Cellulitis
Authors: Imad M. Tleyjeh, M.D., Steven D. Burdette, M.D., Larry M. Baddour, M.D.
Optimal therapy of a disease begins with an accurate and prompt diagnosis. For certain syndromes, modern laboratory or procedural tools are critical in making a correct diagnosis. For other illnesses, including cellulitis, technical modalities are supportive at best in establishing a diagnosis. It is the skilled clinician’s evaluation of a patient that leads to proper diagnosis. The diagnosis of cellulitis relies on the presence of characteristic focal skin and soft tissue changes coupled with a supportive history, to include prior episodes of cellulitis, unique exposures or underlying comorbidities. Thus, both the diagnosis and treatment of cellulitis is based on empiricism.
CLINICAL MANIFESTATIONS
Cellulitis is an acute infection of the skin that involves the subcutaneous
tissues. It is manifested by swelling, erythema, tenderness, and warmth ![]() . Bullae, abscesses and cutaneous hemorrhage may develop in the inflamed skin
. Bullae, abscesses and cutaneous hemorrhage may develop in the inflamed skin ![]() .
Regional lymphadenopathy and lymphangitis can occur. Systemic manifestations are
usually mild, but fever, tachycardia, confusion, hypotension, and leukocytosis may be present.
.
Regional lymphadenopathy and lymphangitis can occur. Systemic manifestations are
usually mild, but fever, tachycardia, confusion, hypotension, and leukocytosis may be present.
The emergence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has significantly impacted the
clinical manifestations of cellulitis. Patients are now presenting to their
health care providers with complaints of having “been bitten by a spider.” The Panton-Valentine leukocidin (PVL)
toxin is felt to be linked to the unique clinical manifestations associated with
CA-MRSA. Examination reveals focal induration and warmth often associated with
surrounding erythema consistent with an abscess ![]() .
CA-MRSA may also present as furuncles (infected hair follicles with spread of
infection through the dermis, aka boils), folliculitis (infection present in the
epidermis only) or carbuncles (multiple furuncles). Focal pain is common while
constitutional symptoms are unusual, but if present, should make a physician
consider an associated bacteremia. These lesions may progress to have a necrotic
and purulent appearing center. Recurrent abscesses, furuncles and carbuncles may
occur despite appropriate therapy. Necrotizing fasciitis and necrotizing soft
tissue infections associated with CA-MRSA have now been reported and must be
considered when evaluating a cellulitis syndrome.
.
CA-MRSA may also present as furuncles (infected hair follicles with spread of
infection through the dermis, aka boils), folliculitis (infection present in the
epidermis only) or carbuncles (multiple furuncles). Focal pain is common while
constitutional symptoms are unusual, but if present, should make a physician
consider an associated bacteremia. These lesions may progress to have a necrotic
and purulent appearing center. Recurrent abscesses, furuncles and carbuncles may
occur despite appropriate therapy. Necrotizing fasciitis and necrotizing soft
tissue infections associated with CA-MRSA have now been reported and must be
considered when evaluating a cellulitis syndrome.
DIFFERENTIAL DIAGNOSIS
There is a plethora of noninfectious diseases that can masquerade as infectious cellulitis. These entities fall into several categories and include vascular, primary dermatologic, rheumatologic, immunologic-idiopathic, malignant, familial and miscellaneous. Table 2 summarizes some important syndromes and their respective distinctive features. Table 3 provides key historical questions that may help differentiate infectious from non-infectious etiologies.
Other diagnostic studies should be sought when noninfectious syndromes are
considered. Skin biopsy is often required to define a noninfectious etiology if
presumed cellulitis syndrome does not respond to antimicrobial treatment. A skin
biopsy, for example, may be needed to differentiate Sweet’s syndrome from a
fungal cellulitis. A duplex ultrasound may be used to rule out a deep vein
thrombosis (while also evaluating for the presence of an abscess). An MRI is
helpful in delineating the depth of the infection such as fasciitis, pyomyositis
or abscess. However, when there is a concern about deep soft tissue infection ![]() , surgical consultation should not be delayed.
, surgical consultation should not be delayed.
ETIOLOGIC AGENTS
The most common causative pathogen varies depending on the location of
cellulitis, associated venous and lymphatic compromise, immune status and
exposure history. Most of the infections arise from streptococci, often group A,
but also from other groups, such as B, C, or G. However, in patients with
associated subcutaneous abscesses ![]() , CA-MRSA must be considered the causative pathogen until a microbiologic
diagnosis is obtained. The source of the pathogens is frequently unclear, but in
many infections of the lower extremities, the responsible streptococci are
present in the macerated or fissured inter-digital spaces while CA-MRSA
colonizes the nares. Beta-hemolytic streptococci,
besides Streptococcus pyogenes (group A beta-hemolytic streptococcus), have a proclivity to produce soft tissue
infections in the setting of venous and/or lymphatic compromise. The initial
choice of antimicrobial therapy is empiric in the majority of cases because
identifying a specific pathogen is the exception rather than the rule.
, CA-MRSA must be considered the causative pathogen until a microbiologic
diagnosis is obtained. The source of the pathogens is frequently unclear, but in
many infections of the lower extremities, the responsible streptococci are
present in the macerated or fissured inter-digital spaces while CA-MRSA
colonizes the nares. Beta-hemolytic streptococci,
besides Streptococcus pyogenes (group A beta-hemolytic streptococcus), have a proclivity to produce soft tissue
infections in the setting of venous and/or lymphatic compromise. The initial
choice of antimicrobial therapy is empiric in the majority of cases because
identifying a specific pathogen is the exception rather than the rule.
Significant attention must be paid to CA-MRSA due to its increasing frequency of causing infection. The frequency of CA-MRSA colonization varies by patient population and associated risk factors. Kuehnert et al reported a MRSA colonization rate in the general population of 0.8% as compared to an MSSA colonization rate of 32.4% in 2001-2002. Moran et al recently report that MRSA is the most common identifiable cause of skin and soft-tissue infections among patients presenting to the emergency department. Risk factors that have been identified include: skin trauma, athletes participating in contact sports, a higher body mass index (BMI), recent antibiotics, cosmetic body shaving, physical contact with a person who has a draining lesion or is a carrier of MRSA and sharing equipment that is not cleaned or laundered between users. With that being said, many patients present with CA-MRSA infections without a single identified risk factor therefore these cannot be relied on when evaluating a cellulitis syndrome.
DIAGNOSIS
Cellulitis is a clinical diagnosis. Erythema, rubor, swelling ![]() and tenderness are present in a majority of patients. In certain circumstances
it may be useful to try to isolate an organism. However, even when attempts are
made to culture blood, skin aspirates, or skin biopsy specimens, no pathogen is
demonstrated in the majority of patients. Skin biopsy cultures have the highest
yield (26% in one study) whereas blood cultures are only positive in a minority
of patients (2% in one large study). Therefore, obtaining cultures in patients
presenting with a first episode of acute cellulitis is not recommended.
Indications for blood or cutaneous cultures include patients who have:
immunosuppression, systemic toxicity, failure to respond to appropriate therapy,
unusual exposures (such as water or animals), purulent drainage or recurrent
cellulitis. Cultures are helpful when there is a risk for CA-MRSA cellulitis
such as a history that other members of the family or other close contacts
(e.g., military personnel, team athletes) have recently had or currently suffer
from skin infections. Swabbing of the superficial skin without the presence of
pus is discouraged. In patients with abscesses suggestive of CA-MRSA, it is
recommended to obtain abscess cultures at least once as there is a significant
amount of antibiotic resistance associated with this pathogen and resistance
seems to be yet still evolving (i.e. increased fluoroquinolone resistance
recently noted). Serologic evaluation using the anti-streptolysin O reaction,
anti-deoxyribonuclease B test, anti-hyaluronidase or Streptozyme antibody assay
can be used to support a diagnosis of cellulitis due to beta-hemolytic
streptococci but are rarely clinically indicated and are not readily available.
and tenderness are present in a majority of patients. In certain circumstances
it may be useful to try to isolate an organism. However, even when attempts are
made to culture blood, skin aspirates, or skin biopsy specimens, no pathogen is
demonstrated in the majority of patients. Skin biopsy cultures have the highest
yield (26% in one study) whereas blood cultures are only positive in a minority
of patients (2% in one large study). Therefore, obtaining cultures in patients
presenting with a first episode of acute cellulitis is not recommended.
Indications for blood or cutaneous cultures include patients who have:
immunosuppression, systemic toxicity, failure to respond to appropriate therapy,
unusual exposures (such as water or animals), purulent drainage or recurrent
cellulitis. Cultures are helpful when there is a risk for CA-MRSA cellulitis
such as a history that other members of the family or other close contacts
(e.g., military personnel, team athletes) have recently had or currently suffer
from skin infections. Swabbing of the superficial skin without the presence of
pus is discouraged. In patients with abscesses suggestive of CA-MRSA, it is
recommended to obtain abscess cultures at least once as there is a significant
amount of antibiotic resistance associated with this pathogen and resistance
seems to be yet still evolving (i.e. increased fluoroquinolone resistance
recently noted). Serologic evaluation using the anti-streptolysin O reaction,
anti-deoxyribonuclease B test, anti-hyaluronidase or Streptozyme antibody assay
can be used to support a diagnosis of cellulitis due to beta-hemolytic
streptococci but are rarely clinically indicated and are not readily available.
Patients with signs of deep soft-tissue infection (such as necrotizing fasciitis) should be hospitalized with rapid imaging of the infected area and early surgical evaluation. Signs of deep tissue infection include the following: (1) pain disproportionate to the physical findings, (2) violaceous bullae, (3) cutaneous hemorrhage, (4) skin sloughing, (5) skin anesthesia, (6) rapid progression, and (7) gas in the tissue.
TREATMENT
Generalized Approach
The existing treatment paradigm is that empiric therapy should provide coverage for the most commonly involved gram-positive cocci. The presence or absence of an abscess as well as recent antibiotic exposures also impacts empiric treatment in light of issues regarding CA-MRSA. Treatment options and common pathogens are detailed in Table 1 for different cellulitis syndromes. Algorithms 1-4 provide extensive detail on dealing with the various types of cellulitis and associated conditions. Whether initial empiric therapy administered parenterally or orally is based on the clinician’s decision and not on evidence-based data which are not available. A list of indications for inpatient antibiotic treatment are listed in Table 4 and includes: comorbidities, signs of systemic toxicity (tachycardia, hypotension), those who cannot tolerate oral antibiotics or those who are not reliable for early follow up should be hospitalized and treated with parenteral therapy. Once symptomatic improvement occurs, therapy can be switched to oral (usually within 2-5 days) to complete 10-14 days course.
Individualized Approach
Although the antibiotic regimens listed in Algorithms 1-4 should be efficacious in most cases of cellulitis in the immunocompetent patient; pivotal epidemiologic and host factors must be reviewed case-by-case so that therapy for less frequent or more resistant pathogens is provided when unique factors are present. In each case, however, empiric coverage should be selected that will provide coverage for beta-hemolytic streptococci and methicillin-sensitive Staphylococcus aureus. The following sections address treatment options in those cases where epidemiologic or host-related factors impact choice of empiric therapy of cellulitis. Treatment options are detailed in Table 1.
Recurrent Abscesses
The major method of controlling recurrent abscesses ![]() is the use of antibacterial agents to eradicate staphylococcal carriage. There
is scant data available on the efficacy of the various decolonization regimens. Mupirocin (bactroban) is the most commonly utilized topical antibiotic, often
applied to the nares on the first 5 days of each month for 6 consecutive months.
Chlorhexedine body wash is often used in conjunction with
mupirocin, but tolerating it is challenging due to drying and irritation of
the skin. Decolonization of household contacts may decrease recurrent episodes
and should be considered. If these regimens fail, then low dose systemic
antibiotics may be employed. Daily single dose clindamycin (150 mg) for 3 months has been shown to be 80% effective in
decreasing staphylococcal colonization. Single dose TMP-SMX or doxycycline for 3 months has also been used, though clinical data is scarce.
Some endorse the previous mentioned systemic antibiotics combined with rifampin for a shorter course of therapy to attempt to eradicate
colonization. Several small studies have shown that tea tree oil (4% nasal
ointment and 5% body wash) is at least as effective as a mupirocin based
regimen. Polysporin may also have a role in the decolonization process.
is the use of antibacterial agents to eradicate staphylococcal carriage. There
is scant data available on the efficacy of the various decolonization regimens. Mupirocin (bactroban) is the most commonly utilized topical antibiotic, often
applied to the nares on the first 5 days of each month for 6 consecutive months.
Chlorhexedine body wash is often used in conjunction with
mupirocin, but tolerating it is challenging due to drying and irritation of
the skin. Decolonization of household contacts may decrease recurrent episodes
and should be considered. If these regimens fail, then low dose systemic
antibiotics may be employed. Daily single dose clindamycin (150 mg) for 3 months has been shown to be 80% effective in
decreasing staphylococcal colonization. Single dose TMP-SMX or doxycycline for 3 months has also been used, though clinical data is scarce.
Some endorse the previous mentioned systemic antibiotics combined with rifampin for a shorter course of therapy to attempt to eradicate
colonization. Several small studies have shown that tea tree oil (4% nasal
ointment and 5% body wash) is at least as effective as a mupirocin based
regimen. Polysporin may also have a role in the decolonization process.
Host-Related Factors
Diabetes
Cellulitis is a common diagnosis in
diabetic patients, though most studies have not demonstrated that itis a risk
factor. The etiology of cellulitis without an ulcer is typically staphylococcal (MSSA or MRSA) or beta-hemolytic streptococcus.
Gram negative and anaerobic bacteria are only a concern in the presence of an ulcer ![]() (such as occurs with diabetic
foot infections) or gangrene
(such as occurs with diabetic
foot infections) or gangrene ![]() .Other risk factors for gram negative cellulitis,
such as water or animal exposures, should be assessed in every patient with
cellulitis. Algorithm 2 provides
recommendationsfor the treatment of cellulitis without an ulcerin diabetics.
Refer to the monograph on diabetic foot
infections for treating cellulitis that is associated with a diabetic ulcer.
.Other risk factors for gram negative cellulitis,
such as water or animal exposures, should be assessed in every patient with
cellulitis. Algorithm 2 provides
recommendationsfor the treatment of cellulitis without an ulcerin diabetics.
Refer to the monograph on diabetic foot
infections for treating cellulitis that is associated with a diabetic ulcer.
Lymphedema
Anatomical areas complicated by lymphedema ![]() are prone to the development of cellulitis, often occurring on a recurrent
basis. Moreover, each episode of cellulitis may lead to further compromise of
the lymphatic system by induction of inflammation, swelling, and lymphatic
vessel fibrosis. Physicians must also keep in mind 2 common clinical
masqueraders in patients with chronic lymphedema. Venous stasis and stasis
dermatitis commonly may appear visually to be “cellulitis” but upon further
questioning the appearance of the skin has not changed recently and palpation of
the area lacks warmth or tenderness.
are prone to the development of cellulitis, often occurring on a recurrent
basis. Moreover, each episode of cellulitis may lead to further compromise of
the lymphatic system by induction of inflammation, swelling, and lymphatic
vessel fibrosis. Physicians must also keep in mind 2 common clinical
masqueraders in patients with chronic lymphedema. Venous stasis and stasis
dermatitis commonly may appear visually to be “cellulitis” but upon further
questioning the appearance of the skin has not changed recently and palpation of
the area lacks warmth or tenderness.
Two key observations warrant emphasis. First, beta-hemolytic streptococci have a
unique proclivity to cause cellulitis ![]() in the setting of venous and lymphatic compromise. Second, bacteremia may occur
more commonly in patients with cellulitis complicating lymphedema. Although
identification of a blood culture isolate permits a revision of empiric
antibiotic therapy to pathogen-specific treatment, only a minority (less than
30%) of patients will be bacteremic in the setting of lymphedema and cellulitis.
in the setting of venous and lymphatic compromise. Second, bacteremia may occur
more commonly in patients with cellulitis complicating lymphedema. Although
identification of a blood culture isolate permits a revision of empiric
antibiotic therapy to pathogen-specific treatment, only a minority (less than
30%) of patients will be bacteremic in the setting of lymphedema and cellulitis.
Empiric treatment options are listed in Table 1 and Algorithm 1 and 2. If a beta-hemolytic streptococcus is isolated, then parenteral or oral penicillin can be given. Susceptibility testing should be done if therapy with clindamycin or a macrolide is considered. Patients with lymphedema also require non-antibiotic therapy such as compression stockings and extremity elevation to help expedite improvement.
Post-saphenous venectomy
Cellulitis complicates a small minority of patients who undergo saphenous
venectomy for coronary artery bypass grafting ![]() .
The initial bout usually occurs months to years following the surgical
procedure. For some patients, recurrent bouts of cellulitis occur and can
account for considerable morbidity and serial hospitalizations. Patients who
undergo saphenous venectomy do not, in general, have clinically obvious
deficiencies in venous or lymphatic function in the involved lower extremity.
However, it is presumed that vascular and lymphatic dysfunction is present, and
that subclinical lymphedema occurs due to surgical disruption of lymphatic
drainage along the medial aspect of the lower extremity and in close proximity
to the saphenous vein. Beta-hemolytic streptococci account for the majority of
cellulitis cases in the post-saphenous venectomy setting and therefore the
aforementioned "Generalized Approach" should be appropriate.
.
The initial bout usually occurs months to years following the surgical
procedure. For some patients, recurrent bouts of cellulitis occur and can
account for considerable morbidity and serial hospitalizations. Patients who
undergo saphenous venectomy do not, in general, have clinically obvious
deficiencies in venous or lymphatic function in the involved lower extremity.
However, it is presumed that vascular and lymphatic dysfunction is present, and
that subclinical lymphedema occurs due to surgical disruption of lymphatic
drainage along the medial aspect of the lower extremity and in close proximity
to the saphenous vein. Beta-hemolytic streptococci account for the majority of
cellulitis cases in the post-saphenous venectomy setting and therefore the
aforementioned "Generalized Approach" should be appropriate.
Immunocompromised
Immunocompromised hosts include patients with neutropenia and those with cellular immune deficiencies (lymphoma, transplant recipients, AIDS and medications such as corticosteroids and immunosuppressant’s). They are predisposed to a diverse spectrum of etiologic agents that cause skin and systemic infection.
In addition to beta-hemolytic streptococci and S. aureus, a variety of
atypical pathogens may invade the skin of immunocompromised patients after local
trauma including fungi (Paecilomyces, Penicillium, Scedosporium, Trichosporon, Fusarium, Alternaria), and mycobacteria (Mycobacterium
marinum, Mycobacterium abscessus). Localized
or disseminated nodules, papules, or necrotizing infections are more commonly
due to unusual organisms. Ecthyma gangrenosum ![]() is a necrotizing vasculitis classically associated with Pseudomonas (with or
without bacteremia), however, has been reportedly caused by numerous pathogens.
The cutaneous findings of ecthyma gangrenosum are characterized by an
erythematous halo surrounding a dark gray or black nodule.
is a necrotizing vasculitis classically associated with Pseudomonas (with or
without bacteremia), however, has been reportedly caused by numerous pathogens.
The cutaneous findings of ecthyma gangrenosum are characterized by an
erythematous halo surrounding a dark gray or black nodule.
Gangrenous cellulitis is caused by a variety of organisms. Pseudomonas bacteremia, for example, can produce a gangrenous cellulitis in immunocompromised hosts. Gangrenous skin lesions may also occur with disseminated Aspergillis. Mucormycotic necrotizing angioinvasive cellulitis can be caused by the zygomycete Apophysomyces elegans after traumatic injury potentially contaminated with soil or by Rhizopus spp. from contaminated tape. The infection may exhibit an indolent course with minimal fever and a slowly enlarging black ulcer, or it may follow a rapidly progressive febrile course. The characteristic lesion consists of a central black, necrotic area with a surrounding raised zone of violaceous cellulitis and edema. Superficial vesicles and blistering may occur in the gangrenous area. Identification of the cause is best obtained via biopsy.
Cryptococcus can cause cellulitis in the severely immunocompromised host but it is extremely rare. If Cryptococcus is considered than an effort should be made to secure a diagnosis (skin biopsy, serum cryptococcal antigen) rather than empiric administration of antifungal therapy. If a diagnosis is confirmed, one must consider evaluation of the CNS (lumbar puncture and CT scan of the head) and pulmonary system (chest radiograph).
Immunocompromised hosts with cellulitis should be treated with broad spectrum antimicrobial therapy. Causative organisms may be multidrug-resistant and may be identified in blood cultures. Drug resistance is more likely in patients who have received prior antibiotic therapy. Therefore, empiric antibiotic regimens should be used that provide coverage for both aerobic gram-negative bacilli (especially P. aeruginosa in the neutropenic host) in addition to the standard, gram-positive cocci (beta-hemolytic streptococci and S. aureus) (Table 1 and Algorithm 4).
Post-Surgical Treatment of Breast Cancer
There are two distinct cellulitis syndromes that can complicate the surgical treatment of breast cancer. In one, ipsilateral upper extremity cellulitis occurs, usually months to years, after mastectomy with axillary lymph node dissection. Recurrences are common. In the other syndrome, ipsilateral breast cellulitis complicates breast conservation therapy (lumpectomy, axillary lymph node dissection and local irradiation to the remaining breast). With increasing use of breast conservation therapy for early-stage breast cancer, the expected number of breast cellulitis cases will continue to escalate. The therapy outlined in the "Generalized Approach" section is applicable to either.
Morbid Obesity
Obesity has been identified as a risk factor for the development of lower extremity cellulitis. Abdominal wall cellulitis in morbidly obese patients is also well described. In one study, of those with a body mass index (BMI) that averaged 62.3, nearly one in three suffered recurrent bouts of abdominal wall cellulitis. The microbiology of this syndrome is yet to be defined but treatment regimens described in the "Generalized Approach" section should be adequate.
Trauma in the Setting of Water Exposure or Aquatic Creatures
Aeromonas species, Vibrio vulnificus, Erysipelothrix rhusiopathiae, Streptococcus iniae, Edwardsiella tarda, Pseudomonas aeruginosa, and Mycobacterium marinum are common causes of the cellulitis syndrome after water or aquatic creature exposure. For the latter organism, a subacute lymphadenitis syndrome is more characteristic. For the other remaining pathogens, cellulitis is well-recognized as a disease manifestation and often occurs as a complication of a traumatic wound which can range from trivial to severe.
The following associations are often made: Aeromonas species with fresh water exposure, Vibrio vulnificus with salt water exposure, Erysipelothrix rhusiopathiae and Streptococcus iniae with fish handling or other marine life for meal preparation, Pseudomonas aeruginosa with hot tubs, Mycobacterium fortuitum with pedicure tubs, and Edwardsiella tarda from a variety of marine exposures. Regardless of the type of marine exposure, empiric coverage should include antibiotics that also harbor activity against beta-hemolytic streptococci and S. aureus.
Animal and Human Bites
Cellulitis and other infectious complication that can occur
following animal or human bites are linked with polymicrobial processes due to
the "mixed" normal flora of the oral cavity. Animal bites related cellulitis can
be caused by Pasteurella sp.![]() , Staphylococcus aureus and Streptococcal sp., Capnocytophaga canimorsus, Bacteroides sp., Fusobacterium sp., Porphyromonas sp., Prevotella heparinolytica, Propionibacterium sp.,
and Peptostreptococcal sp.
Cellulitis associated with human bites related cellulitis are caused by
streptococci (especially viridans group streptococci), Staphylococcus aureus, Haemophilus species, Eikenella, anaerobes, including Fusobacterium nucleatum and other Fusobacterium species, Prevotella species, and Porphyromonas species. Broader spectrum
antibiotics with anaerobic coverage are required.
, Staphylococcus aureus and Streptococcal sp., Capnocytophaga canimorsus, Bacteroides sp., Fusobacterium sp., Porphyromonas sp., Prevotella heparinolytica, Propionibacterium sp.,
and Peptostreptococcal sp.
Cellulitis associated with human bites related cellulitis are caused by
streptococci (especially viridans group streptococci), Staphylococcus aureus, Haemophilus species, Eikenella, anaerobes, including Fusobacterium nucleatum and other Fusobacterium species, Prevotella species, and Porphyromonas species. Broader spectrum
antibiotics with anaerobic coverage are required.
Erysipelas
Erysipelas is a distinct superficial cellulitis ![]() with prominent lymphatic involvement. Erysipelas is a painful lesion with a
bright red, edematous, indurated with raised borders that are sharply demarcated
from the adjacent normal skin. It is almost always caused by Streptococcus pyogenes.
Erysipelas is more common in infants, young children, and older adults. Seventy
to 80% of the lesions are on the lower extremities, and 5% to 20% are on the
face. Streptococcal bacteremia occurs in about 5% of patients.
with prominent lymphatic involvement. Erysipelas is a painful lesion with a
bright red, edematous, indurated with raised borders that are sharply demarcated
from the adjacent normal skin. It is almost always caused by Streptococcus pyogenes.
Erysipelas is more common in infants, young children, and older adults. Seventy
to 80% of the lesions are on the lower extremities, and 5% to 20% are on the
face. Streptococcal bacteremia occurs in about 5% of patients.
Mild early cases of erysipelas in an adult may be treated with oral penicillin V (Table 1). Although erysipelas can often times be distinguished from cellulitis, the differentiation occasionally may not be simple. When this occurs, broadening of antibiotic coverage to include improved staphylococcal coverage (dicloxacillin, amoxicillin-clavulonate or moxifloxacin) would be indicated. In some circumstances, it may be prudent to treat with both a beta-lactam and an anti-MRSA agent. Many oral antibiotics used to treat MRSA (specifically TMP-SMX and doxycycline) do not provide adequate streptococcal coverage. Treatment with clindamycin alone may suffice, but with increasing resistance among both staphylococcal and streptococcal species, close follow-up must be ensured.
Lymphangitis
Acute lymphangitis is a bacterial infection in the lymphatic vessels which is characterized by painful, red streaks along the lymphatic vessels. It is often associated with the cellulitis syndromes. Classically, the causative organisms are β-hemolytic streptococcus. However, there has recently been an increased association with CA-MRSA. Primary treatment includes antibiotic coverage against streptococcus, but when there is an associated abscess, bursitis, history of “skin popping” or intravenous drug abuse, anti-MRSA antibiotics should be considered.
PATHOGEN SPECIFIC THERAPY
Antibiotic spectrum should be narrowed once a pathogen is identified. In most cases of cellulitis, however, culture results are negative or cultures are not obtained, thus, empiric therapy often becomes destination therapy (antibiotic chosen to complete the therapy) with continuation of a broader spectrum agent. Failure of therapy is usually due to factors that are related either to host, pathogen, or errors in diagnosis (such as non-infectious syndrome). Host-related factors include immunocompromised state, medical noncompliance, abscess formation, or foreign body-related infection. The predominant pathogen-related factor is antimicrobial resistance. This is pertinent currently because a variety of skin and soft tissue infections, including cellulitis, is now seen in CA-MRSA which are generally refractory to beta-lactam antibiotics that are commonly chosen as initial empiric therapy.
ADJUNCTIVE THERAPY
Elevation of the affected area is an important aspect of cellulitis treatment. An aggressive approach to resolve underlying dermatologic diseases, such as tinea pedis, stasis dermatitis, lymphedema or extremity edema, should be undertaken. Prompt treatment of tinea pedis in patients with cellulitis involving a lower extremity is mandatory because there is evidence that resolution of these infections diminishes the likelihood that recurrent bouts of lower extremity cellulitis will occur. Topical and systemic antifungal agents have been shown to be fairly effective in the treatment of tinea pedis. Management of lymphedema and extremity edema includes both pharmacologic (such as diuretics) but emphasis must be placed non-pharmacologic interventions (exercise, compression stockings, massage therapy, and, in some cases, external pneumatic compression). Abscesses should be drained once identified and cultures obtained during the procedure. Adequately drained abscesses, less than 5 centimeters in diameter, do not require systemic antibiotics unless significant cellulitis is present.
REFERENCES
1. Baddour LM. Cellulitis syndromes: an update. Int J Antimicrob Agents. 2000; 14(2):113-6. [PubMed]
2. Baddour LM. Recent Considerations in Recurrent Cellulitis. Curr Infect Dis Rep. 2001; 3(5):461-465. [PubMed]
3. Baddour LM, Bisno AL. Non-group A Beta-hemolytic streptococcal cellulitis: association with venous and lymphatic compromise. Am J Med 1985; 79:155-9. [PubMed]
4. Baddour LM, Bisno AL. Recurrent cellulitis after saphenous venectomy for coronary bypass surgery. Ann Intern Med. 1982; 97(4):493-6. [PubMed]
5. Bjornsdottir S, Gottfredsson M, Thorisdottir AS, Gunnarsson GB, Rikardsdottir H, Kristjansson M, Hilmarsdottir I. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis 2005; 41:1416-22. [PubMed]
6. Bommer J, Vergetis W, Andrassy K, Hingst V, Borneff M, Huber W. Elimination of Staphylococcus aureus in hemodialysis patients. Asaio J 1995;41:127-131. [PubMed]
7. Brewer VH, Hahn KA, Rohrbach BW, Bell JL, Baddour LM. Risk factor analysis for breast cellulitis complicating breast conservation therapy. Clin Infect Dis. 2000; 31 (3):654-9. [PubMed]
8. Burdette SD and Bernstein JM. The gift that keeps on giving. Skinmed. 2005; 4(6): 381-384. [PubMed]
9. Caelli M, Porteous J, Carson CF, Heller R, Riley TV. Tea tree oil as an alternative topical decolonization agent for methicillin-resistant Staphylococcus aureus. J Hosp Infect 2000;46:236-237. [PubMed]
10. Chen SF. Staphylococcus aureus decolonization. Pediatr Infect Dis J 2005;24:79-80. [PubMed]
11. Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med. 2005; 142(1):47-55. [PubMed]
12. Fung S, O’Grady S, Kennedy C, Dedier H, Campbell I, Conly J. The utility of polysporin ointment in the eradication of methicillin-resistant Staphylococcus aureus colonization: a pilot study. Infect Control Hosp Epidemiol 2000;21:653-655. [PubMed]
13. Lipsky BA, Pecoraro RE, Ahroni JH, Peugeot RL. Immediate and longterm efficacy of systemic antibiotics for eradicating nasal colonization with Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 1992; 11: 43–7. [PubMed]
14. Harbarth S, Dharan S, Liassine N, Herrault P, Auckenthaler Pittet D. Randomized, placebo-controlled, double-bind trial to evaluate the efficacy of mupirocin for eradicating carriage of mthicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 1999; 43:1412-6. [PubMed]
15. Mertz KR, Baddour LM, Bell JL, Gwin JL. Breast cellulitis following breast conservation therapy: a novel complication of medical progress. Clin Infect Dis. 1998; 26(2):481-6. [PubMed]
16. Miller LG, Perdeau-Remington F, Rieg G, Mehdi S, Perloth J, Bayer A, Tang A, Phung TO, Spellberg B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005; 352 (14): 1445-1453. [PubMed]
17. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006; 355 (7): 666-674.[PubMed]
18. Raz R, Miron D, Colodner R, Staler Z, Samara Z, Keness Y. A 1-year trial of nasal mupirocin in the prevention of recurrent staphylococcal nasal colonization and skin infection. Arch Intern Med 1996; 156: 1109–12. [PubMed]
19. Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan EL, Montoya JG, Wade JC. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005; 41 (10):1373-406. [PubMed]
20. Swartz MN. Cellulitis. N Engl J Med 2004; 350:904-12. [PubMed]
21. Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005; 37(8):605-608. [PubMed]
Table 1: Specific Cellulitis Syndromes: Organisms and Empirical Therapy.
| Syndrome | Location | Likely organisms | Source | Empiric parenteral therapy | Oral regimens |
|---|---|---|---|---|---|
Extremity cellulitis without evidence of abscess |
Lower extremity | Beta-hemolytic streptococci, S. aureus(evaluate for risk factors for MRSA) | Macerated or fissured interdigital toe spaces | Cefazolin
ORnafcillin In the penicillin-allergic patient* clindamycinORdaptomycinORmoxifloxacin |
Dicloxacillin
ORamoxicillin-clavulonate In the penicillin-allergic* clindamycinORmoxifloxacinORlinezolid |
| Extremity cellulitis with abscess | Lower extremity | MRSA | Nasal colonization |
Daptomycin ORvancomycinORlinezolid |
TMP/SMZ ORdoxycyclineORlinezolidORclindamycin |
| Preseptal cellulitis | Periorbital | Streptococcus pneumoniae, Staphylococcus aureus, coagulase-negative Staphylococcus, and anaerobes, Haemophilus influenzae type b, S. pyogenes | Contiguous infection of the soft tissues of the face and eyelids secondary to local trauma, insect bites, or foreign bodies |
[Ceftriaxone + metronidazole +/- vancomycin]OR[ertapenem (Invanz®) +/- vancomycin]ORmoxifloxacin |
Amoxicillin-clavulanateOR[levofloxacin + metronidazole]ORmoxifloxacin |
| Orbital cellulitis | Orbital | Streptococci, S. aureus, and non-spore-forming anaerobes | Sinusitis, orbital trauma with fracture or foreign body, dacryocystitis, and infection of the teeth, middle ear, or face | Same as preseptal cellulitis | Same as preseptal cellulitis |
| Erysipelas | Face and lower extremity | Group A streptococci | Antecedent streptococcal respiratory tract infection, skin ulcers, local trauma or abrasions, psoriatic or eczematous lesions | Same as extremity cellulitis without abscess | Same as extremity cellulitis without abscess |
| Post-mastectomy cellulitis | Ipsilateral arm | Non-group A beta-hemolytic streptococci | Complication of surgeries which involve lymph node dissection | Same as extremity cellulitis without abscess | Same as extremity cellulitis without abscess |
| Post-lumpectomy cellulitis | Ipsilateral breast (may extend to shoulder, back, and arm) | Non-group A beta-hemolytic streptococci | Complication of surgeries which involve limited lymph node dissection and breast conservation and radiation | Same as extremity cellulitis without abscess | Same as extremity cellulitis without abscess |
| Post-saphenous venectomy cellulitis | Lower extremity | Beta-hemolytic streptococci | Occur months to years after saphenous venectomy: Disruption of the cutaneous barrier, lymphedema, venous insufficiency | Same as extremity cellulitis without an abscess | Same as extremity cellulitis without an abscess |
| Perineal or post-gynecologic surgery cellulitis | Abdominal wall, inguinal area, and/or the proximal thigh | Non-group A beta-hemolytic streptococci, Staphylococcus aureus, enterococci, Escherichia coli, Peptostreptococcus, Prevotella and Porphyromonas, Bacteroides fragilis group, and Clostridium. | Surgical procedures with local lymph node dissection and radiation for several types of gynecologic cancer | Same as preseptal cellulitis | Same as preseptal cellulitis |
| Abdominal wall cellulitis | Abdominal wall, may extend to thighs | Beta-hemolytic streptococci | Morbid obesity, abdominal wall lymphedema | Same as extremity cellulitis | Same as extremity cellulitis |
| Bacterial cellulitis in the immuno-suppressed patient | Varies | Group A streptococci, S aureus, P. aeruginosa, Serratia, Proteus, and the Enterobacteriaciae | Immuno-suppression, bacteremia |
Cefepime ORimipenemORpiperacillin-tazobactamIn penicillin-allergic*[aztreonam + MRSA agent]: Consider the addition of tobramycin or ciprofloxacin to above until pathogen identifiedIn patients with possible MRSA: Same as extremity cellulitis with abscess |
Not recommended initially |
| Dog and cat bite cellulitis | Varies | Pasteurella , S. aureus, streptococci, Capnocytophaga, Bacteroides species, Fusobacterium, Porphyromonas species, Prevotella heparinolytica, Proprionibacterium, and Peptostreptococcus | Oral organisms and skin flora | Ampicillin-sulbactam ORertapenem (Invanz®)OR[clindamcyin + levofoxacin]OR[metronidazole + levofloxacin] |
Amoxicillin-clavulonateORmoxifloxacinOR[clindamycin + TMP/SX]OR[levofloxacin + metronidazole] |
| Human bite cellulitis | Varies | Normal oral flora of biter: Streptococci (especially viridans streptococci), S. aureus, Haemophilus, Eikenella and anaerobes | Oral organisms and skin flora | Same as dog and cat bite cellulitis | Same as dog and cat bite cellulitis |
| Fresh water exposure cellulitis | Varies | Aeromonas hydrophilia | Fresh water | Levofloxacin ORcefepimeORcarbapenems |
Same fluoroquinolones as listed for “parenteral” |
| Saltwater exposure cellulitis | Varies | Vibrio species, particularly V. vulnificus | Saltwater, ingestion of raw or undercooked seafood | [Minocycline + cefotaxime]
ORlevofloxacin |
Minocycline
+levofloxacin |
| Aquaculture cellulitis | Varies | Streptococcus iniae | Fish | Penicillin G but empiric coverage is same as extremity cellulitis | Penicillin V but empiric coverage is same as extremity cellulitis |
| Meat handling cellulitis | Hands | Erysipelothrix rhusiopathiae | Meat, poultry, hides, saltwater fish and shellfish | Penicillin G
ORceftriaxoneORimipenemIf penicillin* allergic:levofloxacinORclindamycin |
Penicillin V
If penicillin* allergic:ciprofloxacinORclindamycinORazithromycin |
*:Immediate-type hypersensitivity reaction
+: Nafcillin and oxacillin can be used interchangeably
Table 2: Syndromes That Mimic Cellulitis
| Syndrome | Mechanism | Distinctive features |
|---|---|---|
| Stasis dermatitis | Chronic venous insufficiency, chronic edema and inflammation | Subacute clinical course, no fever, bilateral lower extremities |
| Cutaneous expansion syndrome | Rapid expansion of edema with stretching of the skin | No fever, bilateral extremities |
| Superficial thrombophlebitis | Intravenous needle or catheter | Tender and palpable cord |
| Deep vein thrombosis | Vein thrombosis with edema, warmth and erythema | Palpable cord, engorgement of veins, Duplex U/S, risk factors (stasis, trauma, malignancy) |
| Contact dermatitis | Toxic or irritant agent related inflammation | No fever, typically known or suspected exposure, confined to area of exposure |
| Insect bites | Local inflammatory reaction | Pruritis, history |
| Sweet syndrome | Neutrophilic dermatosis | Associated with malignancies (AML) |
| Gouty arthritis | Arthritis with Cutaneous erythema | Location, history, joint exam |
Table 3: Important Historical Information in Evaluation of the Cellulitis Syndrome
|
Table 4: Indications for Inpatient Treatment of Cellulitis
|
Algorithm 1: Empiric Intravenous Antibacterial Agent Therapy Based on Anatomic Site. [Download PDF]

Algorithm 2: Empiric Intravenous Antibacterial Therapy for Community-Acquired Cellulitis Based on Host Status and in Patients without Preceding Antibiotic Therapy. [Download PDF]
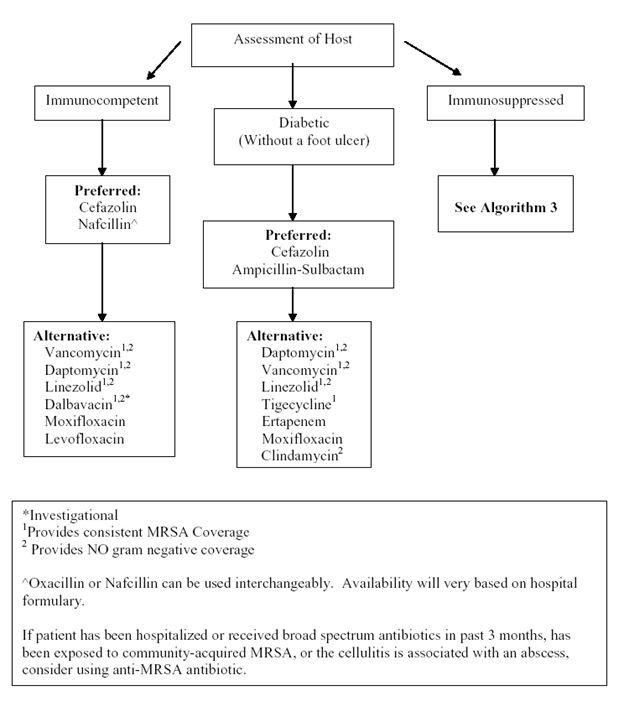
Algorithm 3: Empiric Intravenous Antibacterial Therapy for Cellulitis Based on Degree of Immunosuppression. [Download PDF]
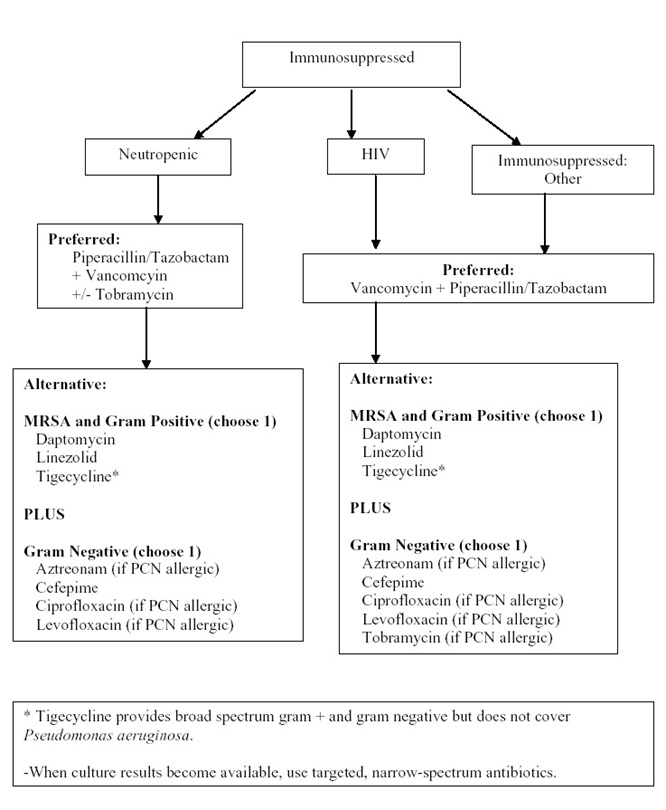
Algorithm 4: Empiric Antibacterial Agent Therapy Based on Type of Exposure. [Download PDF]

Williams HC, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med 2013;369:881-882.
Bader MS, et al. Risk factors of cellulitis treatment failure with once-daily intravenous cefazolin plus oral probenecid. South Med J 2011;104:789-793.
GUIDED MEDLINE SEARCH FOR
Marina Morgan,Venkata G. Meka. Panton-Valentine leukocidin
Hirschmann JV, et al. Lower limb cellulitis and its mimics: part I. Lower limb cellulitis. J Am Acad Dermatol 2012;67:e1-12.
Elliott DJ, Zaoutis TE, et al. Empiric Antimicrobial Therapy for Pediatric Skin and Soft-Tissue Infections in the Era of Methicillin-Resistant Staphylococcus aureus. Pediatrics. 2009 Jun;123:e959-66. Epub 2009 May 26.
Van Dort M, Shams WE, Costello PN, Sarubbi FA. Recognizing Less Common Causes of Bacterial Cellulitis. Infect Med 2007;24:340-345.
Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C, and the Linezolid CSSTI Study Group. Linezolid versus Vancomycin in Treatment of Complicated Skin and Soft Tissue Infections. Antimicrobial Agents and Chemotherapy 2005;49:2260-2266.
Burdette SD, Bernstein JM.The Gift that Keeps on Giving (Recurrent cellulitis). Skinmed 2005;4:381-384.
Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJC, Gorbach SL, Hirschmann JV, Kaplan EL, Montoya JG, Wade JC. Practice Guidelines for the Diagnosis and Management of Skin and Soft-Tissue Infections. Clin Infect Dis 2005;41:1371-406.
Kusne S, Eibling DE, et al. Gangrenous Cellulitis Associated with Gram-Negative Bacilli in Pancytopenic Patients. Am J Med. 1988 Oct;85(4):490-4.
