Table 1. In Vitro Susceptibility of Selected Fungi to Amphotericin B
|
Organism |
MIC Range (mg/ml) |
|
Candida albicans |
0.05-4 |
|
C. tropicalis |
0.04-16 |
|
C. parapsilosis |
0.025->6.25 |
|
C. krusei |
0.05->6.25 |
|
C. lusitaniae |
0.39-50 |
|
C. guilliermondii |
0.02-2 |
|
Cryptococcus neoformans |
0.04-2.8 |
|
Aspergillus fumigatus |
0.14->25 |
|
A. flavus |
12.5->25 |
|
A. niger |
0.12->25 |
|
Absidia corymbifera |
0.39-100 |
|
Rhizopus oryzae |
0.5->100 |
|
R. rhizopodiformis |
0.2->5 |
|
Blastomyces dermatitidis |
0.05-0.78 |
|
Coccidioides immitis |
0.15-96 |
|
Histoplasma capsulatum |
0.001->100 |
|
Paracoccidioides brasiliensis |
1.56->100 |
|
Sporothrix schencki |
0.4->100 |
|
Malasezzia furfur |
0.3-2.5 |
|
Pseudallescheria boydii |
1.56->100 |
Adapted from George D, Kordick D, Miniter T, et al. Combination therapy in experimental invasive aspergillosis. J Infect Dis 1993;168:692-698 (59).
Table 2 In vitro Susceptibilities of Selected Fungi to Four Amphotericin B Formulations
|
Organism (number of strains) |
Agents |
MIC Range (μg/ml) |
MIC50 (μg/ml) |
|
A. flavus (10) |
ABLC |
2->8 |
8 |
|
|
Amphotericin B desoxycholate |
0.5-2 |
1 |
|
|
ABCD |
8->8 |
>8 |
|
|
Liposomal amphotericin B |
>8 |
>8 |
|
|
|
|
|
|
A. fumigatus (30) |
ABLC |
0.12-0.25 |
0.25 |
|
|
Amphotericin B desoxycholate |
0.5-2 |
1 |
|
|
ABCD |
8->8 |
>8 |
|
|
Liposomal amphotericin B |
2-8 |
4 |
|
|
|
|
|
|
C. albicans (40) |
ABLC |
0.06-0.25 |
0.12 |
|
|
Amphotericin B desoxycholate |
0.25-0.5 |
0.25 |
|
|
ABCD |
1-2 |
1 |
|
|
Liposomal amphotericin B |
2-8 |
4 |
|
|
|
|
|
|
C. glabrata (20) |
ABLC |
0.12-0.5 |
0.25 |
|
|
Amphotericin B desoxycholate |
0.25-0.5 |
0.25 |
|
|
ABCD |
1-4 |
2 |
|
|
Liposomal amphotericin B |
4->8 |
8 |
|
|
|
|
|
|
C. lusitaniae (10) |
ABLC |
0.06-0.25 |
0.12 |
|
|
Amphotericin B desoxycholate |
0.5-2 |
0.5 |
|
|
ABCD |
4-8 |
4 |
|
|
Liposomal amphotericin B |
4->8 |
8 |
|
|
|
|
|
|
C. neoformans (20) |
ABLC |
0.03-0.25 |
0.06 |
|
|
Amphotericin B desoxycholate |
0.12-0.5 |
0.25 |
|
|
ABCD |
1-4 |
2 |
|
|
Liposomal amphotericin B |
4->8 |
8 |
Adapted from Johnson EM, Ojwang JO, Szekely A, Wallace TL, Warnock DW. Comparison of
in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four
amphotericin B formulations. Antimicrob Agents Chemother 1998; 42:1412-6 (73).
Table 3. Approved Indications and Dosage Regiments for Amphotericin B Formulations
|
Name |
Approved indication |
Approved dosage |
|
Amphotericin B desoxycholate |
Progressive and potentially life threatening fungal infections: aspergillosis, cryptococcosis (torulosis), North American blastomycosis, systemic candidiasis, histoplasmosis, zygomycosis including mucormycosis due to susceptible species of the genera Absidia, Mucor and Rhizopus, and infections due to related susceptible species of Conidiobolus and Basidiobolus, and sporotrichosis |
1 mg/kg q.d. |
|
Liposomal amphotericin B |
Empirical therapy for presumed fungal infection in febrile, neutropenic patients; treatment of cryptococcal meningitis in HIV-infected patients; treatment of patients with Aspergillus, Candida, and/or Cryptococcus infections refractory to AmB deoxycholate, or in patients in whom renal impairment or unacceptable toxicity precludes the use of AmB deoxycholate; and treatment of visceral leishmaniasis |
3 mg/kg q.d. for empirical therapy, 3-5 mg/kg q.d. for systemic fungal infections, 6 mg/kg q.d. for cryptococcal meningitis in HIV-infected patients |
|
Amphotericin B lipid complex (ABLC) |
Treatment of invasive fungal infections in patients who are refractory to or intolerant of conventional AmB therapy |
5 mg/kg q.d. |
|
Amphotericin B colloidal dispersion (ABCD) |
Treatment of invasive aspergillosis in patients in whom renal impairment or unacceptable toxicity precludes the use of AmB deoxycholate in effective doses, and in patients with aspergillosis for whom previous AmB deoxycholate therapy has failed |
3-4 mg/kg q.d. |
Table 4: Dosing During Continuous Renal Replacement Therapy
|
|
CVVH |
CVVHD or CVVHDF |
|
Amphotericin B deoxycholate |
0.4-1mg/kg q24h |
0.4-1mg/kg q24h |
|
Amphotericin B lipid complex |
3-5mg/kg q24h |
3-5mg/kg q24h |
|
Amphotericin B liposomal |
3-5mg/kg q24h |
3-5mg/kg q24h |
Abbreviations:
CVVH (Continuous venovenous hemofiltration)
CVVHD (Continuous venovenous hemodialysis)
CVVHDF (Continuous venovenous hemodiafiltration)
Note: CVVH is mainly for fluid removal alone. Many institutions will employ more CVVHD or CVVHDF which combine dialysis with fluid removal.
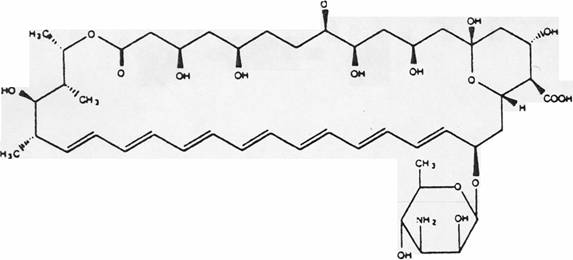
Figure 1. Structure of Amphotericin B.
