Acute Sinusitis
Authors: Itzhak Brook, M.D., MSc
Previous authors: Jack B. Anon, MD, FACS
Acute rhinosinusitis is a common illness. Viral upper respiratory tract infection is the most common presentation of rhinosinusitis. Most individuals resolve the infection spontaneously and only a small proportion develops a secondary bacterial infection. Acute bacterial rhinosinusitis (ABRS) is generally diagnosed in the presence of more than 7-10 days of nasal discharge. The most common bacterial isolates from acute bacterial rhinosinusitis are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Group A beta-hemolytic streptococci, andStaphylococcus aureus. Anaerobes predominate in rhinosinusitis of dental origin, and fungi and Pseudomonas aeruginosa in immunocompromised patients. The appropriate choice of antimicrobial therapy depends on the likely infecting pathogens, bacterial antibiotic resistance and antibiotics’ pharmacologic profiles. Amoxicillin with or without clavulanate is currently recommended as the empiric treatment in those requiring antibiotic therapy. Those who failed initial treatement can be treated with either a high dose amoxicillin plus clavulante, doxycycline, a respiratory fluoroquinolone (levofloxacin or moxifloxacin) or the combination of clindamycin plus a third-generation oral cephalosporin (cefixime or cefpodoxime). Recovery of the causative agents should be considered in those who failed the initial treatment. In addition to antibiotics, adjuvant therapies and surgery may be administered.
Acute rhinosinusitis (ARS) is one of the most common health problems and had increased in prevalence and incidence (14). It causes significant physical symptoms, negatively affects the quality of life, and can substantially impair daily functioning. A US survey found that rhinosinusitis was diagnosed in one of 7 of adults within a year (82). The incidence rate within adults were higher for women than men (about 1.9-fold), and adults between 45 and 74 years are most commonly affected (82).
The pathophysiological cause of rhinosinusitis may be due to obstruction of sinus drainage pathways (sinus ostia), ciliary impairment, and altered mucus quantity and quality.
Acute rhinosinusitis is defined as up to 4 weeks of purulent nasal drainage accompanied by nasal obstruction, facial pain-pressure-fullness, or both. It can be caused by a variety of factors including environmental irritants, allergy, and infection (viral, bacterial or fungal). The most frequent cause of acute rhinosinusitis is a viral upper respiratory infection (URI). The incidence of viral upper respiratory infection is 6 episodes/year in children, and 2-3 episodes/year in adults (49). Secondary acute bacterial rhinosinusitis following an antecedent viral upper respiratory infection occurs in 0.5%–2% of adult cases (54, 83) and about 5% in children (103).
HISTORY
Suspicion of acute bacterial rhinosinusitis is based on clinical symptoms and signs when at least two major or one major and two minor criteria are present. (Table 1) (70). The most common presentation is a persistent (and non improved) nasal discharge or cough (or both) lasting more than 10 days (103).
Differentiating viral upper respiratory tract infection from secondary acute bacterial rhinosinusitis is important and remains difficult. The main feature of viral upper respiratory tract infectionis the presence of nasal symptoms (discharge and congestion/obstruction) or cough or both, and sometimes also a scratchy throat. Fever is absent in most patients and when present it occurs early in the illness. Fever and constitutional symptoms generally disappear within 24 to 48 hours after which the respiratory symptoms predominate. Most with acute viral rhinosinusitis improved spontaneously after 7 to 12 days. Generally the nasal discharge is clear and watery initially, but often, its quality changes over time. In most individuals, the discharge turns thicker and more mucoid and purulent (16). After several days these changes are reversed with the purulent discharge turning mucoid and then clear or dry. These changes occur in uncomplicated viral upper respiratory tract infections without the use of antimicrobials (30).
The characteristic presenting symptoms associated with a bacterial rather than viral infection were evaluated by several consensus expert panels (3, 6, 7, 33, 37, 59, 63, 76, 97).
The panels highlighted three clinical presentations that should prompt consideration of acute bacterial rhinosinusitis rather than a viral upper respiratory tract infection:
1) Onset with persistent symptoms (respiratory symptoms present for more than 10 but less than 30 days with no improvement). The criterion of >10 days duration of symptoms or signs and worsening of symptoms within 10 days after initial improvement (double-sickening) is used to differentiate between bacterial vs. viral acute rhinosinusitis (76, 77, 84). Patients manifest low grade or non-resolving respiratory symptoms. Nasal discharge and daytime cough are common, while headache, facial pain and fever are variable. Confirmation of bacterial infection by sinus aspiration was achieved in about two thirds of adults with symptoms > 7-10 days (53, 55). This suggest that additional qualifying clinical features are needed to differentiate viral from acute bacterial rhinosinusitis.
2) Onset with severe symptoms: ill appearance with fever of at least 390 C (1020 F) and purulent nasal discharge for at least 3-4 consecutive days at the beginning of illness (Table 2). The onset of fever, headache, and facial pain differs from an uncomplicated viral upper respiratory tract infection as the elevated temperature and purulent nasal discharge in acute bacterial rhinosinusitis occur at the beginning of the illness (18).
3) Worsening symptoms after initial improvement with a new onset of fever, an increase in nasal discharge or cough, or the onset of severe headache ( also called “double sickening”).
The symptoms and signs of acute bacterial infection can be divided into non-severe and severe (Table 2) (40). The severe form carries a higher risk of complications and mandates earlier use of antimicrobial therapy. The combination of high fever and purulent nasal discharge that lasts for at least 3 to 4 days suggests acute bacterial rhinosinusitis.
Individuals with acute bacterial rhinosinusitis often have nasal mucous membranes edema, mucopurulent nasal discharge, persistent postnasal drip, fever, and malaise. The quality of the nasal discharge varies, and can be thin or thick, clear mucoid, or purulent. Tenderness and pain of the involved sinus can be induced by percussion of the affected sinus. Cellulitis can also be present overlying the affected sinus. Other findings, especially in acute ethmoiditis, are periorbital cellulitis, edema, and proptosis. Failure to transilluminate the sinus and the presence of nasal voice can be present in many patients. Direct smear of nasal secretions usually shows the predominance of neutrophils, and the observation of numerous eosinophils suggests allergy.
Symptoms are generally protracted and vary considerably in subacute or chronic bacterial sinusitis. Fever can be of low grade or be absent. The patient may complain of malaise, easy fatigability, irregular nasal or postnasal discharge, frequent headaches, difficulty in mental concentration, anorexia, and pain or tenderness to palpation over the affected sinus. Cough and nasal congestion can persist, and a sore throat (because of mouth-breathing) is frequent.
ETIOLOGY
The most common organism isolated from patients with community-acquired, acute bacterial rhinosinusitis are Streptococcus pneumoniae, non-typeable Haemophilus influenzae, Moraxella catarrhalis, Group A beta-hemolytic streptococci, and Staphylococcus aureus (18, 19, 20, 21, 53, 105). The vaccination of children with the 7-valent pneumococcal vaccine introduced in 2000 in the United States brought about the overall decline in the recovery rate of S. pneumoniae and an increase in H. influenzae (26, 32). S. aureus is a common pathogen in sphenoid sinusitis (27). Recent data illustrates a significant increase in the rate of recovery of methicillin resistant S. aureus (MRSA) in patients with acute bacterial rhinosinusitis (22, 25).
The infection is polymicrobial in about a third of the patients. Enteric bacteria are rarely isolated, and anaerobes account for about 8% of isolates and are usually recovered from acute bacterial rhinosinusitis associated with an odontogenic origin, mainly as an extension of the infection from the roots of the premolar or molar teeth (21, 27). Pseudomonas aeruginosa and other aerobic and facultative Gram-negative rods are mainly isolated from nosocomial rhinosinusitis (mostly in those with nasal tubes or catheters), the immunocompromised, those with human immunodeficiency virus (HIV) infection, (42) and cystic fibrosis (85).
Brook et al (26) obtained middle meatus cultures from 156 adults with acute bacterial rhinosinusitis between 1997 and 2000 (prevaccination) and 229 between 2001 and 2005 (postvaccination). The recovery of S. pneumoniae was significantly reduced (46% prevaccination vs 35% postvaccination; P <.05), whereas that of H. influenzae was significantly increased (36% prevaccination vs 43% postvaccination; P <.05). The proportion of beta-lactamase–producing H. influenzae also increased slightly (from 33% to 39%), (p > 0.5).
The frequency of penicillin resistant S. pneumoniae varies regionally: it is highest in the Southeast (about 25%) of the U.S. and lowest in the Northwest (about 9%) (40). The susceptibility profiles changed in some locations after the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7), and resulted in the emergence of highly virulent and resistant nonvaccine serotypes of S. pneumoniae such as serotypes 14 and 19A (34, 41). In 2010, PCV13 that contains 6 additional pneumococcal serotype antigens including serotype 19A replaced the PCV7 (80). The new vaccine is expected to dramatically reduce penicillin non sensitive S. pneumoniae disease (35, 79).
A decrease in the rate of penicillin resistant S. pneumoniae occurred after the introduction of PCV7. Surveillance study by the CDC in 10-state between 2006–2007 showed that 15% and 10% of all invasive S. pneumoniae isolates were penicillin intermediate and penicillin-resistant, respectively (79). Lower penicillin resistance for S. pneumoniae (8-11%) were reported by three recent studies (40, 57, 87, 111).
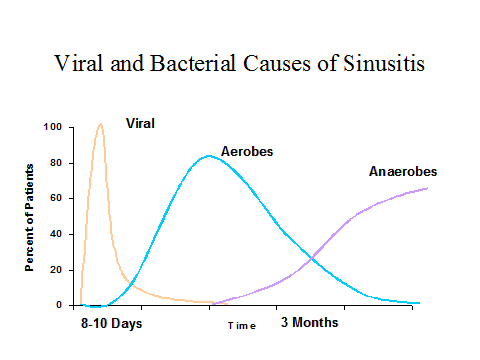
The dynamics of sinusitis as well as otitis media progresses through several phases (Figure 1). The early one is generally viral (mostly rhinovirus, adenovirus, influenza and parainfluenza viruses) that generally lasts up to 10 days where complete recovery occurs in 99% of individuals (88). In a small number of patients, a secondary acute bacterial infection may emerge, generally caused by aerobic bacteria (i.e., S. pneumoniae, H. influenzae and M. catarrhalis). If resolution does not take place, oropharyngeal anaerobic bacteria become predominant over time (28). The mechanism by which viruses predispose to bacterial sinusitis may involve viral-bacterial synergy, induction of local inflammation that blocks the sinus ostia, increase of bacterial attachment to the epithelial cells, and disruption of the local immunity.
DIAGNOSIS
History
Recorded medical history should include: previous episodes of sinusitis, and other respiratory tract infections; previous use of antibiotics; the potential of nasal foreign bodies; having a child attend a day care center; immunizations; history of allergy; exposure to cigarette smoke; co-morbidities; and previous hospitalization. The presence of any swelling and pain especially in the facial, forehead, temporal, orbital area or any other site in the head should be noted. Information about what makes the symptoms worse or better should be obtained. The length of symptoms such as cough, nasal secretions, headaches, pain, fever, hyposmia, dental pain or problems should be recorded.
Physical Examination
Physical examination should include:
• A thorough and complete general and head and neck examination (including the orbit, extra ocular motility, the response of the pupils, vision, and cranial nerve function).
• Palpation and/or percussion (over the frontal sinuses, cheeks [maxillary sinuses], and medial orbit [ethmoid sinuses].
• The nasopharynx should be assessed for postnasal drip and obstruction caused by adenoid hypertrophy, choanal atresia, malignancy, polyps, and septal deviation.
• Nasal examination including anterior rhinoscopy with a good light source looking for edema, erythema, crusting, purulent secretion, and presence of a foreign body.
• Bend the patient’s head forward (when sitting) and holding it at knee level for 45–60 seconds can elicit a sensation of fullness and pain at the involved sites (compliance in young patients may be difficult).
• Endoscopic examination performed by an otolaryngologist may localize pus within the nasal cavity directing the examiner to the involved sinus(es); bacterial cultures can also be obtained; however, the specimens may contain nasal mucosal flora.
• Transillumination is infrequently utilized because the findings do not always correlate with the disorder, and reproducibility between observers is poor.
• Indications for referral to an otolaryngologist for maxillary sinus aspiration are: failure to improve on antimicrobial therapy, severe facial pain, orbital or intracranial complications, and in the immunocompromised host (because of their unique microbiology).
• The ears should be otoscopically examined, the oral cavity should be observed for any post nasal drip, all teeth (especially the upper molars and premolars) should be inspected for cavities and tenderness, and pressure should be applied on the maxillary sinuses by the examiner’s thumbs.
Signs of sinus infection that can be observed by physical examination are:
• Mucopurulent nasal or posterior pharyngeal discharge.
• Erythematous nasal mucosa that can be pale and boggy.
• Signs of throat infection that can be associated with malodorous breath.
• Acute otitis media can be present in association with acute bacterial rhinosinusitis.
• Cervical lymphadenitis is rarely present
• Facial tenderness is inconsistent and nonspecific.
• Periorbital edema with skin discoloration may be present, especially with ethmoid sinusitis.
• Upper molar teeth pathology may be the source of maxillary sinusitis.
Clinical Findings
The location of the facial pain can point to the involved sinus. Maxillary rhinosinusitis is commonly associated with cheeks, frontal with the forehead pain, ethmoid with medial canthus, and sphenoid with occipital pain. In patients with chronic infection, changes in motion or position can worsen or alleviate the sinus symptoms.
The gold standard for the diagnosis of acute bacterial rhinosinusitis is the isolation of bacteria in high density (>104 colony-forming units/mL) from the paransal sinus cavity (44, 54, 56). Sinus aspiration is an invasive and painful procedure that is impractical in the office setting (13). Endoscopically guided cultures of the middle meatus may be considered as an alternative in adults (13). Benninger et al (15) performed a meta-analysis that included 126 adult patients from 3 studies and additional unpublished data. Endoscopically directed cultures of the middlemeatus had a sensitivity of 81%, specificity of 91%, positive predictive value of 83%, negative predictive value of 89%, and overall accuracy of 87% (95% CI, 81.3%–92.8%).
Imaging
Imaging studies such as plain radiographs or computed tomography (CT) are often utilized for the diagnosis of acute bacterial rhinosinusitis. However, they are non-specific and can not differentiate viral from bacterial rhinosinusitis.
More than 50% of patients with viral urinary tract infection had abnormal maxillary sinus radiographs (91). Sinus CTs are often abnormal in healthy individuals (43, 91) and those having CT for non respiratory reasons (52). CT performed on young adults recovering from a cold illustrated that 87% had significant maxillary sinuses abnormalities (69). Magnetic resonance imagining (MRI) illustrated that 68% of symptomatic patients with URI (68) and 42% of healthy ones (96) had significant sinus abnormalities.
The above findings illustrate that imaging studies in most individuals with uncomplicated viral URI, show abnormalities that are indistinguishable from those associated with acute bacterial rhinosinusitis. Therefore, these studies can only be useful when they are negative as they confirm the absence of acute bacterial rhinosinusitis. However, abnormal radiographic studies cannot assist in diagnosing acute bacterial rhinosinusitis, and are therefore not required in uncomplicated acute bacterial rhinosinusitis (37). Imaging is helpful in determining the disease location and extent beyond the site of the original source. They may help in supporting the diagnosis or determine the degree of mucosal involvement (96).
CT or MRI should be generally performed only in those with recurrent or complicated sinusitis or when suppurative complications are suspected. Suppurative complications of acute bacterial rhinosinusitis are infrequent, occurring in 3.7% -11% of hospitalized patients with sinusitis. They are mainly associated with potential orbital and intracranial complications of sinusitis (109). CT is best for the assessment of bony and anatomical changes associated with sinusitis and is also helpful in surgical planning and for intraoperative image-guided navigation. MRI is most effective in evaluating the extent of soft tissue inflammation and abnormalities (2, 58, 61, 72, 75, 92, 108).
The contrast-enhanced CT scan has been the preferred imaging method when complications of sinusitis are suspected (42, 85). Because there are instances where a contrast-enhanced CT scan has not revealed the pathology responsible for the clinical presentation and the MRI with contrast has, especially for intracranial complications and rarely for orbital complications (26,40). American College of Radiology recommends both MRI with contrast and contrast-enhanced CT as complementary examinations when evaluating potential complications of sinusitis (67). However, the Infectious Diseases Society of America (IDSA) panel favored contrast-enhanced CT over MRI because of its greater value, relative availability, speed, and lack of need for sedation (37). CT is especially advantageous in children because their sinuses are often asymmetrical and smaller than those in adults (1).
Differential Diagnosis
Acute bacterial rhinosinusitis has to be differentiated chronologically from other types of rhinosinusitis. These include recurrent acute, subacute, chronic, and acute exacerbation of chronic rhinosinusitis. The symptoms and signs of acute bacterial rhinosinusitis can be divided into non-severe and severe forms (Table 2) (38). The severe form has a greater risk of complications and mandates earlier use of antimicrobial treatment. In patients with subacute or chronic bacterial sinusitis the symptoms are protracted, fever is uncommon, cough and nasal congestion persist, and a sore throat is common. Differentiation must be made between allergic rhinitis, other causes of head or facial pain, asthma, and dental disorders. An allergic etiology can be confirmed by history of nasal symptoms and a history of allergy (38).
TREATMENT
The medical management of acute bacterial rhinosinusitis includes the use of antibiotics and adjuvant agents. The goals of therapy are to get rid of infection, decrease the severity and duration of symptoms, and prevent complications.
The management of acute bacterial rhinosinusitis has become a challenging endeavor because the choice of appropriate antimicrobials has become more complex in recent years. This is because many of the predominant bacterial pathogens have developed resistance to commonly used antibiotics (40, 47, 111).
Culture obtained through direct aspiration or endoscopy can direct the selection of antimicrobials in the treatment of patients who fail to respond (37).
The emerging antimicrobial resistance among respiratory pathogens leads to the empirical over utilization of broad-spectrum antibiotics which generates selective pressure that promotes the emergence of greater antimicrobial resistance (10, 73).
Several practice guidelines for the treatment of acute bacterial rhinosinusitis have been published in the U.S. within the past decade (3, 6, 7, 33, 59, 63, 83, 97). These guidelines present varying opinions about the clinical criteria for initiation and choice of empiric antimicrobial regimens. The most recent guideline, developed by the IDSA, (37) addresses some of the more controversial areas concerning initial choice of empiric management of acute bacterial rhinosinusitis in children and adults.
Empiric antimicrobial therapy should be started as soon as the clinical diagnosis of acute bacterial rhinosinusitis is made. Pharmacokinetic/pharmacodynamic (PK/PD) principles should guide adequate dosing for respiratory tract infections (39). The utility of these diagnostic criteria for initiating antimicrobial treatment has been validated by three randomized clinical trials (50, 102,104). These studies demonstrated significantly higher cure rates in those treated with antibiotics compared to placebo. Some individuals patients with mild but persistent symptoms can be observed without giving antimicrobial therapy (104). These individuals need close observation and antimicrobial agents should be given if improvement has not occurred within three days.
The justification for amoxicillin as first-line therapy for patients with acute bacterial rhinosinusitis relates to its safety, efficacy, low cost, and narrow microbiologic spectrum (94). When used in sufficient doses amoxicillin is effective against susceptible and intermediate resistant pneumococci. Amoxicillin is ineffective, however, against beta-lactamase producing M. catarrhalis andH. influenzae (about 90% and 30% of isolates, respectively).
Consideration to prescribe amoxicillin with clavulanate for adults with acute bacterial rhinosinusitis should be given to those at a high risk of being infected by an amoxicillin resistant microrganism(s) (17) (Table 3).
The use high-dose amoxicillin with clavulanate (2 g orally twice daily or 90 mg/kg/day orally twice daily) is recommended by the IDSA Practice Guideline for acute bacterial rhinosinusitis (37) for adults at a high risk of being infected by amoxicillin resistant organism. High-dose amoxicillin is preferred over standard-dose amoxicillin primarily to cover penicillin non susceptible (NPS) S. pneumoniae. This risk exists in those from geographic regions with high endemic rates (>10%) of invasive penicillin non susceptible S. pneumoniae, those with severe infection (e.g., evidence of systemic toxicity with fever of 39oC [102oF] or higher, and threat of suppurative complications), age > 65 years, recent hospitalization, antibiotic use within the past month, or who are immunocompromised (29). The primary disadvantages of using the “high-dose” amoxicillin-clavulanate is the added cost and potentially higher incidence of adverse effects.
The recommendation of administering amoxicillin-clavulanate is based on in vitro susceptibility data that show a high recovery of amoxicillin resistant organisms in adult patients with acute bacterial rhinosinusitis, and the current prevalence rates of beta-lactamase production among H. influenzae (26).
For penicillin-allergic adult patients, either doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is recommended as an alternative agent for empiric antimicrobial therapy. Combination therapy with clindamycin plus a third-generation oral cephalosporin (cefixime or cefpodoxime) is recommended in adults with a history of non–type I hypersensitivity to penicillin.
The high prevalence of macrolide-resistant S. pneumoniae in the U.S. (>40%) (66) and the high rates of resistance to trimethoprim-sulfamethoxazole among both S. pneumoniae (50%) andH. influenzae (27%) excludes these agents for treatment of acute bacterial rhinosinusitis (111).
Studies determining S. pneumoniae penicillin resistance using the revised Clinical Laboratory Standards Institute (CLSI) breakpoints defining penicillin-intermediate (MIC 4 micg/ml; treatable with “high-dose” amoxicillin) and penicillin-resistant S. pneumoniae (MIC >8 micg/ml; untreatable with amoxicillin), illustrated a higher rate of penicillin susceptibility (89-93%) (35, 48, 51, 57, 71, 87, 111). These suggest that unless the rate of penicillin-resistant S. pneumoniae in the community is high (>10%), “standard-dose” amoxicillin-clavulanate should be adequate for the treatment of non-meningitis S. pneumoniae infections including acute bacterial rhinosinusitis.
Oral cephalosporons are inactive against penicillin-resistant S. pneumoniae (45, 86). The activity of second and third generation oral cephalosporins (i.e. cefaclor, cefuroxime axetil, cefpodoxime, cefprozil, cefdinir and cefixime) is variable against penicillin-intermediate and resistant S. pneumoniae. Cefpodoxime, cefuroxime axetil and cefidnir are moderately active against this organism (<50% susceptible), and cefixime is less effective (45, 46, 51, 86). The parenteral third generation cephalosporins cefotaxime and ceftriaxone are active against all S. pneumoniae including penicillin-resistant ones and are the recommended second-line empiric therapy (in place of high-dose amoxicillin-clavulanate) for hospitalized children. The most active oral cephalosporin against both H. influenzae and M. catarrhalis (beta-lactamase positive and negative), is cefpodoxime followed by cefixime, cefuroxime and cefdinir (65, 86). Cefaclor and cefprozil are least effective.
Because of the variableactivity of second and third generation oral cephalosporins against S. pneumoniae and H. influenzae they are no longer adequate as monotherapy for the initial empiric treatment of acute bacterial rhinosinusitis. If an oral cephalosporin is used, a third generation cephalosporin (e.g. cefpodoxime or cefixime) combined with clindamycin is recommended in regions with high isolation rates of penicillin-nonsusceptible S. pneumoniae (>10%).
The increase recovery of MRSA in acute bacterial rhinosinusitis requires consideration of the need for coverage against these organisms (22, 25, 60). A comparison of the rate of recovery ofMRSA between 2001-2003 to 2004-2006 in 244 patients with acute bacterial rhinosinusitis illustrated a significant increase in the rate of recovery of this organism in patients (from 3% of patients to 10%, p< .01) (25). This finding suggests the use of greater index suspicion for the presence of MRSA in sinusitis and greater use of sinus cultures especially in patients who do not improve or fail antimicrobial after 48 hours of therapy. Because the nose can be a reservoir for S. aureus, there is a concern that the recovery of S. aureus could be due to contamination by the nasal flora during sinus aspiration or acquisition of middle meatus cultures. Accurate diagnosis of MRSA rhinosinusitis by microbiological cultures is essential for appropriate antimicrobial treatment.
Currently there is insufficient evidence to support the empiric coverage for MRSA in acute bacterial rhinosinusitis. However, in seriously ill individuals with suspected orbital or intracranial complication, and hospitalized patients with nosocomial sinusitis, empiric coverage for MRSA is helpful. Although vancomycin is considered the gold standard for therapy of MRSA, the increasing in vitro resistance to vancomycin (60), and reports of clinical failures underscore the need for alternative therapies. Other agents with good in vitro activity against MRSA include trimethoprim-sulfamethoxazole, clindamycin, linezolid, quinupristin-dalfopristin, daptomycin, and tigecycline.
The current treatment guidelines for acute bacterial rhinosinusitis that generally recommend a course of antimicrobial therapy for 10-14 days, are derived from the length of therapy in many of the randomized control studies in adults (84). Some recommend that treatment for 7 days beyond the time symptoms had resolved (100). Data about the optimal duration of therapy, are non conclusive because the efficacy of shorter courses has not been studied in a rigorous randomized manner (81). In children with acute bacterial rhinosinusitis, the longer treatment duration of 10-14 days is still recommended (37).
Most pathogens are eliminated from the maxillary sinuses by the third day of adequate antimicrobial therapy (4, 5, 7, 8, 9). A correlation was noted between time to bacterial eradication and time to clinical resolution (5) resistant pathogens, structural abnormalities or a non-infectious etiology. Similarly, if there is no clinical improvement within 3-5 days despite initial empiric antimicrobial therapy, an alternate management strategy should be considered.
Consecutive endoscopic cultures from maxillary sinus were performed of aspirates obtained from 20 patients with acute bacterial rhinosinusitis who failed initial empiric antimicrobial therapy (29). Increased level of resistance with MIC at least 2-fold higher than for the pre-treatment isolate was identified in half of patients. These findings show that bacterial resistance should be considered in all patients who fail to respond to initial empiric antimicrobial therapy.
If the patient worsens or fails to improve with the initial management option by 3-5 days, the clinician should reassess the patient to confirm acute bacterial rhinosinusitis, exclude other causes of illness and treatment failure and detect complications including orbital or intracranial spread of infection. A nonbacterial cause or infection with drug resistant bacteria (29) should be considered and should prompt a switch to alternate antibiotic therapy and reevaluation of the patient. When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent (94) and the choice of antibiotic should provide adequate coverage of anticipated bacteria. Beta-lactams, flouroquinoloes, and doxycycline resistant S. pneumoniaeand beta-lactamase producing H. influenzae and M. catarrhalis are more common following previous antibiotic exposure (57, 64, 95). If bacterial cultures are obtained treatment can be tailored to the bacterial isolates and their antimicrobial susceptibility.
In choosing a second-line treatment in a those who failed initial antimicrobial choice, an agent with broader spectrum of activity and in a different antimicrobial class should be considered (90, 100). Antimicrobials selected should be active against PNS S. pneumoniae and ampicillin-resistant H. influenzae as well as other beta-lactamase producing respiratory pathogens. Patients who were initially treated with amoxicillin without clavulanate can be treated with either high dose amoxicillin plus clavulante (4 g per day amoxicillin equivalent), doxycycline, a respiratory fluoroquinolone (levofloxacin or moxifloxacin) or the combination of clindamycin plus a third-generation oral cephalosporin (cefixime or cefpodoxime). These agents would also cover less common pathogens, such as S. aureus and anaerobic bacteria. Conversely, oral cephalosporins and macrolides are predicted to offer inadequate coverage for S. pneumoniae or H. influenzae.
ADJUVANT THERAPIES
In addition to antibiotics, other therapies have been utilized in the management of bacterial sinusitis. These included topical and systemic decongestants, corticosteroids, anti-inflammatory agents, mucolytic agents, humidification, antihistamines, nasal irrigation, saline nasal spray, spicy food, and hot dry air (24, 89). These agents induce rapid vasoconstriction, improve ostial potency, reduce swelling and congestion of the turbinates, and decrease inflammation at the osteomeatal, thus facilitating sinus drainage. Use of any intranasal medications in children may not be well tolerated.
Neither topical nor oral decongestants and/or anti-histamines are recommended as adjunctive treatment in patients with acute bacterial rhinosinusitis. Topical decongestants may induce rebound congestion and inflammation, while oral antihistamines may induce drowsiness, xerostomia and other adverse effects.
Even though decongestants and antihistamines are frequently used by those with acute bacterial rhinosinusitis, there is minimal evidence supporting that they enhance recovery. Although patients may subjectively feel improvement after using these agents, objective rhinometric measurements do not support this impression (12, 62, 74, 95, 107).
The recommendation against the use of decongestants or antihistamines as adjunctive therapy in acute bacterial rhinosinusitis places a relatively high value on avoiding their adverse effects, and a relatively low value on the incremental clinical improvement. However, these agents may still provide symptom relief in some individuals with viral rhinosinusitis.
Reduction in the viscosity and improvement in the quality of mucus can assist in resolution of the infection. Several methods achieve this goal, including nasal saline spray or irrigation, air humidification, adequate hydration, and mucolytic agents (98, 106).
Antihistamines are generally not used to treat acute bacterial rhinosinusitis, because they can thicken and dry the secretions, which lead to crusting and further blocks the osteomeatal complex. They can be useful, however, if the underlying cause is allergic.
Intranasal corticosteroids offer modest symptomatic improvement and minimal adverse effects with short-term use (11, 78, 111). These agents are recommended as an adjunct to antibiotics in the empiric treatment of acute bacterial rhinosinusitis, mainly in those with a history of allergic rhinitis. Steroids have a delayed onset of action, and clinical improvement may take 7 to 10 days. They are always used in conjunction with antimicrobial therapy.
Systemic corticosteroids are rarely necessary in the treatment of allergic rhinitis, because of the generally good efficacy of topical corticosteroids or which immunotherapy may be effective (37).
SURGICAL TREATMENT
Surgical drainage may be needed in those who fail medical therapy especially when complications occur. The goals of surgery are to allow drainage of purulent material, prevent persistence, recurrence, progression and complications. This is accomplished by removing diseased tissue, and promoting drainage (or obliteration if this is not possible) while considering the cosmetic outcome. Functional endoscopic sinus surgery (FESS) has become the main surgical technique used. Endoscopic surgery achieves up to success rate in over ¾ of patients in both adults and children (99). Radical procedures are used when rhinosinusitis is complicated by orbital or intracranial involvement.
COMPLICATIONS
When not treated promptly and properly sinus infection can spread via anastomosing veins or by direct extension to nearby structures (23). Orbital complications are categorized (101) into five stages according to their severity. Contiguous spread to the orbital area can result in periorbital cellulitis, subperiosteal abscess, orbital cellulitis, and abscess. Sinusitis can extend to the central nervous system, where it can cause cavernous sinus thrombosis, retrograde meningitis, and epidural, subdural, and brain abscesses (29, 93). Orbital symptoms often precede intracranial extension (101). Osteomyelitis of the frontal bone often originates from a spreading thrombo-phlebitis (23). A periostitis of the frontal sinus causes an osteitis and a periostitis of the outer membrane, which produces a tender, puffy swelling of the forehead.
Complications of sinusitis are rare, but can be life threatening. Diagnosis is assisted by observing local tenderness and dull pain, and is confirmed by CT and nuclear isotope scanning. The most common bacterial causes are anaerobic bacteria and S. aureus. Management includes surgical drainage and antimicrobial therapy (23). Antibiotics should be administered for at least 6 weeks.
SUMMARY
Viral infection of the upper respiratory tract is the most common presentation of rhinosinusitis and the vast majority of cases resolve spontaneously. Only a small proportion develops a secondary bacterial infection which will benefit from antimicrobial therapy. Acute bacterial rhinosinusitis is generally diagnosed in the presence of more than 7-10 days and less than 30 days of nasal discharge. The most common bacterial isolates from acute rhinosinusitis are S. pneumoniae, H. influenzae, M.catarrhalis, Group A beta-hemolytic streptococci, and S. aureus. Aerobic Gram-negative rods including Pseudomonas aeruginosa are common in nosocomial sinusitis, the immunocompromised, those with human immunodeficiency virus infection and cystic fibrosis. Fungal and P. aeruginosa are common causes of sinusitis in neutropenic patients.
The proper choice of antibiotic therapy depends on the likely infecting pathogens, bacterial antibiotic resistance and antibiotics’ pharmacologic profiles. In addition to antibiotics, adjuvant therapies and surgery are utilized in the management of bacterial sinusitis.
Since there are currently no good markers that define viral rhinosinusitis from acute bacterial rhinosinusitis many clinicians elect when in doubt to administer antimicrobials to their patients. However, this approach is one of the main contributors to the increase of resistance to antimicrobials of respiratory pathogens that has made the management of true bacterial rhinosinusitis more challenging. The introduction of new vaccinations against S. pneumoniae and the expected new vaccines against other potential sinus pathogens (i.e. non-type b H. influenzae ) may change the bacterial etiology of acute bacterial rhinosinusitis. The increase recovery of MRSA is an example of such a change. Continuous monitoring of the evolving bacterial etiology of acute bacterial rhinosinusitis are therefore of great importance.
REFERENCES
1. Aalokken TM, Hagtvedt T, Dalen I, Kolbenstvedt A. Conventional sinus radiography compared with CT in the diagnosis of acute sinusitis. Dentomaxillofac Radiol 2003;32:60-2. [PubMed]
2. Adame N, Hedlund G, Byington CL. Sinogenic intracranial empyema in children. Pediatrics 2005;116:e461-e467. [PubMed]
3. Agency for Health Care Research and Quality. Update on acute bacterial rhinosinusitis. Evidence Report/Technololgy Assessment: Number 124, Contract No. 290-02-0022. [http://www.ahrq.gov/] 2005;1-7.
4. Ambrose PG, Anon JB, Bhavnani SM, Okusanya OO, Jones RN, Paglia MR, Kahn J, Drusano GL. Use of pharmacodynamic endpoints for the evaluation of levofloxacin for the treatment of acute maxillary sinusitis. Diagn. Microbiol Infect Dis 2008;61:13-20. [PubMed]
5. Ambrose PG, Anon JB, Owen JS, Owen JS, Van Wart S, McPhee ME, Bhavnani SM, Piedmonte M, Jones RN. Use of pharmacodynamic end points in the evaluation of gatifloxacin for the treatment of acute maxillary sinusitis. Clin. Infect. Dis. 2004;38:1513-1520. [PubMed]
6. American Academy of Pediatrics. Subcommittee on Management of Sinusitis and Committee on Quality Improvement. Clinical practice guideline: management of sinusitis. Pediatrics 2001; 108:798-808. [PubMed]
7. Anon JB, Jacobs MR, Poole MD, Ambrose PG, Benninger MS, Hadley JA, Craig WA; Sinus And Allergy Health Partnership. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol.Head Neck Surg. 2004; 130:1-45. [PubMed]
8. Anon JB, Paglia M, Xiang J, Ambrose PG, Jones RN, Kahn JB. Serial sinus aspirate samples during high-dose, short-course levofloxacin treatment of acute maxillary sinusitis. Diagn Microbiol Infect Dis 2007; 57:105-107. [PubMed]
9. Ariza H, Rojas R, Johnson P, Gower R, Benson A, Herrington J, Perroncel R, Pertel P. Eradication of common pathogens at days 2, 3 and 4 of moxifloxacin therapy in patients with acute bacterial sinusitis. BMC Ear Nose Throat Disord. 2006;6:8. [PubMed]
10. Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U.S.A 1999;96:1152-1156. [PubMed]
11. Barlan IB, Erkan E, Bakir M, Berrak S, Başaran MM. Intranasal budesonide spray as an adjunct to oral antibiotic therapy for acute sinusitis in children. Ann Allergy Asthma Immunol. 1997;78:598-601. [PubMed]
12. Bende M, Fukami M, Arfors KE, Mark J, Stierna P, Intaglietta M. Effect of oxymetazoline nose drops on acute sinusitis in the rabbit. Ann. Otol. Rhinol. Laryngol. 1996; 105:222-225. [PubMed]
13. Benninger MS, Appelbaum PC, Denneny JC, Osguthorpe DJ, Stankiewicz JA. Maxillary sinus puncture and culture in the diagnosis of acute rhinosinusitis: The case for pursuing alternative culture methods. Otolaryngology - Head and Neck Surgery 2002; 127:7-12. [PubMed]
14. Benninger MS, Sedory-Holzer SE, Lau J. Diagnosis and treatment of uncomplicated acute bacterial rhinosinusitis: Summary of the Agency for Health Care Policy and Research evidence-based report. Otolaryngol Head Neck Surg 2000,122:1–7. [PubMed]
15. Benninger MS, Payne SC, Ferguson BJ, Hadley JA, Ahmad N. Endoscopically directed middle meatal cultures versus maxillary sinus taps in acute bacterial maxillary rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg 2006;134:3–9. [PubMed]
16. Brook I. Aerobic and anaerobic bacteriology of purulent nasopharyngitis in children. J Clin Microbiol. 1988;26:592–4. [PubMed]
17. Brook I. Sinusitis. Periodontology 2000;49:126-139. [PubMed]
18. Brook I. Bacteriology of acute and chronic frontal sinusitis. Arch Otolaryngol Head Neck Surg 2002;128:583-5. [PubMed]
19. Brook I. Bacteriology of acute and chronic sphenoid sinusitis. Ann Otol Rhinol Laryngol 2002;111:1002-4.[PubMed]
20. Brook I. Acute and chronic frontal sinusitis. Curr Opin Pulm Med. 2003;9:171-174. [PubMed]
21. Brook I. Microbiology of acute and chronic maxillary sinusitis associated with an odontogenic origin. Laryngoscope. 2005;115:823-5. [PubMed]
22. Brook I. Role of methicillin-resistant Staphylococcus aureus in head and neck infections. J Laryngol Otol. 2009 ;11:1-7. [PubMed]
23. Brook I. Microbiology and antimicrobial treatment of orbital and intracranial complications of sinusitis in children and their management. Int J Pediatr Otorhinolaryngol. 2009;73:1183-6.[PubMed]
24. Brook I. Treatment modalities for bacterial rhinosinusitis. Expert Opin Pharmacother. 2010;11:755-69. [PubMed]
25. Brook I, Foote PA, Hausfeld JN. Increase in the frequency of recovery of methicillin-resistant Staphylococcus aureus in acute and chronic maxillary sinusitis. J Med Microbiol. 2008;57:1015-7.[PubMed]
26. Brook I, Foote PA, Hausfeld JN. Frequency of recovery of pathogens causing acute maxillary sinusitis in adults before and after introduction of vaccination of children with the 7-valent pneumococcal vaccine. J Med Microbiol 2006;55:943–6. [PubMed]
27. Brook I, Frazier EH, Gher ME. Microbiology of periapical abscesses and associated maxillary sinusitis. J Periodontal. 1996;67:608–10. [PubMed]
28. Brook I, Frazier EH, Foote PA. Microbiology of the transition from acute to chronic maxillary sinusitis. J Med Microbiol 1996;45: 372–5. [PubMed]
29. Brook I, Gober AE. Resistance to antimicrobials used for therapy of otitis media and sinusitis: effect of previous antimicrobial therapy and smoking. Ann Otol Rhinol Laryngol 1999; 108:645–7. [PubMed]
30. Brook I, Gober AE. Dynamics of nasopharyngitis in children. Otolaryngol Head Neck Surg. 2000;122:696-700. [PubMed]
31. Brook I, Gober AE. Antimicrobial resistance in the nasopharyngeal flora of children with acute maxillary sinusitis and maxillary sinusitis recurring after amoxicillin therapy. J Antimicrob Chemother. 2004;53:399-402. [PubMed]
32. Brook I, Gober AE. Frequency of recovery of pathogens from the nasopharynx of children with acute maxillary sinusitis before and after the introduction of vaccination with the 7-valent pneumococcal vaccine. Int J Pediatr Otorhinolaryngol. 2007;71:575-9. [PubMed]
33. Brook I, Gooch WM, Jenkins SG, Pichichero ME, Reiner SA, Sher L, Yamauchi T. Medical Management of Bacterial Sinusitis. Recommendation of Clinical Advisory Committee on Pediatric and adult Sinusitis. Ann Otol Rhinol Laryngol Suppl. 2000;182:1-20. [PubMed]
34. Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 2010;29:304–9. [PubMed]
35. Centers for Disease Control and Prevention. Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae—United States, 2006–2007. MMWR Morb Mortal Wkly Rep 2008;57:1353–5. [PubMed]
36. Chandler JR, Langenbrunner DJ, Stevens EF. The pathogenesis of orbital complications in acute sinusitis.Laryngoscope. 1970;80:1414–28. [PubMed]
37. Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, Pankey GA, Seleznick M, Volturo G, Wald ER, File TM Jr. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112. [PubMed]
38. Clement PAR, Bluestone CD, Gordts F, Lusk RP, Otten FW, Goossens H, Scadding GK, Takahashi H, van Buchem FL, Van Cauwenberge P, Wald ER. Management of rhinosinusitis in children. Consensus Meeting, Brussels, Belgium, September 13 1996. Arch Otolaryngol Head Neck Surg 1998;124:31–4. [PubMed]
39. Craig WA. The hidden impact of antibacterial resistance in respiratory tract infection. Re-evaluating current antibiotic therapy. Respir. Med 2001;95 Suppl A:S12-S19. [PubMed]
40. Critchley IA, Brown SD, Traczewski MM, Tillotson GS, Janjic N. National and regional assessment of antimicrobial resistance among community-acquired respiratory tract pathogens identified in a 2005–2006 U.S. faropenem surveillance study. Antimicrob Agents Chemother 2007; 51:4382–9. [PubMed]
41. Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect 2009; 15(Suppl 3):16–20. [PubMed]
42. Decker CF. Sinusitis in the Immunocompromised Host. Curr Infect Dis Rep.1:27-32, 1999. [PubMed]
43. Diament MJ, Senac MO, Gilsanz V, Baker S, Gillespie T, Larsson S. Prevalence of incidental paranasal sinuses opacification in pediatric patients: a CT study. J Comput Assist Tomogr 1987; 11:426-431. [PubMed]
44. Evans FO, Sydnor JB, Moore WE, Manwaring JL, Brill AH, Jackson RT, Hanna S, Skaar JS, Holdeman LV, Fitz-Hugh S, Sande MA, Gwaltney JM Jr. Sinusitis of the maxillary antrum. N Engl J Med 1975; 293:735-739. [PubMed]
45. Fenoll A, Gimenez MJ, Robledo O, Aguilar L, Tarragó D, Granizo JJ, Gimeno M, Coronel P. In vitro activity of oral cephalosporins against pediatric isolates of Streptococcus pneumoniaenon-susceptible to penicillin, amoxicillin or erythromycin. J. Chemother. 2008;20:175-179. [PubMed]
46. Fenoll A, Gimenez MJ, Robledo O, Aguilar L, Tarragó D, Granizo JJ, Martín-Herrero JE. Influence of penicillin/amoxicillin non-susceptibility on the activity of third-generation cephalosporins against Streptococcus pneumoniae. Eur.J.Clin.Microbiol.Infect.Dis. 2008; 27:75-80. [PubMed]
47. Flamm RK, Sader HS, Jones RN. Spectrum and potency of ceftaroline against leading pathogens causing community-acquired respiratory tract and skin and soft tissue infections in Latin America, 2010. Braz J Infect Dis. 2013;17:564-72. [PubMed]
48. Flamm RK, Sader HS, Farrell DJ, Jones RN. Antimicrobial Activity of Ceftaroline Tested against Drug-Resistant Subsets of Streptococcus pneumoniae from U.S. Medical Centers. Antimicrob Agents Chemother. 2014;58:2468-71. [PubMed]
49. Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl 2007: 1–136. [PubMed]
50. Garbutt JM, Goldstein M, Gellman E, et al. A randomized, placebo-controlled trial of antimicrobial treatment for children with clinically diagnosed acute sinusitis. Pediatrics 2001; 107:619-625. [PubMed]
51. Ginsburg AS, Tinkham L, Riley K, Kay NA, Klugman KP, Gill CJ. Antibiotic non-susceptibility among Streptococcus pneumoniae and Haemophilus influenzae isolates identified in African cohorts: a meta-analysis of three decades of published studies. Int J Antimicrob Agents. 2013;42:482-91. [PubMed]
52. Gwaltney J, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25-30. [PubMed]
53. Gwaltney JM Jr, Scheld WM, Sande MA, Sydnor A. The microbial etiology and antimicrobial therapy of adults with acute community-acquired sinusitis: a fifteen-year experience at the University of Virginia and review of other selected studies. J Allergy Clin Immunol. 1992;90:457–62. [PubMed]
54. Gwaltney JM Jr, Wiesinger BA, Patrie JT. Acute community acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis 2004; 38:227–33. [PubMed]
55. Hadley JA, Mosges R, Desrosiers M, et al. Moxifloxacin five-day therapy versus placebo in acute bacterial rhinosinusitis. Laryngoscope 2010; 120:1057-1062. [PubMed]
56. Hamory BH, Sande MA, Sydnor A, Jr., et al. Etiology and antimicrobial therapy of acute maxillary sinusitis. J Infect Dis 1979; 139:197-202. [PubMed]
57. Harrison CJ, Woods C, Stout G, Martin B, Selvarangan R. Susceptibilities of Haemophilus influenzae, Streptococcus pneumoniae, including serotype 19A, and Moraxella catarrhalis paediatric isolates from 2005 to 2007 to commonly used antibiotics. J Antimicrob Chemother 2009; 63:511–19. [PubMed]
58. Herrmann BW, Forsen JW, Jr. Simultaneous intracranial and orbital complications of acute rhinosinusitis in children. Int J Pediatr Otorhinolaryngol. 2004;68:619-625. [PubMed]
59. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med 2001;134:498-505. [PubMed]
60. Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antmicrob Agents Chemother. 2006;50:3039-47. [PubMed]
61. Hurley MC, Heran MK. Imaging studies for head and neck infections. Infect Dis Clin North Am. 2007; 21:305-3vi. [PubMed]
62. Inanli S, Ozturk O, Korkmaz M, Tutkun A, Batman C. The effects of topical agents of fluticasone propionate, oxymetazoline, and 3% and 0.9% sodium chloride solutions on mucociliary clearance in the therapy of acute bacterial rhinosinusitis in vivo. Laryngoscope 2002; 112:320-325. [PubMed]
63. Institute for Clinical Systems Improvement (ICSI) Healthcare Guidelines: Diagnosis and Treatment of Respiratory Illness in Children and Adults, 2nd ed. ICSI 2008.
64. Jansen WT, Verel A, Beitsma M, Verhoef J, Milatovic D. Surveillance study of the susceptibility of Haemophilus influenzae to various antibacterial agents in Europe and Canada. Curr Med Res Opin 2008; 24:2853-2861. [PubMed]
65. Jacobs MR. Antimicrobial-resistant Streptococcus pneumoniae: trends and management. Expert Rev Anti Infect Ther 2008;6:619-635. [PubMed]
67. Karmazyn BK. ACR Appropriateness Criteria - sinusitis. Expert Panel on Pediatric Imaging. 2009; 1-5.
66. Jenkins SG, Farrell DJ. Increase in pneumococcus macrolide resistance, United States. Emerg Infect Dis 2009;15:1260–4. [PubMed]
68. Kristo A, Alho OP, Luotonen J, Koivunen P, Tervonen O, Uhari M. Cross-sectional survey of paranasal sinus magnetic resonance imaging findings in schoolchildren. Acta Paediatr. 2003; 92:34-36. [PubMed]
69. Kristo A, Uhari M, Luotonen J, Koivunen P, Ilkko E, Tapiainen T, Alho OP. Paranasal sinus findings in children during respiratory infection evaluated with magnetic resonance imaging. Pediatrics 2003; 111:e586-e589. [PubMed]
70. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997;117:51-7. [PubMed]
71. Lee S, Bae S, Lee KJ, Yu JY, Kang Y. Changes in serotype prevalence and antimicrobial resistance among invasive Streptococcus pneumoniae isolates in Korea, 1996-2008. J Med Microbiol. 2013;62:1204-10. [PubMed]
72. Mafee MF, Tran BH, Chapa AR. Imaging of rhinosinusitis and its complications: plain film, CT, and MRI. Clin. Rev. Allergy Immunol. 2006;30:165-186. [PubMed]
73. Magee JT, Pritchard EL, Fitzgerald KA, Dunstan FD, Howard AJ. Antibiotic prescribing and antibiotic resistance in community practice: retrospective study, 1996-8. BMJ 1999; 319:1239-1240. [PubMed]
74. McCormick DP, John SD, Swischuk LE, Uchida T. A double-blind, placebo-controlled trial of decongestant-antihistamine for the treatment of sinusitis in children. Clin Pediatr (Phila) 1996;35:457-460. [PubMed]
75. McIntosh D, Mahadevan M. Failure of contrast enhanced computed tomography scans to identify an orbital abscess. The benefit of magnetic resonance imaging. J Laryngol Otol 2008; 122:639-640. [PubMed]
76. Meltzer EO, Bachert C, Staudinger H. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol 2005;116:1289-1295. [PubMed]
77. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Adinoff AD, Bachert C, Borish L, Chinchilli VM, Danzig MR, Ferguson BJ, Fokkens WJ, Jenkins SG, Lund VJ, Mafee MF, Naclerio RM, Pawankar R, Ponikau JU, Schubert MS, Slavin RG, Stewart MG, Togias A, Wald ER, Winther B; Rhinosinusitis Initiative. Rhinosinusitis: Developing guidance for clinical trials. Otolaryngol. Head Neck Surg. 2006;135:S31-S80. [PubMed]
78. Meltzer EO, Teper A, Danzig M. Intranasal corticosteroids in the treatment of acute rhinosinusitis. Curr Allergy Asthma Rep. 2008;8:133-8. [PubMed]
79. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 2011; 29:9127–31. [PubMed]
80. MMWR Recomm Rep Advisory Committee on Immunization Practices (ACIP). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children. MMWR 2010;59:258–61. [PubMed]
81. Pichichero ME. Short course antibiotic therapy for respiratory infections: a review of the evidence. Pediatr Infect Dis J 2000;19:929-937. [PubMed]
82. Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat 10, 2009:1–157. [PubMed]
83. Rosenfeld RM, Andes D, Bhattacharyya N, Cheung D, Eisenberg S, Ganiats TG, Gelzer A, Hamilos D, Haydon RC 3rd, Hudgins PA, Jones S, Krouse HJ, Lee LH, Mahoney MC, Marple BF,Mitchell CJ, Nathan R, Shiffman RN, Smith TL, Witsell DL. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg 2007;137:S1–31. [PubMed]
84. Rosenfeld RM, Singer M, Jones S. Systematic review of antimicrobial therapy in patients with acute rhinosinusitis. Otolaryngol. Head Neck Surg. 2007; 137:S32-S45. [PubMed]
85. Ryan MW, Brooks EG. Rhinosinusitis and comorbidities. Curr Allergy Asthma Rep. 2010;10:188-93. [PubMed]
86. Sader HS, Jacobs MR, Fritsche TR. Review of the spectrum and potency of orally administered cephalosporins and amoxicillin/clavulanate. Diagn Microbiol Infect Dis 2007;57:5S-12S. [PubMed]
87. Sahm DF, Brown NP, Draghi DC, Evangelista AT, Yee YC, Thornsberry C. Tracking resistance among bacterial respiratory tract pathogens: summary of findings of the TRUST Surveillance Initiative, 2001–2005. Postgrad Med 2008;120:8–15. [PubMed]
88. Sande MA, Gwaltney JM. Acute community-acquired bacterial sinusitis – continuing challenges and current management. Clin Infect Dis 2004;39(Suppl 3):S151-8. [PubMed]
89. Scarupa MD, Kaliner MA. Adjuvant therapies in the treatment of acute and chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:251-62. [PubMed]
90. Scheid DC, Hamm RM. Acute bacterial rhinosinusitis in adults: part II. Treatment. Am Fam Physician 2004;70:1697-1704. [PubMed]
91. Shopfner CE, Rossi JO. Roentgen evaluation of the paranasal sinuses in children. Am.J.Roentgenol.Radium.Ther.Nucl.Med. 1973; 118:176-186. [PubMed]
92. Sievers KW, Dietrich U, Zoller E, Dost P, Jahnke K. Diagnostic aspects of isolated sphenoid sinusitis. HNO 1992;40:464-467. [PubMed]
93. Siira L, Jalava J, Kaijalainen T, Ollgren J, Lyytikäinen O, Virolainen A. Antimicrobial Resistance in Relation to Sero- and Genotypes Among Invasive Streptococcus pneumoniae in Finland, 2007-2011. Microb Drug Resist. 2014;20:124-30. [PubMed]
94. Sinus and Allergy Health Partnership (SAHP). Antimicrobial treatment guidelines for ABRS. Otolaryngol Head Neck Surg 2004;130(Suppl):1– 45.
95. Sipila J, Suonpaa J, Silvoniemi P, Laippala P. Correlations between subjective sensation of nasal patency and rhinomanometry in both unilateral and total nasal assessment. ORL J. Otorhinolaryngol. Relat Spec. 1995;57:260-263. [PubMed]
96. Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005; 116:S13-S47. [PubMed]
97. Snow V, Mottur-Pilson C, Hickner JM. Principles of appropriate antibiotic use for acute sinusitis in adults. Ann Intern Med. 2001;134:495-497. [PubMed]
98. Tomooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope 2000; 110:1189-1193. [PubMed]
99. Vashishta R, Soler ZM, Nguyen SA, Schlosser RJ. A systematic review and meta-analysis of asthma outcomes following endoscopic sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3:788-94. [PubMed]
100. Wald ER. Sinusitis. Pediatric Annals 1998;27:811-818. [PubMed]
101. Wald ER. Periorbital and orbital infections. Infect Dis Clin North Am. 2007;21:393–408. [PubMed]
102. Wald ER, Chiponis D, Ledesma-Medina J. Comparative effectiveness of amoxicillin and amoxicillin-clavulanate potassium in acute paranasal sinus infections in children: a double-blind, placebo-controlled trial. Pediatrics. 1986;77:795-800. [PubMed]
103. Wald ER, Guerra N, Byers C. Upper respiratory tract infections in young children: duration of and frequency of complications. Pediatrics. 1991;87:129-33. [PubMed]
104. Wald ER, Nash D, Eickhoff J. Effectiveness of amoxicillin/clavulanate potassium in the treatment of acute bacterial sinusitis in children. Pediatrics 2009; 124:9-15. [PubMed]
105. Wald ER, Reilly JS, Casselbrant M, Ledesma-Medina J, Milmoe GJ, Bluestone CD, Chiponis D. Treatment of acute maxillary sinusitis in childhood - a comparative study of amoxicillin and cefaclor. J. Pediatrics 1984; 104:297-302. [PubMed]
106. Wang YH, Yang CP, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in children. Int. J. Pediatr .Otorhinolaryngol. 2009; 73:1696-1701. [PubMed]
107. Wiklund L, Stierna P, Berglund R, Westrin KM, Tönnesson M. The efficacy of oxymetazoline administered with a nasal bellows container and combined with oral phenoxymethyl-penicillin in the treatment of acute maxillary sinusitis. Acta Otolaryngol.Suppl 1994; 515:57-64. [PubMed]
108. Younis RT, Anand VK, Davidson B. The role of computed tomography and magnetic resonance imaging in patients with sinusitis with complications. Laryngoscope 2002; 112:224-229. [PubMed]
109. Younis RT, Lazar RH, Anand VK. Intracranial complications of sinusitis: a 15-year review of 39 cases. Ear Nose Throat J. 2002; 81:636-2, 644. [PubMed]
110. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013 Dec 2;12:CD005149. [PubMed]
111. Zhang X, Wang R, Di X, Liu B, Liu Y. Different microbiological and clinical aspects of lower respiratory tract infections between China and European/American countries. J Thorac Dis. 2014 ;6:134-42. [PubMed]
Tables
Table 1: Major and minor clinical criteria suggestive of bacterial sinusitis*
| Major criteria | Minor criteria |
|---|---|
| Facial pain or pressure (requires a second major criterion to constitute a suggestive history) | Headache |
| Facial congestion or fullness | Fever (for subacute and chronic sinusitis) |
| Nasal congestion or obstruction | Halitosis |
| Nasal discharge, purulence or discoloured postnasal drainage | Fatigue |
| Hyposmia or anosmia | Dental pain |
| Fever (for acute sinusitis; requires a second major criterion to constitute a strong history) | Cough |
| Purulence on intranasal examination | Ear pain, pressure or fullness |
| * A strongly suggestive history requires the presence of two major criteria or one major and two or more minor criteria. A suggestive history requires the presence of one major criterion or two or more minor criteria. | |
Table 2: Severity of symptoms and signs in acute bacterial sinusitis
| Non-severe | Severe |
|---|---|
| Rhinorrhea (of any quality) | Purulent (thick, colored, opaque) rhinorrhea |
| Nasal congestion | Nasal congestion |
| Cough | Facial pain or headache |
| Headache, facial pain, and irritability (variable) | Periorbital edema (variable) |
| Low-grade or no fever | High fever (temperature > 39°C) |
Table 3: Risk factors prompting prescribing amoxicillin and clavulanic acid
Bacterial resistance is likely • Antibiotic use in the past month, or close contact with a treated individual(s) • Resistance common in community • Failure of previous antimicrobial therapy • Infection in spite of prophylactic treatment • Child in daycare facility • Winter season • Smoker or smoker in family |
Presence of moderate to severe infection • Presentation with protracted (> 30 days) or moderate to severe symptoms • Complicated ethmoidal sinusitis • Frontal or sphenoidal sinusitis • Patient history of recurrent acute sinusitis |
Presence of co-morbidity and extremes of life • Co-morbidity ( i.e. chronic cardiac, hepatic or renal disease, diabetes) • Immunocompromised patient • Older than 65 years |
Figure 1. The Chronology of Viral and Bacterial Causes of Sinusitis.
What's New
Mandal R. Role of antibiotics in sinusitis. Curr Opin Infect Dis 2012 Apr;25(2):183-92
Wald ER. Staphylococcus aureus: Is It a Pathogen of Acute Bacterial Sinusitis in Children and Adults? Clin Infect Dis 2012;54:826-831.
Wald ER, Nash D, et al. Effectiveness of Amoxicillin/Clavulanate Potassium in the Treatment of Acute Bacterial Sinusitis in Children. Pediatrics. 2009 Jul;124:9-15.
Guided Medline Search For:
Reviews
None
