Osteomyelitis
Authors: Nalini Rao, M.D., FACP, FSHEA, Bruce H. Ziran, M.D., Sandra Arnold, M.D.
Osteomyelitis is an infection of bone and bone marrow caused by hematogenous or contiguous spread of the organism from local infection and or open traumatic fracture. Acute osteomyelitis frequently evolves into a chronic disease. The term cure is not used since the bone infection may relapse after many years of successful remission. In this viewpoint, osteomyelitis behaves much like a benign tumor, in that, it rarely kills, but tends to return without complete ablation. Furthermore, the management of osteomyelitis involves diagnosis, staging, treatment, and reconstruction, using methods similar to those used in tumor surgery. The present section will discuss the pathogenesis, diagnosis, and treatment of osteomyelitis.
Pathogenesis and Biofilms
The precise definition of a biofilm has continued to evolve as this area of research progressively expands. Today a biofilm can best be described as a dynamic and heterogeneous community of sessile bacteria which are surrounded by an extra cellular matrix of polymers. These bacteria by definition exhibit an altered phenotype in comparison to their free floating planktonic counterparts. This entire conglomeration, known as the biofilm, is adherent to an inert surface (e.g. plates, bony sequestra, or a prosthetic joint replacement). The structure of a biofilm consists of three basic elements: bacteria, extra cellular matrix, and other interstitial metabolic components. The biofilm is a dynamic microenvironment with continual ebbs and flows. Pockets of grouped sessile bacteria are surrounded by exopolysaccharide glycocalyces. These micro colonies are found with interspersed water channels which provide for the flow of various metabolites, chemicals and nutrients. Biofilms appear to tend to form in areas of high mechanical shear. Here the colonies formed show remarkable resistance to mechanical breakage.
Numerous bacterial species have been described to form biofilms. Most commonly in orthopaedic surgery, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, and Pseudomonas aeruginosa are the underlying causative organisms of musculoskeletal infections. The clinical significance of biofilms in orthopaedic surgery lies in the fact that biofilms form on implanted hardware and cause infections that are difficult to eradicate. This apparent antibiotic resistance does not appear to be mediated through traditional cellular modes of antibiotic resistance such as efflux pumps, deactivating enzymes, or target protein mutations. The extra cellular glycocalyx matrix portion of the biofilm constitutes one potential area of antibiotic resistance. In some instances, diffusion from outside of the biofilm inwards to the sessile bacteria may present a barrier which limits the efficacy of a given antibiotic. Likewise specific antibiotics may be bound by the extra cellular portion of the biofilm and hence rendered ineffective. The polymeric extra cellular portion of the biofilm can also serve to create an altered microenvironment in which certain compounds may be less able to exert specific effects. Sessile bacteria within biofilms appear to be phenotypically different from their planktonic counterparts.
Sessile bacteria have a slower rate of growth. From an evolutionary perspective it appears that these cells serve to function like spores, which are more adept at persisting over time but are also less metabolically active. As such, these less metabolically active cells naturally are less susceptible to chemotherapeutic agents designed to limit cell reproduction. This ability of sessile bacteria to persist may have a role in chronic infections. As bacteria survive for longer periods there is more opportunity for acquiring traditional methods of cellular antimicrobial resistance. This combined with the unique properties of biofilm resistance can lead to an infection which is difficult to conquer. As planktonic bacteria are intermittently released from the biofilm over time, there are multiple opportunities for the development of a secondary infection. As the biofilm matures, there is a greater probability that the planktonic bacteria which are released will be better equipped for survival.
Several immunologic proposals have been made which contribute to our understanding of biofilms and the role that they play in device-related infections. A foreign material elicits a cellular and/or humoral immune response, however, if this response persists over time, the macrophages can cease there normal activity, so, as the biofilm develops and the immune system fails to clear the infection, valuable chemical mediators are expended. Later when specific enzymes are needed, the reserves are dry and hence a clinically symptomatic infection may occur. Similarly, as the immune system reacts but is unable to attack sessile bacteria hidden within the auspices of a biofilm, frustrated phagocytosis may occur. Reactive oxidative species can then be released which cause damage to normal intact tissues which are adjacent to the infection-causing biofilm.
TYPES OF OSTEOMYELITIS
Acute Hematogenous Osteomyelitis
This is seen commonly in children (boys more often than girls) involving metaphysis or epiphysis of long bones most often in the lower extremity. The anatomy of metaphysical region explains the clinical localization of the disease. Non-anastamotic capillary ends of nutrient artery make sharp loops and enter a system of venous sinusoids with low and turbulent blood flow. These capillaries of the nutrient artery are essentially end arteries. Any capillary obstruction therefore causes avascular necrosis. In addition, the metaphysial capillaries and sinusoidal veins lack phagocytes. The cumulative effects of these factors when preceded by minor trauma in an infant or a child produce a small hematoma, vascular occlusion and bone necrosis. This can be invaded by blood-borne pathogen leading to a focus of medullary osteomyelitis.
Bacteria responsible for hematogenous osteomyelitis in infants include S. aureus, Streptococcus agalactiae and Escherichia coli. Whereas in children over the age of 1 to 4 years staphylococcus aureus, Streptococcus pyogenes and H. influenzae are most commonly isolated. With the recent immunization program, the overall incidence of H. influenza osteomyelitis has markedly decreased. In adults with hematogenous osteomyelitis, Staphylococcus aureus is the most common organism isolated. Pseudomonas osteomyelitis is seen in drug addicts and has predilection for spine (Table 1).
Clinical features include chills, fever, malaise, pain and local swelling of the affected limb. Children may present with abrupt fever, irritability, and lethargy along with inability to move the affected extremity and pain or passive movement. In approximately 50% of the cases there may be minimal to no fever, vague complaints including pain of the involved extremity over several months. In general, the outcome of acute osteomyelitis in children is good if antibiotic therapy is directed at the responsible pathogen within seven to ten days of the onset of illness.
Vertebral Osteomyelitis
Vertebral osteomyelitis is usually of hematogenous origin rather than retrograde spread via Batsons’s venous plexus. It involves lower dorsal and lumbar spine with an occasional involvement of the cervical spine. The infection leads to a thrombosis of one of the segmental arteries supplying two adjacent vertebrae and the intervertebral disc resulting in Ischemia, infarction and bone necrosis. Posterior extension can lead to epidural abscess and meningitis ![]() . Staphylococcus aureus is the most common organism although Pseudomonas is seen in drug addicts. A variety of other unusual organisms such as Actinobacillus actinomycetemcomitans, Kingella spp., and Moraxella catarrhalis have been implicated in vertebral osteomyelitis in rare occasions.
. Staphylococcus aureus is the most common organism although Pseudomonas is seen in drug addicts. A variety of other unusual organisms such as Actinobacillus actinomycetemcomitans, Kingella spp., and Moraxella catarrhalis have been implicated in vertebral osteomyelitis in rare occasions.
Disease usually presents with intractable back pain. Localized tenderness of the involved segment is present in majority of the patients. Fever and leucocytosis may be seen in 50% of the patients. Erythrocyte sedimentation rate is usually elevated and may be used as a prognostic guide during treatment. Radiographs are usually normal early in the disease course. Magnetic resonance imaging ![]() is the most sensitive and specific to diagnose disc space infection. Complications of vertebral osteomyelitis include epidural abscess, spinal cord compression, paravertebral and retroperitoneal abscess. Motor and sensory deficits occur in six to fifteen percent of patients.
is the most sensitive and specific to diagnose disc space infection. Complications of vertebral osteomyelitis include epidural abscess, spinal cord compression, paravertebral and retroperitoneal abscess. Motor and sensory deficits occur in six to fifteen percent of patients.
Osteomyelitis Secondary to Contiguous Infection
Contiguous osteomyelitis occurs when the microorganisms are introduced into bone by trauma, nosocomial contamination following surgical procedure ![]() and extension from adjacent soft tissue infection. Predisposing factors include open fractures, internal fixation devices
and extension from adjacent soft tissue infection. Predisposing factors include open fractures, internal fixation devices ![]() , prosthetic devices
, prosthetic devices ![]() and chronic soft tissue infection
and chronic soft tissue infection ![]() . Multiple organisms are usually isolated from the bone although Staphylococcus aureus and Staphylococcus epidermidis are the most prevalent pathogens.
. Multiple organisms are usually isolated from the bone although Staphylococcus aureus and Staphylococcus epidermidis are the most prevalent pathogens. 
Osteomyelitis Secondary to Vascular Insufficiency
This is a special category seen predominantly in patients with diabetes and peripheral vascular disease and is localized almost exclusively to lower extremities. Neuropathy, Ischemia ![]() and biomechanical dysfunction lead to foot ulcers
and biomechanical dysfunction lead to foot ulcers ![]() . Infection is seen as a consequence of ulcer, which progressively burrows its way into small bones of the feet. Multiple organisms are usually isolated although S. aureus and S. agalactiae still predominate. Patients may present with non-healing foot ulcer, cellulitis
. Infection is seen as a consequence of ulcer, which progressively burrows its way into small bones of the feet. Multiple organisms are usually isolated although S. aureus and S. agalactiae still predominate. Patients may present with non-healing foot ulcer, cellulitis ![]() or deep abscess
or deep abscess ![]() . Fever and systemic toxicity are often absent unless there is severe limb threatening infection with gangrene and fasciitis. Pain may be absent in patients with severe neuropathy. Osteomyelitis should be suspected when bone is exposed before or after debridement or probing the ulcer with a stainless steel probe, bone is encountered. Resection of the infected bone is almost always necessary for a favorable outcome.
. Fever and systemic toxicity are often absent unless there is severe limb threatening infection with gangrene and fasciitis. Pain may be absent in patients with severe neuropathy. Osteomyelitis should be suspected when bone is exposed before or after debridement or probing the ulcer with a stainless steel probe, bone is encountered. Resection of the infected bone is almost always necessary for a favorable outcome.
Acute Posttraumatic Osteomyelitis
Posttraumatic osteomyelitis results from pathogenic organisms that proliferate in traumatized tissue. Traumatized tissue also results in compromised blood supply, leading to tissue and bone necrosis, which promotes infection. Moreover, the fixation devices ![]() that are required in the management of fractures serve as additional foci for bacterial colonization. Other factors that contribute to the development of osteomyelitis are the presence of hypotension, inadequate debridement of the fracture site, malnutrition, alcoholism, and smoking. Trauma has been reported to delay the inflammatory response to bacteria, to depress cell-mediated immunity, and to impair function of PMNs, including chemotaxis, superoxide production, and microbial killing.
that are required in the management of fractures serve as additional foci for bacterial colonization. Other factors that contribute to the development of osteomyelitis are the presence of hypotension, inadequate debridement of the fracture site, malnutrition, alcoholism, and smoking. Trauma has been reported to delay the inflammatory response to bacteria, to depress cell-mediated immunity, and to impair function of PMNs, including chemotaxis, superoxide production, and microbial killing.
Open Fractures and Trauma
The presence of bacteria in an open wound is not sufficient to cause infection. Approximately 60% to 70% of open fractures are contaminated by bacteria, but a much smaller percentage develops infection and the risk of subsequent infection is highly correlated with the degree of soft tissue injury (Table 2). With type IIIB open fractures, those in which extensive soft tissue stripping does not allow for adequate coverage over the site, infection can occur in up to 40% of cases. Moreover, the bacteria recovered from clinical infections are most likely to be hospital acquired pathogens such as S. aureus or gram-negative bacilli (including Pseudomonas aeruginosa). Clinical studies indicate that open fractures that are properly debrided and covered definitively within 10-14 days have a much lower incidence of late osteomyelitis. This supports the contention that many infections are hospital acquired. However, other bacteria should be considered, depending on the environment, specifically, C. perfringens when there is soil contamination, Pseudomonas and Aeromonas hydrophilia following fresh water injury, and vibrio and erysipelothrix in salt water injury.
Overall, the incidence of infection in Grade I open fractures is nearly zero, that of grade II open fractures is below 5% and depending on the degree of contamination, in Grade III fractures, the infection rate is between 5-40%. Again, the hallmark of treatment is adequate debridement and soft tissue coverage. With recent advance such as vacuum assisted closure (V.A.C.), definitive free tissue transfer may not always be necessary. Furthermore, with complex bone regeneration techniques, an osteo-myo-cutaneous flap can be slowly transported into the defect. In the end, open fractures remain one of the most challenging problems to overcome and treat.
Chronic Osteomyelitis
Acute osteomyelitis that is inappropriately treated can become chronic osteomyelitis. Chronic osteomyelitis resulting from acute osteomyelitis is often caused by S. aureus; however, chronic osteomyelitis occurring after a fracture can be poly-microbial. Gram-negative organisms are now seen in about 50% of all cases of chronic osteomyelitis. The fundamental problem in chronic osteomyelitis is devascularization of bone, leaving protected pockets of necrotic material to support bacterial growth in relative seclusion from systemic antibiotic therapy. This collection of necrotic tissue, bone and bacteria is what is termed the sequestrum and the body’s attempt to wall off the offending material with a reactive and inflammatory tissue (either bone or soft-tissue) is termed involucrum. It should be noted that this involucrum can be highly vascular and potentially viable and structural, which should be taken into consideration during surgical debridement.
Chronic Sclerosing Osteomyelitis
Chronic sclerosing osteomyelitis primarily involves the diaphyseal bones of the adolescents. Typical features include intense proliferation of the periosteum leading to bony deposits and progressive sclerosis on the radiographs. Localized pain and tenderness are the hallmarks. In all cases malignancy must be ruled out.
Brodie’s Abscess
Brodie’s abscess is characterized by local pain and low-grade fever. Radiographic changes include central destruction surrounded by sclerosis usually in the metaphysis of the long bones. Majority of cases occur in the lower extremities. The differential diagnosis includes Ewing’s sarcoma and chondroblastoma especially when localized to epiphysis.
Tuberculosis Osteomyelitis
The incidence of tuberculosis osteomyelitis varies based on the geographic region and patient risk factors for the disease. While it can involve any bone due to its hematogenous dissemination, it does have a predilection for affecting the spine (Pott’s disease). Clinical presentation includes a subacute onset and often time’s consideration for the disease occurs in patients with negative bacterial cultures with risk factors for tuberculosis. Risk factors include: HIV, IV drug abuse, alcoholism, homelessness, immunosupression, history of a positive PPD and former residence in an endemic area. Evaluation for TB associated osteomyelitis includes acid-fast bone cultures and ziehl-neelsen (or Kinyoun) stains, histopathology (granulomatous inflammation), chest radiograph (looking for active or evidence of previous TB) and either PPD or QuantiFERON testing. Treatment involves an extended course of multiple anti-mycobacterial agents and should be tailored based on sensitivity testing.
Osteomyelitis In A Specific Clinical Setting
Based upon a certain clinical setting, specific organisms as well as bone sites may be involved (Table 3).
CLASSIFICATION
Historically, osteomyelitis was classified as either acute or chronic depending on the duration of symptoms. Several classification systems have been proposed but the most widely used system is that of Cierny and Mader (Table 4) (Figure 1) ![]() , which identifies the extent of infection as well as the condition of the host. This system is based on four factors: the degree of osseous involvement, the site of involvement, the degree of impairment caused by the disease, and the general condition of the host. Type I is medullary osteomyelitis
, which identifies the extent of infection as well as the condition of the host. This system is based on four factors: the degree of osseous involvement, the site of involvement, the degree of impairment caused by the disease, and the general condition of the host. Type I is medullary osteomyelitis ![]() (examples of which include hematogenous osteomyelitis and infections of IM nails). Type II is superficial osteomyelitis
(examples of which include hematogenous osteomyelitis and infections of IM nails). Type II is superficial osteomyelitis ![]() confined to the bone surface. Type III is localized osteomyelitis
confined to the bone surface. Type III is localized osteomyelitis ![]() involving the full thickness of the cortex but without the loss of axial stability. Type IV is diffuse osteomyelitis
involving the full thickness of the cortex but without the loss of axial stability. Type IV is diffuse osteomyelitis ![]() involving the circumference of the cortex and loss of axial stability. The general condition of the patient is based on those factors that affect the response to infection and treatment. Class A patients have normal systemic defenses, metabolic capabilities, and vascular supply to the limb. Class B patients have a local (trauma, prior surgery, local inflammation) or systemic (immunosuppressed, on corticosteroids, peripheral vascular disease) deficiency. Class C patients are those in whom the treatment of the disease (the infection) is worse than the infection itself. In other words, there is greater potential morbidity or mortality from treatment, than from the infection (or non curative treatment such as suppression). Pairing the four types of osteomyelitis with the three host classes, Cierny et al proposed a detailed treatment regimen, which defined optimal treatment modalities for each stage. As one would expect, class A hosts fared the best with success rates of 98% were achieved, even in type IV osteomyelitis. For compromised, class B hosts, success rates were far lower. Depending on anatomic type, success ranged from 79% to 92%.
involving the circumference of the cortex and loss of axial stability. The general condition of the patient is based on those factors that affect the response to infection and treatment. Class A patients have normal systemic defenses, metabolic capabilities, and vascular supply to the limb. Class B patients have a local (trauma, prior surgery, local inflammation) or systemic (immunosuppressed, on corticosteroids, peripheral vascular disease) deficiency. Class C patients are those in whom the treatment of the disease (the infection) is worse than the infection itself. In other words, there is greater potential morbidity or mortality from treatment, than from the infection (or non curative treatment such as suppression). Pairing the four types of osteomyelitis with the three host classes, Cierny et al proposed a detailed treatment regimen, which defined optimal treatment modalities for each stage. As one would expect, class A hosts fared the best with success rates of 98% were achieved, even in type IV osteomyelitis. For compromised, class B hosts, success rates were far lower. Depending on anatomic type, success ranged from 79% to 92%.
CLINICAL MANIFESTATIONS
A history of infection or inter-current illness as well as remote surgery or trauma should raise the clinical suspicion for osteomyelitis. Normal signs of inflammation (rubor, calor, dolor, tumor) may be absent and thus the diagnosis of infection when clinical signs are masked can be difficult. Patients can often have a history of infection of another site, such as the lungs, bladder, or skin, especially with a history of trauma. They usually complain of substantial pain in the affected area. Moreover, reduced activity, malaise, and anorexia may be exhibited. Local findings include swelling and warmth, occasional redness, tenderness to palpation, drainage ![]() , and restricted range of motion of adjacent joints. With a history of trauma, clinical risk factors for infection include a history of open fracture, severe soft tissue injury, a history of substance abuse and smoking, inadequate previous treatment, and an immunocompromised state.
, and restricted range of motion of adjacent joints. With a history of trauma, clinical risk factors for infection include a history of open fracture, severe soft tissue injury, a history of substance abuse and smoking, inadequate previous treatment, and an immunocompromised state.
DIAGNOSIS
Laboratory Findings
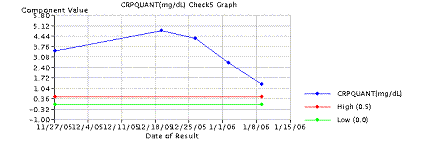
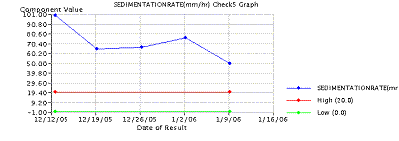
Routine blood cultures are of little help unless the patients have manifestations of systemic disease, such as in hematogenous osteomyelitis (Cierny and Mader Type I). Blood cultures are positive in about 50% to 75% in such cases. Laboratory changes suggestive of infection include elevations in the white blood cell (WBC) count, and elevations in the C-reactive protein (CRP) and Erythrocyte Sedimentation Rate (ESR) levels. The erythrocyte sedimentation rate may be normal in the first 48 hours but rises to levels above 100 mm/h, and may remain elevated for weeks. The CRP/ESR can be an excellent screening tool to measure response to treatment ( Figure 2).
Radiographic Imaging
Radiographic changes may not be seen for at least 10 days. When present, it usually signifies trabecular bone destruction ![]() . If the infection spreads to the cortex (usually within 3 to 6 weeks), a periosteal reaction may be evident on plain radiographs. Unfortunately, radiologic findings in the initial presentation of acute osteomyelitis are often normal. The most common radiographic sign of bone infection is rarefaction, representing diffuse demineralization secondary to inflammatory hyperemia soft tissue swelling with obliteration of tissue planes, trabecular destruction, lysis and cortical permeation, periosteal reactions and involucrum formation. One study reported that in cases of eventually proven osteomyelitis, 5% of radiographs were abnormal initially, 33% were abnormal by 1 week, and 90% were abnormal by 4 week.
. If the infection spreads to the cortex (usually within 3 to 6 weeks), a periosteal reaction may be evident on plain radiographs. Unfortunately, radiologic findings in the initial presentation of acute osteomyelitis are often normal. The most common radiographic sign of bone infection is rarefaction, representing diffuse demineralization secondary to inflammatory hyperemia soft tissue swelling with obliteration of tissue planes, trabecular destruction, lysis and cortical permeation, periosteal reactions and involucrum formation. One study reported that in cases of eventually proven osteomyelitis, 5% of radiographs were abnormal initially, 33% were abnormal by 1 week, and 90% were abnormal by 4 week.
Nuclear Medicine
Radionuclide Scintigraphy is a very useful diagnostic tool and can be performed with various algorithms. Technetium-99m is the principal radioisotope employed in most bone scans. After intravenous injection, there is rapid distribution of this agent throughout the extra cellular fluid. Within several hours, more than half the dose will accumulate in bone, while the remainder is excreted in the urine. There is evidence to suggest that the technetium phosphates bind to both the organic and the inorganic matrix. There is preferential incorporation into metabolically active bone. Following the initial injection, dynamic images are captured over the specified region. These are followed by static images at later time points. The first phase represents the blood flow phase, the second phase immediately post-injection represents the bone pooling phase, and the third phase is a delayed image made at 3 hours when there is decreased soft tissue activity. Classically, osteomyelitis presents as a region of increased blood flow and should appear “hot” on all phases, with focal uptake in the third phase. Other processes such as a healing fracture, a loose prosthesis, and degeneration do not appear hot ![]() in the early phase despite a hot appearance in the delayed phase. Reported sensitivities of bone scintigraphy for the detection of osteomyelitis vary considerably from 32% to 100%. Reported specificities have ranged from 0% to 100%.
in the early phase despite a hot appearance in the delayed phase. Reported sensitivities of bone scintigraphy for the detection of osteomyelitis vary considerably from 32% to 100%. Reported specificities have ranged from 0% to 100%.
Gallium-67 citrate binds rapidly to serum proteins, particularly transferrin. There is uptake in the blood, especially by leukocytes. Gallium has been used in conjunction with technetium-99 to increase the specificity of the bone scan. Several mechanisms have been postulated to explain the increased activity at sites of inflammation. Bacteria have high iron requirements and thus avidly take up gallium. Gallium is strongly bound to bacterial siderophores and leukocyte lactoferins. In a typical study, gallium is injected intravenously and delayed images are acquired (at 48 to 72 hours). The hallmark of osteomyelitis is focal increased uptake of gallium. Unfortunately, gallium's non-specific bone uptake can be problematic since any processes causing reactive new bone formation will “light up.” In the case of patients with fractures or prosthesis, osteomyelitis cannot be diagnosed with gallium alone. Most authors will interpret gallium images along with bone scans. The reported sensitivities and specificities for the diagnosis of osteomyelitis range from 22% to 100% and 0% to 100%, respectively.
Indium-111 or 99mTC-HMPAO (Ceretec) labeled leukocyte scan is useful as a confirmatory test following a positive Tc-RBC bone scan. The leukocytes migrate to the region of active infection so that the scan can confirm the presence of an active inflammatory reaction. The use of a combined red cell and white cell scan increases both the sensitivity and specificity significantly, and now represents the gold standard of radionuclide testing for infection. Leukocytes are labeled and then re-injected, where they redistribute in the intravascular space. Immediate images thereafter show activity in the lungs, liver, spleen, and blood pool. The half-life is about 7 hours. After 24 hours, only the liver, spleen, and bone marrow show activity. Normal-healing wounds and fully treated infections show no increase in uptake. Leukocytes that migrate to an area of active bone infection will show increased uptake. Most results show improved sensitivity (80–100%) and specificity (50–100%) for the diagnosis of osteomyelitis. Indium-labeled WBC scans are generally superior to bone scans and gallium scans in the detection of infection. In one study, evaluating the diagnostic utility of indium scans in 39 patients with suspected osteomyelitis, confirmed by bone biopsy, Indium scans were 97% sensitive and 82% specific for osteomyelitis. The few false-positive results occurred in patients with overlying soft tissue infections. An accompanying bone scan can help to differentiate bone infection from soft tissue infection. In these situations, the indium scan should be performed before the bone scan to avoid false-positive results (from the remaining technetium uptake). With both tests, the sensitivities and specificities are in excess of 90%. Until recently, a clinician investigating for the site of infectious foci using nuclear medicine had a choice between 67Ga-citrate imaging and 111In-oxine leukocyte imaging, but scientific advances (especially in nuclear medicine) have increased these choices considerably, and continue to increase them. Several techniques in nuclear medicine significantly aid infection diagnosis, including imaging with 99mTc-hexamethylpropyleneamine oxime (99mTc-HMPAO (Ceretec) and 99mTc-stannous fluoride colloid-labelled leukocytes. Each radiopharmaceutical has specific advantages and disadvantages that make it suitable to diagnose different infectious processes (e.g., soft-tissue sepsis, osteomyelitis, abscesses, and infections commonly found in immunocompromised patients).
Marrow scanning ![]() is also increasingly used for diagnosis of infection. With use of microcolloid bone marrow scans, more information is available to determine whether there is truly an infection. There is the possibility of leukocyte accumulation with certain inflammatory conditions that could result in a false positive indium scan. An infection will tend to suppress marrow activity and thus render the marrow scan cold, while the white cell scan will still be hot. If the white cell scan is hot as is the marrow scan, it is possible that an infection may not be present. One study examined technetium labeled white cell scans (Tc-HMPAO) versus Tc microcolloid marrow scans in total joints. They found that in 77 patients, the white cell scans had a sensitivity of 96% and specificity or 30% by itself. When the colloid scan was added, the sensitivity went down to 93% but the specificity went up to 98%. The addition of a regular red cell scan was not helpful. In another study, an indium white cell scan was compared to technetium sulphur colloid scans to differentiate infection from Charcot arthropathies. They found that white cell scans were positive in 4 out of 20 cases, of which three were infected. In the sixteen negative white cell scans, the marrow scan was also negative. However, in the four positive cases, the marrow scan was positive in two cases which were confirmed to be infected. They concluded that white cell scans can be positive in hematopoetically active bones which can occur in the absence of infection and that marrow scans should be used to confirm the diagnosis.
is also increasingly used for diagnosis of infection. With use of microcolloid bone marrow scans, more information is available to determine whether there is truly an infection. There is the possibility of leukocyte accumulation with certain inflammatory conditions that could result in a false positive indium scan. An infection will tend to suppress marrow activity and thus render the marrow scan cold, while the white cell scan will still be hot. If the white cell scan is hot as is the marrow scan, it is possible that an infection may not be present. One study examined technetium labeled white cell scans (Tc-HMPAO) versus Tc microcolloid marrow scans in total joints. They found that in 77 patients, the white cell scans had a sensitivity of 96% and specificity or 30% by itself. When the colloid scan was added, the sensitivity went down to 93% but the specificity went up to 98%. The addition of a regular red cell scan was not helpful. In another study, an indium white cell scan was compared to technetium sulphur colloid scans to differentiate infection from Charcot arthropathies. They found that white cell scans were positive in 4 out of 20 cases, of which three were infected. In the sixteen negative white cell scans, the marrow scan was also negative. However, in the four positive cases, the marrow scan was positive in two cases which were confirmed to be infected. They concluded that white cell scans can be positive in hematopoetically active bones which can occur in the absence of infection and that marrow scans should be used to confirm the diagnosis.
In general, a total body bone scan (RBC) alone is of little value. The best potential for determining infection is to add the white cell scan as well as the marrow scan. It is even more important that the radiologist know the clinical history, view the radiographs and interpret the scintigraphs in such context. Otherwise, a rather vague and generic reading will be obtained that has little clinical value to the general practitioner. Ongoing data are being presented that may find the regular total body bone scan to be of lesser value when compared to the pair if marrow scan and white cell scanning. Generally speaking however, all three test should be order and will take approximately one week to obtain.
Magnetic Resonance Imaging
Magnetic resonance imaging continues to play an important role in the evaluation of musculoskeletal infections. The sensitivity and specificity of Magnetic Resonance Imaging (MRI) imaging for osteomyelitis range from 60% to 100% and 50% to 90% respectively. It has the spatial resolution necessary to evaluate accurately the extent of the infection in preparation for surgical treatment and localizes any abscess cavities. T1 and T2 weighted imaging is usually sufficient, fat suppression and STIR (Short TI Inversion Recovery) sequences may be added to better image bone marrow and soft tissue abnormalities. It also has the ability to differentiate between infected bone and involved adjacent soft tissue structures. Images can be acquired in any orientation and there is no radiation exposure. Gadolinium enhancement should be obtained in the postoperative population to better differentiate post surgical artifact from infection-related bone marrow edema patterns. Gadolinium may better differentiate abscess formation from diffuse inflammatory changes and non-infectious fluid collections. Characteristically, active osteomyelitis displays a decreased signal on T1-weighted images ![]() and appears bright on T2-weighted images
and appears bright on T2-weighted images ![]() . The process represents the replacement of marrow fat with water from edema, exudate, hyperemia, and ischemia. The MRI signal characteristics that reflect osteomyelitis are intrinsically non-specific: tumors and fractures can also increase the marrow water content. In patients without prior complications, MRI has been found to be sensitive (but not specific) for osteomyelitis. When a fracture or prior surgery is evident, MRI is less specific in the diagnosis of infection. Furthermore, in the presence of metallic implants, the artifact makes it difficult to comment on areas near the implant, which may be of primary interest. When no metallic implants exist, an MRI should be used to help stage the infection, to help determine the extent of both bone and soft tissue infection, and to differentiate between non-specific marrow changes and infection.
. The process represents the replacement of marrow fat with water from edema, exudate, hyperemia, and ischemia. The MRI signal characteristics that reflect osteomyelitis are intrinsically non-specific: tumors and fractures can also increase the marrow water content. In patients without prior complications, MRI has been found to be sensitive (but not specific) for osteomyelitis. When a fracture or prior surgery is evident, MRI is less specific in the diagnosis of infection. Furthermore, in the presence of metallic implants, the artifact makes it difficult to comment on areas near the implant, which may be of primary interest. When no metallic implants exist, an MRI should be used to help stage the infection, to help determine the extent of both bone and soft tissue infection, and to differentiate between non-specific marrow changes and infection.
CT, PET, SPECT, Biopsy, Culture, and Molecular Diagnostics
Computed tomography has assumed a lesser role in the evaluation of osteomyelitis with the widespread use of MRI. It remains unsurpassed, however, in the imaging of cortical bone. It is especially useful in delineating the cortical details in chronic osteomyelitis, such as sequestra and foreign bodies. It also is useful in evaluating the adequacy of cortical debridement in the staged treatment of chronic osteomyelitis. Thus, it can help differentiate between type III and type IV infections. It should be used with fine cuts and can be ordered with contrast to help with surgical planning. It is most useful when trying to establish the structural integrity or permeation of the infection into the bone. It is not useful when there is a lot of metal implant present unless special software manipulations are done to minimize the artifact.
The use of fluorine-18 labeled 2-fluoro-2-deoxy- d-glucose positron emission tomography FDG (PET) scanning enables non-invasive detection and demonstration of the extent of chronic osteomyelitis with 97% accuracy. Positron emission tomography is especially accurate in the central skeleton within active bone marrow. While it is not yet in widespread use, it remains an adjunctive tool if other methods fail. The overall accuracy of FDG-PET in evaluating infection involving orthopaedic hardware was 96.2% for hip prosthesis, 81% for knee prosthesis, and 100% in 15 patients with other orthopaedic devices. Among the patients having chronic osteomyelitis, the accuracy is 91%. FDG- PET appears to be a sensitive and specific method for the detection of infectious foci due to metallic implants in patients with trauma. Sensitivity, specificity, and accuracy were 100%, 93.3%, and 97%, respectively, for all PET data; 100%, 100%, and 100%, respectively, for the central skeleton; and 100%, 87.5%, and 95%, respectively, for the peripheral skeleton. 18F-FDG-PET used to investigate infection in postoperative spine holds promise to become the standard imaging technique in this difficult patient population, with sensitivity, specificity and accuracy at 100%, 81%, and 86%, respectively, in a recent study. The accurate differentiation between synovitis, loosening or infection is often difficult with conventional X-rays, arthrography, or bone scintigraphy. Results suggest that FDG PET could be a useful tool for differentiating between infected and loose orthopaedic prostheses as well as for detecting only inflammatory tissue such as synovitis. Unfortunately, the study is expensive and not covered by insurance carriers. Ongoing studies may find it to be more efficacious than the sequence of scintigraphy, but until such studies demonstrate cost efficacy, it may be of less value to the clinician.
Single Photon Emission Computed Tomography (SPECT) provides a qualitative and quantitative look at the volume distribution of biologically significant radiotracers after injection into the human body. SPECT has been used to diagnose spinal prosthetic infection. The overall sensitivity of Tc-99m HMPAO leukocyte scan with SPECT to detect bone infection was 92%, with a specificity rate of 85%. The bone biopsy/culture is another important method to identify the bacteria and determine the appropriate antibiotic. The procedure can usually be done under fluoroscopic guidance. While sinus tract cultures can be helpful, they should not be the sole guide for antibiotic treatment. In a prospective study, Mousa found that 88.7% of deep sinus tract isolates were identical to operative specimens in 55 patients with chronic bone infection. These results were dependent on aspiration of material by syringe from the depths of an active flowing sinus and immediate inoculation on culture media. Bone biopsy remains the preferred diagnostic procedure in chronic osteomyelitis. Histological and microbiologic evaluation of percutaneous biopsy samples should be combined in cases of suspected osteomyelitis. The sensitivity of culture in the diagnosis of osteomyelitis could be improved from 42% to 84% by the addition of histological evaluation.
Molecular Diagnostics are being developed for diagnosis in osteomyelitis because some infections remain without an identified pathogen, when using standard techniques. The most commonly used method for the diagnosis of orthopaedic infections is the polymerase chain reaction. Sequences within bacterial 16S ribosomal RNA have served as targets for amplification and detection. The Polymerized Chain Reaction has been used to identify very small remnants of bacteria by identifying their nuclear contents. Unfortunately, it cannot easily delineate between nuclear materials from living or dead bacteria, thus increasing the likelihood of false positive studies. Recent studies have found that bacterial typing and assays that target other characteristics of bacteria, such as RNA, may help differentiate between viable and necrotic bacterial elements. Further investigations are required before these techniques can be widely used as they lack sufficient sensitivity and specificity, but their use remains promising.
MANAGEMENT AND TREATMENT
The management of osteomyelitis relies on a multidisciplinary approach, combining debridement, soft tissue coverage, and antimicrobial therapy to give the patient the best chance of cure (Table 5). The first step involves identifying (staging the host) and optimizing the host (treating the morbidity of the host and optimizing their physiologic condition, such as nutrition, smoking cessation, treatment of diabetes, medical or surgical management of vascular disease). Step two involves classifying the type of osteomyelitis (staging the disease). Then, identifying the organism is required to determine appropriate antimicrobial treatment (the tumor analogy of chemotherapy). Once the extent of disease, the nature of the host and infecting organism are noted, a determination should be made regarding one of several general treatment algorithms. The treatment options available include attempted ablation and cure of the offending infection or in selective cases, such as C-hosts, that are not suitable for this line of treatment , some type of suppressive treatment. Attempted ablation and complete cure has numerous issues and decision making steps but will often require the tumor equivalent of a wide resection with “clean” margins. While a surgically clean bed with extensive resection is desirable, efforts should be made to maintain axial stability when possible. Thus, retention of a well vascularized but affected involucrum, or a viable segment of bone adjacent to infection may be retained. At some point, if adequate resection will result in too extensive a reconstruction that is unsuitable for the host status, amputation is the best option and should not be considered a failure. In some cases of life or limb threatening infection, a “debulking” of the infection may be a suitable first step followed by chronic suppression. In such a circumstance, identification of the infecting bacteria is required to allow use of a narrow-spectrum antibiotic; otherwise, broad-spectrum antibiotics are needed. The increased incidence of methicillin-resistant staphylococcal species adds greater emphasis to the need for a microbiologic diagnosis.
Antibiotic Depot Devices and Techniques
Use of antibiotic depots allows for high local concentrations of antibiotic with little systemic absorption Agents have to be heat stable and in powder form. Tobramycin and Vancomycin are the most commonly used antibiotics for depot delivery. Antibiotic release is bi-phasic, most occurring during the first hours to days post-implantation and the remaining elution persisting for weeks and sometimes for years. Some of the other antibiotics that have been tried with Polymethylmethacralate (PMMA) include clindamycin which elutes well, but is not available as a pharmaceutical grade powder; Fluoroquinolones in cement powder has not been reported; Erythromycin is heat stable but demonstrated inadequate elution from the cement; Tetracycline and colistin fail to elute from the palacos cement in clinically meaningful quantities. Most of the antibiotic cement use in the US has been off label use by the surgeon, and despite very encouraging results from several studies, their approval has been slow. There are also newer types of material available for local delivery of antibiotics which are resorbable and do not require removal ![]() .
.
Debridement Techniques
If surgical treatment is chosen, the hallmark of treatment is debridement. All non-viable and inert structures should be debrided to remove the infected material and debris without destabilizing the bony structure. The goal is to convert a necrotic, hypoxic, infected wound to a viable wound. The critical judgment for the clinician occurs when there is potentially infected bone, that may be partially vascularized, and that is critically needed to maintain the structural stability of the bone. The limits of debridement have classically been determined by the “Paprika sign” which is characterized by punctuate cortical or cancellous bleeding. Reactive new bone surrounding an area of chronic infection is living and usually does not require debridement. The sequestrum needs to be identified and removed, whereas the involucrum may be preserved. When the medullary canal is infected, intramedullary reaming is a good method of debridement. In general, one should over ream the medullary canal by 2 mm. Intramedullary reaming of the canal as a debridement technique has shown favorable results in the treatment of medullary osteomyelitis. In one series, 25 patients with posttraumatic osteomyelitis (of whom 22 were treated with intramedullary reaming) 21 of the 22 patients were free of any recurrent infection. In a more recent study, 40 patients suffering from chronic osteomyelitis were treated with intramedullary reaming. Only four patients suffered a recurrent infection following intramedullary reaming ![]() .
.
If the medullary infection is too proximal (or distal) for a tight reamer fit, a trough must be created to debride the canal directly (saucerization). Greater than 30% loss of circumferential cortical contact or any segmental resection requires stabilization.
Acute Post-operative Infection
Often, infection present with a recent reconstruction with the presence of metallic implants and other inert biologic tissue (compromised bone or bone graft). The question arises regarding the need to intervene operatively or the ability to remove implants (Table 6). If there are obvious signs of infection such as erythema, copious drainage, edema, and fever, acute surgical debridement and irrigation is needed. If there is satisfactory fixation and stability of the construct, only non-essential elements such as excess bone graft, should be removed. Placement of an antibiotic delivery depot (either PMMA or resorbable) with a more aggressive antibiotic regimen may curb the infection until healing has taken place. If this treatment is undertaken, suppression with antibiotics should continue until such time and when adequate healing has occurred, there should be strong consideration of re-debridement, removal of hardware and an intra-operative decision as to whether more antibiotic depot treatment is required (Figure 5). This algorithm is followed with the hypothesis that the original wound was contaminated and probably colonized with bacteria (with biofilm formation on all inert structures in the wound), and that the debulking and suppression was in an effort to prevent the need to “start over”. As such, with our current understanding of bio film bacterial, it would be unlikely that the wound could be free of bacteria, so there is a higher likelihood of recurrence of infection. It is for this reason that the threshold of hardware removal and re-debridement is lower than normal. If this approach is not successful, there should be consideration of “starting over”, which is complete removal and debridement of inert substances, placement of an antibiotic depot system, temporary stabilization with an external fixator or cast, and a staged reconstruction. Unlike the literature in arthroplasty, there is not much scientific literature on success/failure rates in such circumstances for fractures. It should be noted however, that bones can heal in the presence of active infection and unlike arthroplasty, where the implant is considered permanent, the fracture setting allows for implant removal once bone healing has taken place.
Principles of Antimicrobial Therapy
As bacteria become more resistant antibiotic therapy can be challenging, therefore an adequate attempt at identifying a microbiologic pathogen is critical. Rarely will rapid initiation of antibiotic therapy change the clinical course, unless the patient is showing significant signs of systemic toxicity. Therefore, appropriate consultation and attempts to obtain bone cultures should be the first treatment. However, there are times when bone cultures will not be possible or cultures will be negative (despite pathologic evidence suggesting osteomyelitis) and therapy will be empiric. Empiric therapy should be guided based on the risk and host factors mentioned in Tables 1 and 3. Previous history of bacteremia(especially with MRSA) should be taken into account due to the potential for bacterial seeding of bone during episodes of bacteremia. When treatment is empirical, clinical response should be evaluated after 2 weeks of treatment to determine if therapy is adequate. Evidence of response would include, but is not limited to, decreased acute phase reactants along with decreased pain, swelling and tenderness. Radiographic changes will be delayed and are not a reliable means of monitoring response to treatment.
Antibiotics must be nontoxic, convenient to administer and cost-effective. The selection is based upon in-vitro susceptibilities of the microorganisms causing the infection and ability to penetrate the bone. The therapy is often prolonged requiring outpatient management, administered intravenously for at least the first two weeks following surgery. The regimens for the initial treatment of osteomyelitis are listed in Table 7. Antibiotics should be tailored based on the cultures and the specific needs of each patient. Due pharmacological complexities of the available antibiotics, their adverse effects and possible complications, a dedicated infectious disease specialist often times facilitate the treatment process. Although most of the early reports suggest such an arrangement may improve outcomes, this has yet to be supported by level I evidence-based data.
The duration of antibiotic therapy for osteomyelitis varies as some researchers have recommended as few as two weeks of therapy and others suggested long-term therapy and there is no general consensus. Short-term therapy is indicated in otherwise healthy patients with total debridement and healthy host tissue. The long-term antibiotic therapy is based upon biofilm technology data. These recommendations are typically made for patients requiring reconstruction surgery involving a large volume of graft and of inert materials. These inert materials are the potential site for colonization of bacteria from the blood stream or remnants in the tissue bed until complete revascularization takes place. Selecting the appropriate oral antibiotic and monitoring of antibiotic therapy can minimize bacterial seeding of the reconstruction site. In order to increase the patient compliance antibiotic agents selected must be least toxic, least expensive and require administration once or twice daily. The oral antibiotics with excellent oral bioavailability are listed in Table 8. These antibiotics may be substituted for intravenous agents whenever possible provided that the microorganism is susceptible to these agents.
Because of increased incidences of vancomycin-resistant Enterococcus, especially in intensive care units, and vancomycin-resistant Staphylococcus aureus have been recently reported, vancomycin should be used only if there is high rate of infection caused by methicillin-resistant S. aureus or methicillin-resistant S. epidermidis. A single dose of vancomycin administered before surgery and followed by two or three doses postoperatively should provide adequate peri-operative prophylaxis. Vancomycin should only be used with type 1 hypersensitivity to cephalosporins that includes those with urticaria, laryngeal edema, and bronchospasm with or without cardiovascular shock. Clindamycin is considered a good alternative to cefazolin.
Chronic Suppressive Therapy
In certain selective settings when radical surgical debridement cannot be performed (Class C hosts), if the hardware has to be left in place to maintain stability such as an acute infection or if the patient refuses surgical treatment eradication of the infection is unlikely. In such instances, use of chronic suppressive therapy may be in order which typically involves long-term administration of oral antibiotic therapy. Microbiologic diagnosis must be attempted before considering chronic suppressive therapy and the patient must be compliant and be able to tolerate long-term antibiotic therapy. The exact duration of therapy is not clearly defined. However, a six-month course is typically administered to contain and not to eradicate the infection. In patients with fractures suppressive antibiotic therapy is maintained until healing occurs followed by removal of the hardware. In some patients, suppressive antibiotic therapy may be required indefinitely.
PREVENTION
Prevention of osteomyelitis implies decreasing known risk factors in host. For hematogenous osteomyelitis, major risk factors include, indwelling intravascular catheters, distant focus of infection and I.V. drug abuse. For contiguous osteomyelitis, risk factors include trauma, adjacent soft tissue infection, bites and puncture wounds. Patients with diabetes have multiple risk factors inherent to the disease along with vascular insufficiency. Systemic and local co-morbid conditions need to be corrected to avoid osteomyelitis. Smoking cessation and adequate nutrition play an important role in the outcome of osteomyelitis.
Reading List
1. Anglen JO: Wound Irrigation in Musculoskeletal Injury. J Acad Orthop Surg. 2001;9:219-226. [PubMed]
2. Calhoun JH, Manring MM: Adult osteomyelitis. Infect Dis Clin N Am 2005;19:756-786. [PubMed]
3. Cardozo Blum Y, Esterhai, J: The History of the Treatment of Orthopedic Infections, in Operative Techniques in Orthopedics, 2003;12(4):226-231.
4. Cierny G 3rd, Mader JT. Approach to adult osteomyelitis. Orthop Rev. 1987;16(4): 259-70.[PubMed]
5. Cierny G, Mader JT. Adult Chronic Osteomyelitis: An Overview. Orthopaedics 1984;7(109):1557-1564.
6. Cierny G, Zorn KE. Segmental tibial defects. Comparing conventional and Ilizarov methodologies. Clin Orthop. 1994;301:118-23. [PubMed]
7. Conroy BP, Anglen JO, Simpson WA et al: Comparison of castile soap, benzalkonium chloride, and bacitracin as irrigation solutions for complex contaminated
orthopaedic wounds. J Orthop Trauma 1999;13(5): 332-7. [PubMed]
8. Costerton JW, Stewart PS, Greenberg EP: Bacterial biofilms: a common cause of persistent infections. Science. 1999 May 21; 284(5418): 1318-22.[PubMed]
9. Donlan RM, Costerton JW: Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167-193. [PubMed]
10. Kaplan SL: Osteomyelitis in children. Inf Dis Clin N Am 2005;19(4):787-797. [PubMed]
11. Keating JF, Blachut PA, O'Brien PJ et al Reamed nailing of open tibial fractures: does the antibiotic bead pouch reduce the deep infection rate? J Orthop Trauma. 1996;10(5): 298-303. [PubMed]
12. Lazzarini L, de Lalla F: Long bone osteomyelitis. Current Treat Options Inf Dis 2002; 4:303-310. [PubMed]
13. Lazzarini L, Lipsky BA, Mader JT: Antibiotic treatment of osteomyelitis: Int J Inf Dis 2005;9(3):127-138. [PubMed]
14. Mackowiak PA, Jones SR, Smith JW: Diagnostic value of sinus tract cultures in chronic osteomyelitis. JAMA, 1978;239:2772-2775. [PubMed]
15. Mader JT, Calhoun JH: Osteomyelitis. Principles and Practice of Infectious Diseases, 5. Ed. Mandell GL, Douglas RG, Bennett JE 2000;182-1196.
16. Mousa HA. Evaluation of sinus-track cultures in chronic bone infection. J Bone Joint Surg Br. 1997;79(4):567-569.[PubMed]
17. Osmon DR, Berbari E: Outpatient Intravenous Antimicrobial Therapy for the Practicing Orthopaedic Surgeon. Clinical Orthopaedics and Related Research
2002;403: 80-86. [PubMed]
18. Ostermann PA, Seligson D, Henry SL: Local antibiotic therapy for severe open fractures: A review of 1085 fractures. JBJS 1995;77B: 93-97. [PubMed]
19. Rao N, Santa E: Anti-infective therapy in orthopedics. Operative Techniques in Orthopedics, Volume 12, Number 4, pp. 247-252, 2002.
20. Rao N, Ziran BH, Hall RA et al: Successful treatment of chronic bone and joint infections with oral linezolid. Clin Ortho Rel Res 2004;(427):67-71. [PubMed]
21. Shuford JA, Steckelberg JM: Role of oral antimicrobial therapy in the management of osteomyelitis. Current Opinions in Inf Dis 2003;16(6):515-619. [PubMed]
22. Steer AC, Carapetis JR: Acute hematogenous osteomyelitis in children: recognition and management. Paediatric Drugs 2004;6(6):333-346. [PubMed]
23. Stewart PS, Costerton JW: Antibiotic resistance of bacteria in biofilms. Lancet. 2001; 358(9276):135-8. [PubMed]
24. Tehranzadeh J, Wong E, Wang F, et al: Imaging of Osteomyelitis in the mature skeleton. Radiol Clinic of North America 2000;39:223-250. [PubMed]
25. Tetsworth K, Cierny G: Osteomyelitis debridement techniques. Clin Ortho Rel Res 1999;360:87-95. [PubMed]
26. Unger E, Moldofsky P, Gatenby R et al: Diagnosis of osteomyelitis by MR imaging. AJR Am J Roentgenol. 1988;150(3): 605-10. [PubMed]
27. Wininger DA, Fass RJ: Antibiotic-impregnated cement and beads for orthopedic infections. Antimicrob Agents Chemother 1996;40:2675-2679. [PubMed]
28. Zalavras CF, Patzakis MJ, Holtom P: Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Ortho Rel Res 2004;(427):86-93. [PubMed]
Tables
Table 1: Microorganisms in Acute Hematogenous Osteomyelitis
Organism |
Patient Category |
|---|---|
Staphylococcus aureus Group B Streptococcus Escherichia coli |
Infants |
Staphylococcus aureus Streptococcus pyogenes Hemophilus influenzae |
Children (up to 4 years of age) |
Staphylococcus aureus |
21 years or older |
Gram negative rods |
Elderly |
Patients with intravascular devices or history of candidemia |
|
Staphylococcus aureus Pseudomonas aeruginosa |
IV drug abuse |
HIV, IV drug abuse, alcoholism, homelessness, immunosupression, history of a positive PPD and former residence in an endemic area |
Table 2: Classification of Open Fractures
· Minor / Grade I - small punctate wound less than 1 centimeter associated with low velocity trauma. Minimal soft tissue injury. No crushing or comminution.
· Moderate / Grade II - wounds which are extensive in length and width but with relatively little soft tissue damage, and only moderate crushing or comminution.
· Major / Grade III - wounds of moderate or massive size with considerable soft tissue injury and/or foreign body contamination:
o III A - sufficient soft tissue to cover the fracture
o III B - insufficient tissue to cover the fracture; also periosteal stripping and severe comminution
o III C - arterial damage requiring repair. Degree of soft tissue damage not considered
Table 3. Osteomyelitis In A Special Clinical Setting
Risk Factors |
Organism |
Site |
|---|---|---|
I.V. drug abuse |
S. aureus P. aeruginosa Serratia |
Axial skeleton Sterno-clavicular joint Sacro-ileac joint |
Hemoglobinopathy |
Salmonella |
Long bones |
Tooth abscess |
Anaerobes |
Mandible |
Diabetic foot abscess |
Polymicrobial |
Small bones of hands and feet |
Human bite |
Eikenella Corrodens Staph Aureus |
Hands |
Animal Bite |
Pasteurella Multocida |
Hands, feet, face |
Puncture wound of foot |
P. aeruginosa |
Calcaneus |
Median sternotomy |
S. aureus S. epidermidis |
Sternum
|
Meat handlers |
Brucella | Flat bones |
Fishermen |
M. marinum | Small bones of hand |
Hemodialysis |
S. aureus S. epidermidis |
Axial skeleton |
Positive PPD Exposure to TB Resident in endemic setting |
M. tuberculosis |
Thoracic spine |
Prosthetic devices |
S. aureus S. epidermidis |
Extremities and joints |
Table 4. Cierny and Mader Staging System
Anatomic Type |
Description |
|---|---|
Stage 1: medullary |
Infection limited to the intramedullary surfaces of bone (i.e. infected intramedullary rod) |
Stage 2: superficial |
Contiguous focus of infection (i.e. exposed surface of bone at the base of a soft tissue wound) |
Stage 3: localized |
Full-thickness cortical sequestration that can be removed without compromising bony stability |
Stage 4: diffuse |
Through and through infection requiring intercalary resection of bone and loss of stability |
Host Class |
Description |
A host |
Normal host |
B host |
Systemic compromise (Bs) Local compromise (Bl) Malnutrition Chronic lymphedema Renal, hepatic failure Venous stasis Major vessel compromise Diabetes mellitus Arteritis Chronic hypoxia Extensive scarring Immune disease Radiation fibrosis Malignancy Small vessel disease Extremes of age Immunosuppression or Immune deficiency |
C host |
Systemic and local compromise (Bsl) Treatment worse than disease |
Table 5. Treatment Principles of Osteomyelitis
- Stage the disease based upon Cierny-Mader classification
- Establish microbiological diagnosis
- Surgical drainage, debridement and stabilization
- Dead space management, wound protection and tissue transfer
- Appropriate antibiotic therapy
- Set realistic goals with the patient, support medical and psychological state of the patient
Table 6. Algorithm for Acute Infection.
- Hardware Stable+Bone Not-healed = Retain hardware, antibiotics until healed, then hardware removal
- Hardware Unstable + Bone not healed = Remove hardware, antibiotics, temporary stabilization, spacer, and reconstruction when clean
- Hardware Stable + Bone Healed = Remove hardware, debride with effort not to destabilize, control dead space, and antibiotics
- Hardware stable + Bone not healed + systemic effects = Remove hardware, temporary stabilize, spacer, antibiotics, and reconstruction when able, consider amputation if bad host
Table 7. Initial Antibiotic Regimens for Patients with Osteomyelitis
Organism |
Primary Antibiotic |
Alternative Antibiotic |
|---|---|---|
Staphylococcus aureus orcoagulase-negative (methicillin-sensitive) staphylococci |
Oxacillin or cefazolin |
Clindamycin, vancomycin or daptomycin |
S. aureus or coagulase-negative (methicillin-resistant) staphylococci |
vancomycin +/- Rifampin |
Daptomycin, Linezolid,trimethoprim-sulfamethoxazole or minocycline plus rifampin |
Varied streptococci (groups Aand B b-hemolytic organisms or penicillin-sensitive Streptococcus pneumoniae) |
IV Penicillin G |
Clindamycin, azithromycin,vancomycin or ceftriaxone |
Intermediate penicillin-resistant S. pneumoniae |
ceftriaxone |
Clindamycin, Azithromycin or levofloxacin |
Penicillin-resistant S. pneumoniae |
vancomycin |
levofloxacin |
Enterococcus species |
Ampicillin or vancomycin |
Linezolid, daptomycin |
Enteric gram-negative rods |
Fluoroquinolone |
ceftriaxone |
Serratia species |
levofloxacin |
Ertapenem |
Pseudomonas aeruginosa |
Cefepime, piperacillin,ciprofloxacin |
Imipenem |
Anaerobes |
Clindamycin |
amoxicillin-clavulanate, Ertapenem, moxifloxacin or metronidazole |
Mixed aerobic and anaerobic organisms |
amoxicillin-clavulanate, 875 mg and 125 mg, respectively, orally BID |
Ertapenem, moxifloxacin |
Table 8. Selected Oral Antimicrobial Agents with Excellent Oral Bioavailability Commonly Used to Treat Patients with Musculoskeletal Infection
| Antimicrobial Agents |
|---|
| Doxycycline |
| Fluoroquinolones |
Ciprofloxacin |
| Levofloxacin |
| Moxifloxacin |
| Metronidazole |
| Linezolid |
| Rifampin (must give in conjunction with a second antibiotic) |
| Trimethoprim-sulfamethoxazole |
Figures 1. Anatomic classification of adult Osteomyelitis
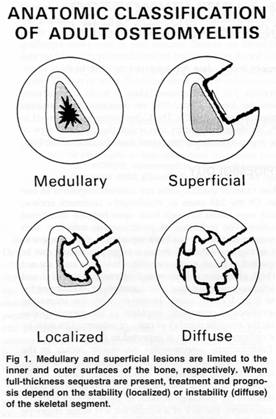
Figure 2: Graph of ESR/CRP response to surgical and antibiotic treatment in chronic osteomyelitis. Note drop of both CRP (top) and ESR (bottom) after surgical intervention red arrow. Antibiotics alone were initiated at black arrow.

What's New
Spellberg, et al. Systemic Antibiotic Therapy for Chronic Osteomyelitis in Adults. J Clinical Infectious Diseases. 2012;54(3): 393-407.
Ritz N, et al. Tuberculosis dactylitis-an easily missed diagnosis. Eur J Clin Microbiol Infect Dis. 2011;30:1301-1310.
Peltola H, et al. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 2010;29:1123-1128.
Zeller V, et al. Continuous Clindamycin Infusion, an Innovative Approach to Treating Bone and Joint Infections. Antimicrobial Agents and Chemotherapy. 2010;54(1):88-92.
Euba G, et al. Long-Term Follow-Up Trial of Oral Rifampin-Cotrimoxazole Combination versus Intravenous Cloxacillin in Treatment of Chronic Staphylococcal Osteomyelitis. Antimicrob Agents Chemother. 2009 Jun;53:2672-6.
Pääkkönen M, Kallio MJ, et al. Sensitivity of Erythrocyte Sedimentation Rate and C-reactive Protein in Childhood Bone and Joint Infections. Clin Orthop Relat Res. 2009 Jun 17. [Epub ahead of print]
Zaoutis, T et al. Prolonged Intravenous Therapy Versus Early Transition to Oral Antimicrobial Therapy for Acute Osteomyelitis in Children. PEDIATRICS Vol. 123 No. 2 February 2009, pp. 636-642 (doi:10.1542/peds.2008-0596).
Guided Medline Search For:
Reviews
Arnold, S. Antibiotic Management of Pediatric Osteomyelitis.
Guided Medline Search For Recent Reviews
Guided Medline Search For Historical Aspects
Table of Contents
- Pathogenesis and Biofilms
- Types of Osteomyelitis
- Acute Hematogenous Osteomyelitis
- Vertebral Osteomyelitis
- Osteomyelitis Secondary to Contiguous Infection
- Osteomyelitis Secondary to Vascular Insufficiency
- Acute Posttraumatic Osteomyelitis
- Open Fractures and Trauma
- Chronic Osteomyelitis
- Chronic Sclerosing Osteomyelitis
- Brodie’s Abscess
- Tuberculosis Osteomyelitis
- Osteomyelitis In A Specific Clinical Setting
- Classification
- Clinical Manifestation
- Diagnosis
- Management and Treatment
- Prevention
