at any interval after first dose | No |
Table 2: Medical conditions or other indications for administration of 13-valent pneumococcal conjugate vaccine (PCV13), and indications for 23-valent pneumococcal polysaccharide vaccine (PPSV23) administration and revaccination for adults aged ≥19 years,1 by risk group – Advisory Committee on Immunization Practices, United States, 2012
| PCV13 | PPSV23 | |||
|---|---|---|---|---|
| Risk Group | Underlying medical condition | Recommended | Recommended | Revaccination 5 yrs after first dose |
| Immunocompetent persons | Chronic heart disease2 | ✓ | ||
| Chronic lung disease3 | ✓ | |||
| Diabetes mellitus | ✓ | |||
| Cerebrospinal fluid leak | ✓ | ✓ | ||
| Cochlear implant | ✓ | ✓ | ||
| Alcoholism | ✓ | |||
| Chronic liver disease, cirrhosis | ✓ | |||
| Cigarette smoking | ✓ | |||
| Persons with functional or anatomic asplenia | Sickle cell disease/other hemaglobinopathy | ✓ | ✓ | ✓ |
| Congential or acquired asplenia | ✓ | ✓ | ✓ | |
| Immunocompromised persons | Congenital or acquired immunodeficiency4 | ✓ | ✓ | ✓ |
| Human immunodeficiency virus infection | ✓ | ✓ | ✓ | |
| Chronic renal failure | ✓ | ✓ | ✓ | |
| Nephrotic syndrome | ✓ | ✓ | ✓ | |
| Leukemia | ✓ | ✓ | ✓ | |
| Leukemia | ✓ | ✓ | ✓ | |
| Lymphoma | ✓ | ✓ | ✓ | |
| Hodgkin disease | ✓ | ✓ | ✓ | |
| Generalized malignancy | ✓ | ✓ | ✓ | |
| Iatrogenic immonosppression5 | ✓ | ✓ | ✓ | |
| Solid organ transplant | ✓ | ✓ | ✓ | |
| Multiple myeloma | ✓ | ✓ | ✓ | |
1 All adults aged ≥65 should receive a dose of PPSV23, regardless of previous history of vaccination with pneumococcal vaccine.
2 Including congestive heart failure and cardiomyopathies, excluding hypertension.
3 Including chronic obstructive pulmonary disease, emphysema, and asthma.
4 Includes B- (humoral) or T-lymphocyte deficiency, complement deficiencies (particularly C1, C2, C3, and C4 deficiencies), and phagocytic disorders (excluding chronic granulomatous disease).
5 Diseases requiring treatment with immunosuppressive drugs, including long-term systemic corticosteroids and radiation therapy.
What's New
CDC/Morbidity and Mortality Weekly. Bridges CB, et al. Advisory Committee on Immunization Practices (ACIP) Recommended Immunization Schedule for Adults Aged 19 Years and Older--United States, 2013. Vol 62, January 28, 2013.
CDC/Morbidity and Mortality Weekly. Geographic Differences in HIV Infection Among Hispanics or Latinos-46 States and Puerto Rico, 2010. Vol 61, No. 40, October 12, 2012.
GUIDED MEDLINE SEARCH FOR
Reviews
McCool L, Burdette SD. Vaccination of Solid Organ Transplant Candidates and Recipients
CDC/Morbidity and Mortality Weekly. Immunization of Health Care Personnel. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Vol 60, No. 7, November 25, 2011.
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
History
Table of Contents
- Live versus Inactivated Vaccines
- Specific Vaccines
- Hepatitis A Vaccine
- Hepatitis B Vaccine (HBV)
- Human Papilloma Virus (HPV) Vaccine
- Influenza Vaccine Indications
- Meningococcal Vaccine Indications
- Measles Mumps and Rubella (MMR)
- Pneumococcal Vaccine Indications
- Tetanus, Diphtheria and Pertussis (DTaP, Td and Tdap) Vaccine
- Varicella Zoster Vaccine Indications
- Zoster Vaccine (Herpes zoster Vaccine) Indications