Linezolid and Other Oxazolidinones
Authors: Brian T. Tsuji, Pharm D., Glenn W. Kaatz, M.D., Michael J. Rybak, Pharm.D.
CLASS
The oxazolidinones are a unique class of synthetic antibiotics, chemically distinct from any commercially available agent (45,136) The first oxazolidinones were reported in the late 1970s by researchers at E.I. Dupont de Nemours & Company (45,86) Further chemical modifications resulted in two 3-aryl-oxazolidinones compounds, DuP-721 and DuP-105, in 1987. These agents demonstrated potent in vitro and in vivo activity versus a variety of antibiotic-susceptible and -resistant gram positive organisms (13,34,72,90,109) However, toxicity issues in animals led to the abandonment of further development of these compounds. Pharmacia Corporation (currently Pfizer, Inc.) revisited this class and examined hundreds of different oxazolidinone subclasses in an effort to find those with good antibacterial activity and low toxicity (134). Two lead compounds, eperezolid (PNU-100592) and linezolid (PNU-100766), were selected for further study (20,22,136) Only linezolid continued beyond Phase I development, and is the first oxazalodinone approved for clinical use in more than 50 countries. AZD-2563 (AstraZeneca) is a newer oxazolidinone with a similar spectrum of activity to that of linezolid and completed phase I trials before it was discontinued in July 2002. The oxazolidinones have several noteworthy attributes including 1) a novel mechanism of action, 2) a spectrum of activity that includes multidrug-resistant gram-positive bacteria, 3) excellent oral bioavailability, 4) difficulty in selecting resistance in vitro, and 5) lack of cross resistance with other antimicrobial agents (12,136).
Chemical Structure
The oxazolidinones are heterocyclic molecules with a nitrogen and oxygen in a five membered ring bridged with a carbonyl group (109). Linezolid is a member of the 3-aryl-2-oxzalidinones, which possess good antibacterial activity. These agents have an acetamidomethyl group attached to the C (5) position of the oxazolidinone ring. Their antibacterial activity is enhanced by the hydroxyactetyl group on the heterocyclic nitrogen and fluorine substitutions at the phenyl 3 position (20). AZD-2563 is a member of a series of compounds which have the C-5 acetamidomethyl group replaced by O- or N-linked 5- or 6-member aromatic heterocycles (58). The chemical structures of DuP-721, DuP105, erperezolid, linezolid, and AZD-2563 are shown in Figure 1.
ANTIMICROBIAL ACTIVITY
Spectrum
Oxazolidinones are active mainly against gram-positive organisms, but they also display modest activity against certain gram negative and anaerobic pathogens. Representativein vitro susceptibilities of various gram-positive, gram-negative and anaerobic bacteria are listed in Table 1.
Gram Positive Activity
The testing of several thousand clinical isolates have demonstrated that the 90% minimum inhibitory concentrations (MIC90 ) of both linezolid and AZD2563 for susceptible and multi-drug resistant enterococci, staphylococci and streptococci are ≤ 4 µg/ml. Linezolid and AZD-2563 are highly effective against methicillin-resistant Staphylococcus aureus (MRSA) with MIC90s ranging from 1-4 and 2 µg/ml, respectively. Both compounds possess activity against methicillin-resistant Staphylococcus epidermidis (MRSE) with MIC90s of 1-4 and 1µg/ml, respectively (23,44,48,72,73,88,91,102,103,122,127,136). In addition, clinical isolates of vancomycin/glycopeptide-intermediate S. aureus (VISA/GISA, vancomycin MIC 8 to 16 µg/mL), and vancomycin-resistant S. aureus (VRSA, vancomycin MIC≥ 32 µg/mL) isolates were susceptible to linezolid with MICs ranging from 1-2 µg/ml (4,25,103). Unlike quinupristin-dalfopristin, oxazolidinones also demonstrate activity against both vancomycin-resistant Enterococcus (VRE) faecalis and faecium. Linezolid and AZD-2563 have consistently reported MIC90s of 1-4 and 2 µg/ml for these organisms, respectively (23,39,40,131,136). Against methicillin-resistant Streptococcus pneumoniae both linezolid and AZD-2563 demonstrate excellent in vitro activity with MIC90’s ≤ 2 µg/ml (15,23,95,110,136). Susceptibility breakpoints for linezolid against enterococci, staphylococci, and streptococci are listed in Table 2. The spectrum of activity of the oxazolidinones also includes unusual gram positive species including Bacillus spp., Corynebacterium spp., Listeria monocytogenes, and Micrococcus spp. (70,72,136)
Gram Negative Activity
Oxazolidinones are less active against aerobic gram-negative pathogens due to rapid efflux mechanisms. Linezolid is not active against Acinetobacter spp, Escherichia coli,Klebsiella pneumoniae, Proteus penneri, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia (72,116,136). In addition, linezolid displays minimal activity against Haemophilus influenzae and Neisseria gonorrhea, with MIC90s of 16 µg/ml (72,130). However, linezolid displays modest activity against Bordetella pertussis, Flavobacterium menigosepticum, Moraxella catarrhalis, and Pasteurella multocida with MIC90s of ≤4 µg/ml (35,53,66,130,136).
Anaerobic Activity
Oxazolidinones display activity against select gram-positive anaerobic bacteria. Linezolid has modest activity against Bacteroides fragilis, Clostridium difficile, Clostridium perfringens, Fusobacterium spp., Peptostreptococcus spp., and Prevotella spp. (29,36,54,55,94,97,127,136). AZD-2563 displays greater activity against Clostridium difficile, Clostridium perfringens, and Peptostreptococcus spp. with MIC90s of ≤2 µg/ml against these species (36).
Miscellaneous Activity
Oxazolidinones possess significant activity against Legionella spp. and Mycobacterium tuberculosis. Oxazolidinones inhibited growth of five multidrug-resistant isolates of M. tuberculosis at concentrations of ≤2 µg/ml (136). Against clinical isolates of M. tuberculosis resistant to first line drugs, the MIC90 of linezolid was 0.5 µg/ml (3). In addition, linezolid has activity against Mycobacterium avium complex as well as several rapidly growing mycobacteria such as M. fortuitum third biovariant complex , M. mucogenicum and M. smegmatisgroup (96,124).
Effects on Virulence Factors
S. aureus produces a number of virulence factors such as α-hemolysin, δ-hemolysin, and coagulase, which play an important role in the infection process. S. pyogenesproduces streptolysin O (SLO), which displays toxic activity for a variety of cell types, and deoxyribonuclease (DNase). Subinhibitory concentrations of linezolid at 1/2, 1/4, and 1/8-fold the MIC impaired the production of α-hemolysin, δ-hemolysin, and coagulase in S. aureus and SLO and DNase in S. pyogenes. In addition, in both S. aureus and S. pyogenes,susceptibility to phagocytosis was enhanced by linezolid at concentrations below the MIC (49). S. pyogenes also produces streptococcal pyrogenic exotoxin A (SPE A), which plays an important role in the pathogenesis of severe group A streptococcal infections. Increasing concentrations of SPE A have been associated with increases in IL-6, which is a marker for systemic inflammation (30). In an in vitro pharmacodynamic model using S. pyogenes, linezolid alone and in combination with penicillin significantly reduced the early release of SPE A and was as effective as clindamycin in reducing its overall production (31).
PHARMACODYNAMICS
Parameters Correlating With Outcome
The percentage of the dosing interval that drug concentrations remain above the MIC (T>MIC) and area under the plasma concentration-time curve to MIC ratio (AUC/MIC) are pharmacodynamic parameters which have been shown to be predictors of outcomes for oxazolidinones. Peak drug concentration level in serum to MIC ratio (peak/MIC) has not been shown to be a predictor of efficacy. In a rat pneumococcal pneumonia model, the major parameters predicting efficacy for linezolid were T>MIC of greater than 39% of the dosing interval and an AUC/MIC ratio of >147 (50). In addition, a T>MIC of ≥40% for linezolid was associated with positive outcomes in a gerbil model of acute S. pneumoniae infection (84). However, in a murine-thigh model of infection, Andes et al demonstrated that the 24-h AUC/MIC ratio was the major parameter determining the efficacy of linezolid against eight different strains of penicillin-susceptible S. pneumoniae. The coefficient of determination (R2) was the highest for the 24 h AUC/MIC in comparison with other pharmacodynamic parameters (R2 = 82% for AUC/MIC, 57% for T>MIC and 59% for peak/MIC, respectively) (Figure 2). In methicillin-susceptible S. aureus, the most relevant pharmacodynamic parameter was difficult to determine; however, a 24 h AUC/MIC demonstrated a slightly better correlation with outcomes in comparison with the other parameters (R2 = 75% for AUC/MIC, 74% for T>MIC, and 65% for peak/MIC, respectively). The mean 24 h AUC/MIC ratio required for a bacteriostatic effect with linezolid over 24 h was 48 for S. pneumoniae and 83 for S. aureus (7). In a similar study by Craig et al, the 24 h AUC/MIC ratio was also highly correlated with efficacy for AZD-2563 in both S. aureus, and S. pneumoniae. (R2 = 84-94% for AUC/MIC, 46-73% for T>MIC, and 59% for peak/MIC, respectively) (32).
In 231 patients treated with linezolid for community acquired pneumonia, T>MIC and AUC/MIC ratio were evaluated as predictors of clinical and microbiologic failure. As serum concentrations of linezolid were above the MIC for 100% of the dosing interval, T>MIC could not be analyzed. However, a low 24 h AUC/MIC was shown to be a significant predictor of failure in these patients (28). In 241 seriously ill patients participating in a compassionate use program for gram positive infections, time to pathogen eradication, pathogen eradication, and clinical cure were predicted by both 24 h AUC/MIC and T>MIC. A T > MIC of ≥85% or a 24 h AUC/MIC ratio of >100 was associated with maximal efficacy (84).
Bactericidal Activity
Time-kill experiments have demonstrated that oxazolidinones are predominately bacteriostatic against staphylococci and enterococci irrespective of the presence of resistance to other drugs (34,72,73,102,103,109,127,136). At concentrations of two, four, ten fold the MIC, linezolid has been shown to have bacteriostatic activity against numerous strains of staphylococci and enterococci (102,103,127,136). In an in vitro pharmacodynamic model by Allen et al, linezolid 600mg every 12 h was bacteriostatic against MRSA, MRSE, and vancomycin-resistant E. faecalis and E. faecium. However, against one strain of GISA modest bactericidal activity was achieved (a 3.52 log10 CFU/mL reduction in colony counts at 48 h) (4). In a similar in-vitro model by Cha et al., linezolid demonstrated significant bactericidal activity against multidrug-resistant and vancomycin-tolerant Streptococcus pneumoniae (24). In time kill experiments employing S. pneumoniae, modest bactericidal activity was also achieved by linezolid (127,136). Other time kill experiments have shown that linezolid demonstrated bactericidal activity against B. fragilis, C. perfringens, and S. pyogenes (136). Data from animal models support some of the in vitro findings. In a murine-thigh model of infection by Andes et al, linezolid demonstrated bacteriostatic activity, producing modest reductions in colony counts of ≤ 0.5 log10 CFU/mL in S. aureus and ≤ 2 log10 CFU/mL in S. pneumonia (7). More recently, AZD-2563 has been shown to have concentration independent, bacteriostatic activity against staphylococci and streptococci (68).
Post-antibiotic Effects
Oxazolidinones exhibit a modest to prolonged post antibiotic effect (PAE) against staphylococci and enterococci. In an in vitro pharmacodynamic model linezolid exhibited a minimal to modest PAE against clinical strains of S. aureus, S. epidermidis, E. faecalis, and E. faecium. The PAE was shown to increase with increasing concentrations, greater at 4-fold the MIC (range, 0.2 to 1.4 h) than at the MIC (range, 0.1 to 0.8 h) (102). In vivo, the PAE of linezolid was slightly longer than when determined using in vitro models and was not concentration dependant. Linezolid exhibited a PAE of 3.2 to 3.4 h in a murine-thigh model against S. aureus at both 20 and 80 mg/kg doses. However, against S. pneumoniae linezolid did not exhibit a post antibiotic effect in vivo (7). In contrast, AZD-2563 exhibits a prolonged in vivo PAE. Against S. aureus and S. pneumoniae, PAE’s for AZD-2563 ranged from 7.4-17.0 h, and 1.6 to 12h, respectively, which support the rationale for once-daily dosing with this agent (32).
Synergy
Several in vitro and in vivo studies have investigated combination therapy with oxazolidinones against a variety of resistant gram-positive pathogens. Sweeney et al examined the effects of linezolid in combination with 35 antimicrobial agents against various strains of gram positive bacteria using the checkerboard technique of detecting synergy. Against multidrug-resistant and –susceptible strains of S. aureus, S. pneumoniae, E. faecalis, and E. faecium greater than 90% of the antimicrobial combinations with linezolid showed indifference. However, against S. aureus linezolid plus amoxicillin and linezolid plus imipenem was synergistic against MRSA and methicillin-susceptible S. aureus (MSSA), respectively. Against vancomycin-susceptible E. faecalis linezolid plus teicoplanin was synergistic. In addition, linezolid plus imipenem or tetracycline demonstrated synergy against vancomycin-resistant E. faecium. In the case of penicillin-intermediate S. pneumoniae, linezolid also achieved synergy with erythromycin (116). Time-kill experiments also have demonstrated synergistic combinations with linezolid. Grohs et al analyzed the effect of linezolid in combination with various antimicrobials against ten strains of S. aureus. Against MRSA and MSSA, no synergy was observed when linezolid was combined with fusidic acid, gentamicin, or rifampin at 4- and 8-fold the MIC. However, when linezolid was combined with vancomycin and ciprofloxacin slight antagonism was observed (60).
Other time-kill experiments support the notion of indifference with linezolid combination therapy. Three strains of MRSA and MRSE showed indifference when linezolid was combined with vancomycin or rifampin at one quarter the MIC (88). Against 10 multidrug-resistant strains of staphylococci and streptococci, AZD-2563 in combination with gentamicin but not vancomycin demonstrated synergy (69). Jacqueline et al evaluated the in vitro activity of linezolid alone and in combination with gentamicin, vancomycin, or rifampicin at one-, four-, and eight-fold the MIC against MRSA by time kill methods in conjunction with scanning electron microscopy. Time kill curves demonstrated that the combination of linezolid and rifampin was additive; however, the addition of linezolid resulted in a decrease of antibacterial activity for vancomycin and gentamicin. Only the combination of linezolid and gentamicin was antagonistic. Scanning electron micrographs of an MRSA strain exposed to linezolid alone and in combination with various antimicrobials display similar results to the time kill curves, shown in 3 (67).
Allen et al evaluated the activities of linezolid simulated at 600 mg every 12 hours in combination with a number of antimicrobials against a variety of multi-drug resistant gram positive bacteria in an in vitro pharmacodynamic model. The combinations of linezolid plus cefepime or vancomycin demonstrated additivity against both MRSA, and MSSA. In addition, against MRSE, VRE faecalis, and VRE faecium linezolid plus vancomycin, quinupristin-dalfopristin, or doxycycline demonstrated additivity. Synergy was also demonstrated with the combinations of linezolid plus quinupristin-dalfopristin against MRSA, cefepime against MRSE, and doxycycline against VRE faecalis (4). Similar studies in vancomycin-tolerant S. pneumoniae also suggest synergistic bactericidal activity with linezolid plus rifampin (24). However, contrary to in vitro studies two recent analyses of combination therapy in rabbit models of MRSA endocarditis have demonstrated antagonism with linezolid plus vancomycin, and indifference with linezolid plus rifampin (26,33).
MECHANISM OF ACTION
The oxazolidinones have a unique mechanism of action that involves the inhibition of bacterial ribosomal protein synthesis at a very early stage (34,41,80,109). Oxazolidinones bind to the 23S portion of the 50S ribosomal subunit preventing the formation of a functional 70S initiation complex formed with the 30S subunit, fMet-RNA, initiation factors (IF2, IF3) and mRNA (34,41,80,109,115). While oxazolidinones may compete with chloramphenicol and lincomycin at the 50S subunit, they have no effect on petidyl transferase or translation termination (80). This mechanism of action differs from that of existing protein synthesis inhibitors such as chloramphenicol, aminoglycosides, pristinamycin, lincosamides, macrolides, and tetracycline, which inhibit peptide elongation (81). Due to this novel mechanism of action, cross resistance between the oxazolidinones and other antimicrobial agents is not expected (43). The mechanism of action of oxazolidinones is illustrated in Figure 4.
MECHANISM OF RESISTANCE
Specific point mutations in the central loop of domain V of 23S rRNA of the 50S subunit of the ribosome have been associated with oxazolidinone resistance in S. aureus, E. faecalis, and E. faecium in clinical and laboratory strains (76,98,107,129). Guanosine is replaced by uracil at nucleotide 2576 (G2576U) of the 23SrRNA in linezolid-resistant E. faecalis. Mutations that have been associated with linezolid resistance in S. aureus include G2447U and G2576U, and in E. faecium G2505A and G2576U (81,98). Domain V is one of the most highly conserved rRNA segments, and is an integral part of the ribosomal peptidyl transferase center (38). Most organisms have multiple copies of the 23S rRNA gene, and the degree of linezolid resistance observed correlates with the ratio of wild type to mutant 23S rRNA (107,135). Interestingly, it has been shown that radiolabeled chloramphenicol and lincomycin are capable of partially competing with the binding of oxazolidinones to the 50S subunit of the ribosome (80). Both of these drugs inhibit the peptidyl transferase reaction, whereas oxazolidinones do not (42,80). Thus, even though the mechanism of protein synthesis inhibition for chloramphenicol, lincosamides, and oxazolidinones differ, their binding site(s) on the 23S rRNA may overlap ( 4).
Video: Mechanism of Resistance -- Mutation
In Vitro
In vitro selection of resistant mutants is difficult to induce with oxazolidinones (73,136). In a study by Zurenko et al, after exposure to linezolid at concentrations 2-fold the MIC, no resistant mutants were found in MRSA and MRSE. The frequency of spontaneous resistance for these organisms was <1 X 10-9 (73). Supporting these results, in an analysis by Kaatz et al, after exposure to linezolid at 2-, 4-, and 8-fold the MIC no resistant mutants were found in S. aureus. The frequency of spontaneous resistance in this study was <8 X 10-11 (136).
In Vivo
Resistance to the oxazolidinones is rare among patients receiving linezolid for VRE or MRSA infections. However, in cases of linezolid-resistant organisms indwelling prosthetic devices, undrained abscesses, prior linezolid therapy, and prolonged duration of therapy appear to be risk factors (11,56,65,93,118). Resistance to linezolid has been reported more frequently in enterococci than in staphylococci. Mutnick et al analyzed 9833 gram-positive cocci during 2001-2002, and identified eight (0.08%) linezolid-resistant strains. Of the eight linezolid-resistant strains, four E. faecium, two E. faecalis, and one each of Streptococcus oralis and S. epidermidis were identified (89). During phase III clinical trials, 15 cases of linezolid-resistant enterococci (14 cases with E. faecium and one with E. faecalis) were encountered (81,135). Several subsequent cases of linezolid-resistant E. faecalis and E. faecium have also been reported (11,56,65,71,89,93). Auckland et al described the first three cases of linezolid-resistant enterococci isolated in the United Kingdom. The MICs were 64 µg/mL inE. faecium and E. faecalis. All resistant isolates demonstrated G2576U mutations of the 23SrRNA subunit. Pulsed-field gel electrophoresis (PGFE) along with sequence analysis of rRNA indicated that resistance developed in previously susceptible strains (11). Gonzales et al reported 5 cases of linezolid-resistant E. faecium. Four cases occurred in transplant patients and all patients received prolonged courses of linezolid ranging from 21 to 40 days. Three cases of linezolid resistance were associated with treatment failure (56). Herrero et al described the nosocomial spread of linezolid-resistant E. faecium isolated from seven transplant patients. One strain was first identified in a liver transplant patient with a VRE intra-abdominal infection, receiving linezolid. This strain was subsequently nosocomially transmitted to six other patients, none of whom had prior treatment with linezolid. All isolates had a G2576T mutation in the 23SrRNA subunit, and exhibited indistinguishable patterns on SmaI PFGE (65). Surprisingly, in a report by Jones et al, a linezolid-resistant E. faecium strain from a bloodstream infection in a diabetic patient with end-stage renal disease was unassociated with prior linezolid treatment. This isolate had a G2676U mutation in the 23SrRNA subunit and the linezolid MIC was 8 µg/ml (71). In the case of linezolid resistance in other oxazolidinones, several isolates of enterococci resistant to linezolid also demonstrated cross-resistance to AZD-2563 (68,93).
Only 2 cases of linezolid-resistant S. aureus have been reported to date (118,126). Tsiodras et al reported the first case of a linezolid-resistant strain in an 85 year old man undergoing peritoneal dialysis who developed MRSA peritonitis and was treated with linezolid. After 1 month of receiving linezolid, and having cultured 11 linezolid-susceptible isolates of MRSA (MIC of 2 µg/mL), 3 MRSA isolates recovered from the peritoneal fluid were resistant to linezolid (MIC of > 32 µg/mL). PGFE revealed that this strain was unrelated to previously identified linezolid-susceptible MRSA, and all isolates showed a G2576T mutation in the 23SrRNA subunit (118). More recently, in a report by Wilson et al a 52 year old man received linezolid for a post-operative MRSA infection for 21 days. Three weeks after completion of linezolid therapy, MRSA resistant to linezolid (MIC 32 µg/mL) was isolated from a wound swab of the drain site and the empyema fluid. However, during treatment, the MIC of linezolid for MRSA was 2 µg/mL. In contrast to the previous report, the linezolid-resistant strain developed from a susceptible strain as PGFE revealed identical banding patterns in both isolates. All linezolid-resistant isolates also demonstrated a G2576T mutation in the 23SrRNA subunit (126).
PHARMACOKINETICS
Absorption
Pharmacokinetic data for linezolid are available from two randomized, double-blind, placebo-controlled phase I trials in 42 adult healthy volunteers (111). Linezolid is rapidly and completely absorbed after oral administration, with a mean absolute bioavailability of approximately 100%. Maximum plasma concentrations (Cmax) are reached 1 to 2 hours after administration (Tmax). Linezolid may be administered without regard to the timing of meals; however, when linezolid is given with high fat food Tmax is delayed from 1.5 hours to 2.2 hours and Cmax is decreased by approximately 17%. The area under the concentration–time curve (AUC) is not affected (137). Linezolid exhibits linear pharmacokinetics as plasma concentrations and AUC increased proportionally with dose, irrespective of the route of administration (111). The dose-normalized mean pharmacokinetic values for linezolid after multiple doses were given intravenously and orally are shown in Table 3.
Distribution
Animal and human data have demonstrated that linezolid readily distributes into well-perfused tissues (47). In healthy adult volunteers, its volume of distribution at steady state is 40 to 50 L (0.6-0.7L/kg) (84,137). Linezolid is moderately bound to plasma proteins (31%) and its binding is independent of drug concentration (84,111,137).
Mean linezolid tissue fluid/plasma concentration ratios in sweat and saliva (Cmax) are 0.55 and 1.2 µg/ml, respectively (137). Lovering et al examined the penetration of intravenously-administered linezolid into fat, bone, and muscle before and after surgery in 12 patients undergoing hip arthroplasty. Linezolid penetrated these tissues rapidly with approximately 30, 50 and 90% penetration, respectively (83).
Penetration of linezolid into cerebrospinal fluid is adequate in patients with severe infections. In a patient with VRE faecium meningitis linezolid 600mg administered intravenously every 12 hours produced a CSF:plasma ratio of 0.8 (61). In some cases of meningitis, CSF concentrations of linezolid can even exceed those found in serum. In a report of 5 patients with post-neurosurgical central nervous system infections due to gram positive pathogens, multiple doses of linezolid 600 mg intravenously produced a mean CSF:plasma ratio of 1.6 (121).
Metabolism
Linezolid is metabolized by non-enzymatic oxidation of the morpholine ring into two inactive carboxylic acid metabolites (PNU-142586 and PNU-142300) which do not possess any antibacterial activity. In a study by Slatter et al in 8 healthy volunteers, the disposition of linezolid following a 500 mg oral dose of radioactive drug was determined. Linezolid circulates in plasma mainly as the parent compound (108). Linezolid is not detectably metabolized by human cytochrome P450 (111,137).
Elimination
The elimination half life of linezolid is 4.5 to 5.5 hours under single dose and steady state conditions (137). Its primary route of elimination is non-renal, accounting for approximately 65% of the total clearance, with the renal route accounting for the remainder (clearance rate 40ml/min) (137). The low renal clearance suggests that linezolid undergoes tubular reabsorption. Under steady state conditions approximately 30% of an administered dose appears in the urine as unchanged drug and 50% as the two inactive carboxylic acid metabolites mentioned previously. Virtually no parent drug appears in the feces, while approximately 9% of the dose appears in the feces as PNU-142586 and PNU 142300. Formation of PNU-142586 is the rate-limiting step in the clearance of linezolid (108).
DOSAGE
Normal
For patients with VRE and MRSA infections the recommended dosage of linezolid is 600 mg every 12 h intravenously or orally for 14 to 28 days (VRE) or 7 to 28 days (MRSA). For patients with nosocomial or community-acquired pneumonia or complicated skin and skin structure infections, the recommended dosage of linezolid is 600 mg every 12 hours for 10 to 14 days. In patients with uncomplicated skin and skin structure infections, the recommended dose is 400 mg orally every 12 h for 10 to 14 days. No dosage adjustment is necessary when switching from intravenous to oral administration. The intravenous formulation of linezolid should be infused over 30 to 120 minutes (137).
Pediatric Patients
The disposition of linezolid in pediatric patients has been studied in 58 children (3 months to 16 years of age) who received a single 10mg/kg or 1.5mg/kg intravenous dose. The pharmacokinetics of linezolid are age dependant, with infants and children having greater plasma clearance, larger volumes of distribution, and lower serum concentrations and serum AUC (75,84). Overall, there is limited data on the pharmacokinetics of linezolid in pediatric patients of any age. Pediatric dosing regimens that provide a pharmacokinetic profile similar to adults have not been determined (137). However, 10mg/kg two to three times a day may be effective (75).
For patients on continuous renal replacement, dosages should be modified (Table 5).
Hepatic Insufficiency
In a matched control study of 7 patients with mild to moderate hepatic insufficiency (Childs-Pugh class A or B) compared with 8 healthy volunteers, no significant differences of the pharmacokinetic parameters of linezolid were observed (64). Based on these data, no dosage adjustment for patients with mild-to-moderate hepatic insufficiency is recommended (137). In patients with severe hepatic insufficiency (Childs-Pugh class C) the pharmacokinetics of linezolid have not been evaluated.
Renal Insufficiency
In a study of 24 patients with varying degrees of renal impairment (creatinine clearance ≥80, 40-79, or 10-39 mL/min and patients on hemodialysis) the AUC, Cmax, Tmax, V, and CLTOTAL of linezolid did not change with decreased renal function (21). However, depending on the degree of renal impairment the two carboxylic acid metabolites of linezolid did accumulate, with the amount increasing with the severity of renal function (137). In patients with severe renal insufficiency, a 7 to 8-fold increase in exposure to both metabolites occurred (84). The clinical significance of the accumulation of PNU-142586 and PNU-142300 has not been determined. Based on these observations, the manufacturer recommends that no dosage adjustment is necessary for linezolid in patients with renal insufficiency; however, the risks of using linezolid in this population must be weighed against the potential benefit (137). Hemodialysis removes approximately 30% of the linezolid dose and in this situation continued administration of the standard every 12 h dosing is recommended and should be scheduled after hemodialysis (84).
Critically Ill Patients
The pharmacokinetics of linezolid was studied in 24 critically ill patients in an intensive care unit who received linezolid 600 mg intravenously every 12 hours. The mean Cmaxand Cmin in these patients were slightly lower than that of healthy volunteers at 12.8±5.0 and 4.7±4.3 g/mL, respectively. The first-dose half life of 3.5 hours was shorter than that of healthy volunteers (84). In 318 patients participating in a compassionate use program the intrinsic clearance of linezolid was increased by 60% and the maximum rate of metabolism was 2-fold higher in these debilitated patients compared to previously studied healthy adult volunteers (p < 0.001), resulting in lower AUC values (18).
Pregnancy
There are no adequate well-controlled studies of oxazolidinones in pregnant women or nursing mothers. In mice and rats linezolid was not teratogenic at 4-fold the human exposure level, based on AUC. However, embryo and fetal toxicities were seen. In lactating rats, linezolid and its metabolites were excreted into milk (137).
ADVERSE EFFECTS
Effects on Hematology
Oxazolidinones have shown the potential for reversible myelosuppression in animals (87). Preclinical phase I and II trials have noted hematologic effects with linezolid administration (100). In animal studies, linezolid caused moderate decreases in white blood cell (WBC), red blood cell (RBC) and platelet counts. In human volunteers, 2.4% of those receiving high doses (>1 g/day) developed significant changes in hematocrit, platelet or RBC counts but these changes were reversible when linezolid was discontinued (46). Post-marketing surveillance data gathered during the first 6 months of approved use of linezolid (55,000 treated patients) indicate that the overall incidence of hematologic abnormalities, thrombocytopenia, leukopenia, and neutropenia was 0.13, 0.06, 0.03, 0.004, and 0.006%, respectively (77, 100).
In phase III trials the incidence of abnormalities of hemoglobin, neutrophils, and WBC were not significantly different from that of the comparator group. However, the incidence of thrombocytopenia (defined as less than 75% of lower limit of baseline) was higher in the linezolid group than in the comparator group, at 2.4 versus 1.5%, only close to statistical significance (p=0.066) (51,100). Thrombocytopenia began at approximately 2 weeks of linezolid therapy, but was reversible upon discontinuation of therapy. Several case studies also suggest that while thrombocytopenia associated with linezolid is reversible, it may occur earlier and at a higher incidence (10,59,92). Attassi et al found that the incidence of thrombocytopenia was as high 32% of patients who received linezolid 600mg twice daily for greater than 10 days. Gastrointestinal bleeding occurred in 5% of patients, and 21% of patients who received linezolid required platelet transfusions. Four to 13 days after linezolid therapy was discontinued, platelet counts returned to normal values (10). In addition to thrombocytopenia, there has also been cases of linezolid-induced pancytopenia. In two patients described by Halpern et al, after seven to 21 days of receiving linezolid 600mg twice daily, WBC and hemoglobin levels decreased significantly from baseline. While there was no evidence of bleeding, one patient required erythropoietin alfa, and both patients required blood transfusions (62). Several other cases of reversible myelosuppression, with anemia, reticulocytopenia and increasing iron saturation have been described in patients receiving more than two weeks of linezolid therapy (2,59).
Safety
The safety profile of linezolid was evaluated in seven multi-center, phase III, comparator-controlled trials involving 4047 adult patients with gram positive bacterial infections (100). A total of 2046 patients were treated with linezolid 600 or 400 mg intravenous and orally twice daily for up to 28 days and 2001 patients were treated with comparator agents including cefpodoxime, ceftriaxone, clarithromycin, dicloxacillin, and vancomycin. The linezolid and comparator groups were similar with respect to age, sex, race, presenting infection, and geographic distribution. Adverse drug events occurred more often in the linezolid group than in the comparator group (21.7 vs 15.7%, respectively [p=0.001]). However, the incidence of serious adverse events, treatment discontinuation due to adverse drug events, and patients who died were not significantly different between the two groups. The most common adverse effects of linezolid, which occurred at rates not significantly different from the comparator group, were diarrhea, nausea, and headache (4.3, 3.4, and 2.2 %, respectively) (Table 4) Few patients (≤0.5%) required discontinuation of the study medication due to these effects. Overall, linezolid has generated little toxicity and produced few adverse effects in phase III clinical trial (46,87,100,137).
MONITORING REQUIREMENTS
Since linezolid causes mild, reversible, time-dependant myelosuppression, monitoring of complete blood counts in patients who receive linezolid is now recommended (100,137). Monitoring is especially important in patients receiving the drug for more than 2 weeks, those with preexisting myelosuppression or receiving concomitant drugs that produce myelosuppression, and those with chronic infections who have received previous antibiotic therapy. Discontinuation of therapy with linezolid should be considered in patients who develop or have worsening myelosuppression (137).
Prolonged use of 28 days or longer is a risk factor for peripheral neuropathy (which is usually irreversible), and optic neuropathy which can lead to blindness.
Lactic acidosis can occur and is not associated with duration of therapy.
DrUG INTERACTIONS
Cytochrome P450
Linezolid does not inhibit human cytochrome P450 isoenzymes 1A2, 2C9, 2C19, 2D6, 2E1, or 3A4. Linezolid also does not induce hepatic microsomal CYP 450 1A, 3A, or 4A (84). Concomitant administration of linezolid did not alter substantially the pharmacokinetics of (S)-warfarin. Drug substrates of the CYP2C9 system may be administered with linezolid without changes in dosages. No drug interactions with the CYP450 system are expected (137).
Monamine Oxidase
Oxazolidinones are weak, non-selective, reversible inhibitors of monoamine oxidase. Therefore, these agents have the potential to interact with adrenergic and serotonergic agents. Coadministration of linezolid with adrenergic, vasopressor, or dopaminergic agents may result in a reversible enhancement of their pressor response. Following coadministration of linezolid in normotensive healthy volunteers, minimal yet significant increases were observed in pseudoephedrine and phenylpropanolamine plasma concentrations. Increases in blood pressure were also observed following coadministrationm (63). When linezolid was administered with dextromethorphan in healthy volunteers, minimal yet significant decreases were observed in dextrophan (the primary metabolite of dextromethorphan, a serotonin reuptake inhibitor) plasma concentrations. Coadministration was not associated with a serotonin syndrome such as confusion, delirium, restlessness, tremor, blushing or hyperexia and no effects on blood pressure were observed (63).
In phase III trials, the incidence of MAO-related adverse events was similar between patients who received linezolid or comparator agents. 30.9% of patients in the linezolid group versus 30.3% in the comparator group received potentially MAO-interacting medications. When specific drug classes were examined, there was no clear pattern indicating a MAO-inhibitory interaction effect with linezolid (100).
There can be significant interactions between linezolid and food. An exaggerated pressor response may be seen with the ingestion of foods high in tyramine content such as aged cheeses (up to 15 mg tyramine per ounce), tap beers (4 mg tyramine per 12 ounces), and red wines (up to 6 mg tyramine per 6 ounces). No significant pressor response was observed in patients receiving both linezolid and <100 mg tyramine concurrently (9). Therefore, ingestion of more than 100 mg of tyramine at a single meal should be avoided (119,123). It is recommended that linezolid should only be administered to patients receiving serotonin reuptake inhibitors, vasopressor agents, dopaminergic agents, pethidine or buspirone if there are facilities to monitor blood pressure (84). Initial doses of adrenergic agents should be reduced and titrated to achieve the desired response (137).
CLINICAL INDICATIONS
Linezolid is currently approved in more than 50 countries for clinical use. FDA approved indications include VRE infections, including those instances of concurrent bacteremia, nosocomial pneumonia caused by S. aureus (including methicillin-resistant strains) and S. pneumoniae (penicillin-susceptible strains), complicated skin and skin structure infections caused by S. aureus (including methicillin-resistant strains), Streptococcus pyogenes, and Streptococcus agalactiae, uncomplicated skin and skin structure infections caused by S. aureus(methicillin-susceptible strains) or S. pyogenes, and community-acquired pneumonia caused by S. pneumoniae or S. aureus (penicillin- and methicillin-susceptible strains, respectively). There have been several phase II and III trials evaluating the effectiveness of linezolid in these infections.
Community Acquired Pneumonia
A multicenter randomized, open label, phase III trial was conducted in 747 hospitalized patients to compare the effectiveness of linezolid versus a cephalosporin regimen for the treatment of community acquired pneumonia (CAP). 381 patients received intravenous linezolid 600 mg twice daily, followed by 600mg orally twice daily, while 366 patients received intravenous ceftriaxone 1 g twice daily, followed by oral cefpodoxime, 200 mg twice daily. Following 12 to 28 days of treatment, microbiologic and clinical efficacy was assessed. Clinical cure was significantly higher in the linezolid group than in the cephalosporin group in all patients (83 vs 76.4%; p=0.04). In the case of all patients who had S. pneumoniaeisolated at baseline, no significant differences in eradication rates were demonstrated between linezolid and ceftriaxone/cefpodoxime (both groups approximately 90%). However, when analyzing a subgroup of patients with bacteremia due to S. pneumoniae, linezolid demonstrated a significantly higher clinical cure rate in comparison to the cephalosporin combination (93.1 vs 68.2%; p=0.021) (104).
Consistent witth adult data a phase II, an open label, multicenter trial was conducted in 66 hospitalized children (12 months to 17 years of age) with CAP, who were treated with intravenous linezolid 10mg/kg every 12 h followed by an oral regimen. Linezolid was administered intravenously for a mean of 4.8 ± 4.3 days, and the mean total duration of therapy was 12.2 ±6.2 days. At the end of therapy a successful outcome was observed in 94% of children. Only one patient failed therapy (74).
Despite the fact that oxazolidinones have demonstrated good cure rates in clinical trials and are indicated for treatment of CAP, based on the limited clinical data presented above it is difficult to recommend the use of linezolid as a first-line agent. It is important to remember that the activity of linezolid against H. influenzae and atypical respiratory pathogens is limited (87). It will be of interest to evaluate newer oxazolidinones with increased H. influenzae activity in the treatment of CAP.
Nosocomial Pneumonia
The efficacy of linezolid in 396 hospitalized patients with nosocomial pneumonia was evaluated in a multi-center, randomized, double-blinded comparator-controlled trial. 203 adult patients empirically received intravenous linezolid 600mg twice daily, plus aztreonam and 193 patients received intravenous vancomycin, 1g twice daily plus aztreonam for 7-21 days. Both groups of patients were severely ill as assessed by their mean Acute Physiology and Chronic Health Evaluation (APACHE) II scores of more than 15. The majority of patients were over 65 years of age, and more than 40% were on a ventilator before enrollment. Clinical and microbiologic outcomes were evaluated 12 to 28 days after treatment. The clinical outcome was not significantly different between groups, as 66.4% of patients in the linezolid group versus 68.1% of patients in the vancomycin group were cured (p=0.79). In the case of microbiologic outcome, there were no significant differences between groups. 67.9% of linezolid-treated patients versus 71.8% of vancomycin-treated patients demonstrated microbiologic success (p=0.69). In addition, eradiation rates of MRSA and S. pneumoniae did not differ significantly (99). In a subsequent continuation study, linezolid and vancomycin were again compared using similar methods and identical regimens of linezolid and vancomycin as described above. However, patients from the previous analysis were not included in this continuation study. 321 patients were treated in the linezolid group and 302 in the vancomycin group. Patients in the continuation study were also severely ill as APACHE II scores were >14 and mean ages were >60 years of age. Supporting the results of the first study, there were no significant differences in cure rates between the treatment groups. Clinical cure rates were 67.9% and 64.9%, and microbiologic success rates were 61.8% and 53.2% in the linezolid and vancomycin groups, respectively (128). Both clinical trials have demonstrated that in severely ill patients linezolid is an effective treatment option for adults with gram-positive nosocomial pneumonia.
Skin and Skin Structure
A randomized, double-blinded, multi-center comparator-controlled trial was performed to evaluate the efficacy of linezolid versus oxacillin-dicloxacillin in 826 patients with complicated skin and soft tissue infections. 403 patients were randomized to receive 600 mg linezolid intravenously every 12 h and 423 patients were to receive oxacillin 2 g intravenously every 6 h, followed by oral linezolid or oral dicloxacillin, respectively. Cure rates were not significantly different between the groups in clinically evaluable patients. Clinical cure rates were 88.6% versus 85.8% for patients treated with linezolid or oxacillin-dicloxacillin, respectively (p=0.30). Similarly, microbiologic cure rates were 88.1% in patients in the linezolid group versus 86.1% in patients in the oxacillin-dicloxacillin group. This study supports the use of linezolid for the treatment of adults with complicated skin and soft tissue infections (113).
VRE
A randomized, double-blinded, multi-center, dose-comparative, phase III study was conducted to evaluate the efficacy of linezolid in patients with VRE infections. 79 patients received 600 mg of intravenous or oral linezolid and 66 patients received 200 mg of intravenous or oral linezolid twice daily for 7 to 28 days. The mean duration of linezolid therapy was similar between groups, ranging from 15.0 to 16.1 days. Clinical cure rates were slightly higher in the high dose linezolid group in patients with bacteremia, urinary tract infections, pneumonia, and all other infections, but the differences were not statistically significant. However, microbiologic cure was significantly greater for the high dose linezolid group (88 vs 62%; p=0.007) (125,137). The results of a compassionate use program in 796 patients with a variety of gram-positive infections treated with linezolid support the above findings. VRE was the causative pathogen in 66.3% of cases. In intention to treat analysis, clinical cure and microbiologic cure were 73.3% and 82.4%, suggesting that linezolid has similar efficacy in controlled clinical and real world environments. (18,125) Another study analyzed 15 patients who were treated with linezolid 600 mg every 12 hours for a mean duration of 20.5 days against a variety of VRE infections. Microbiologic cure occurred in all 10 patients who completed therapy, and all 7 patients alive at long term follow up were considered clinically cured (27).
MRSA
A randomized, open label trial in 240 hospitalized patients compared up to 4 weeks of linezolid or vancomycin in the treatment of known or suspected MRSA infections. 56 patients who received linezolid 600 mg twice daily and 60 patients who received vancomycin 1 g twice daily were evaluated in this study. S. aureus was isolated from 53% of patients, and 93% of isolates were methicillin-resistant. Skin and soft tissue was the common infection site, followed by pneumonia and urinary tract infections. The linezolid treated group showed no significant differences versus the vancomycin treated group, as clinical cure and microbiologic success was 73.2% versus 73.1% (p=0.99) and 58.9% versus 63.2% (p=0.65), respectively. MRSA eradication rates were similar in both groups (112).
In addition to the clinical utility in using linezolid for MRSA infections, a number of studies support its use to reduce length of stay (LOS) if therapy is switched to the oral route of administration. In 468 hospitalized patients with known or suspected MRSA infections, for patients who received intravenous followed by oral linezolid instead of vancomycin, the median LOS for patients with skin and soft tissue infections was 8 days shorter for the linezolid-treated group (p=0.003) (78,79). Finally, in patients with serious gram positive bacterial infections, including those caused by MRSA, patients treated with intravenous to oral linezolid demonstrated a 1.6-day shorter LOS and a 66% greater odds of early discharge compared with patients treated with teicoplanin (P = 0.049) (82).
Other Infections
The efficacy of linezolid has also been evaluated against a number of other gram positive infections. A growing number of case reports cite success when linezolid is used for meningitis caused by VRE (57,61,106,120). In addition, the evidence of successfully treating CSF infections due MRSE with linezolid is also growing (52,120). Prosthetic hip infections associated with osteomyelitis due to VRE faecium and MRSA have also been successfully treated with linezolid (14,117). In the case of endocarditis there have been reports of clinical success when linezolid was used as part of combination therapy against multi-drug resistant pathogens. In a 52 year old patient with mitral-valve endocarditis due to GISA clinical response was achieved only after linezolid was added to vancomycin (8). Other successful cases of treatment with linezolid for VRE endocarditis have been reported (27). However, there are also a number of case reports citing failures of linezolid as monotherapy in MRSA and E. faecalis endocarditis (101,133).
Future Oxazolidinones
Oxazolidinones fill an important void in infectious disease chemotherapy. Within the past few years gram-positive organisms have emerged that are resistant to nearly every available antimicrobial agent. The oxazolidinones include such organisms within their spectrum of activity. Oxazolidinones have many favorable attributes with activity against multidrug-resistant bacteria, 100% bioavailability, a long serum half life allowing twice-daily dosing, a low incidence of the emergence of primary resistance and no cross-resistance to existing compounds. AZD-2563 also has favorable characteristics, with a spectrum of activity similar to that of linezolid and a prolonged post antibiotic effect, allowing for once a day dosing. However, as resistance appears to be a class effect with the oxazolidinones, pathogens resistant linezolid are likely to be resistant to other oxazolidinones, including AZD-2563. Several other oxazolidinones exist as potential candidates in the battle against multidrug-resistant pathogens, but other than AZD-2563 and linezolid none have progressed to clinical trials. PNU-183247 (Pfizer) and VRC 3599 (Versicor) are two newer oxazolidinones which have increase anti-Haemophilus activity and good activity against S. pneumoniae (81). RBX7644 (Ranbaxy) is a newer oxazolidinone which demonstrates very good activity against both gram negative and gram positive anaerobic pathogens (37). The development these second generation oxazolidinones and other new oxazolidinones is anxiously awaited.
REFERENCES
1. Abb J. Comparative activity of linezolid, quinupristin-dalfopristin and newer quinolones against Streptococcus pneumoniae. Int J Antimicrob Agents 2003; 21(3):289-291. [PubMed]
2. Abena PA, Mathieux VG, Scheiff JM, Michaux LM, Vandercam BC. Linezolid and reversible myelosuppression. JAMA 2001; 286(16):1973. [PubMed]
3. Alcala L, Ruiz-Serrano MJ, Perez-Fernandez Turegano C, Garcia De Viedma D, Diaz-Infantes M, Marin-Arriaza M, Bouza E. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob Agents Chemother 2003; 47(1):416-417. [PubMed]
4. Allen GP, Cha R, Rybak MJ. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2002; 46(8):2606-2612. [PubMed]
5. Anderegg TR, Biedenbach DJ, Jones RN. In vitro evaluation of AZD2563, a new oxazolidinone, tested against beta-haemolytic and viridans group streptococci. J Antimicrob Chemother 2002; 49(6):1019-1021. [PubMed]
6. Anderegg TR, Biedenbach DJ, Jones RN. In vitro evaluation of AZD2563, a novel oxazolidinone, against 603 recent staphylococcal isolates. Antimicrob Agents Chemother 2002; 46(8):2662-2664. [PubMed]
7. Andes D, van Ogtrop ML, Peng J, Craig WA. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 2002; 46(11):3484-3489. [PubMed]
8. Andrade-Baiocchi S, Tognim MC, Baiocchi OC, Sader HS. Endocarditis due to glycopeptide-intermediate Staphylococcus aureus: case report and strain characterization. Diagn Microbiol Infect Dis 2003; 45(2):149-152. [PubMed]
9. Antal EJ, Hendershot PE, Batts DH, Sheu WP, Hopkins NK, Donaldson KM. Linezolid, a novel oxazolidinone antibiotic: assessment of monoamine oxidase inhibition using pressor response to oral tyramine. J Clin Pharmacol 2001; 41(5):552-562. [PubMed]
10. Attassi K, Hershberger E, Alam R, Zervos MJ. Thrombocytopenia associated with linezolid therapy. Clin Infect Dis 2002; 34(5):695-698. [PubMed]
11. Auckland C, Teare L, Cooke F, Kaufmann ME, Warner M, Jones G et al. Linezolid- resistant enterococci: report of the first isolates in the United Kingdom. J Antimicrob Chemother 2002; 50(5):743-746. [PubMed]
12. Bain KT, Wittbrodt ET. Linezolid for the treatment of resistant gram-positive cocci. Ann Pharmacother 2001; 35(5):566-575. [PubMed]
13. Barry AL. In vitro evaluation of DuP 105 and DuP 721, two new oxazolidinone antimicrobial agents. Antimicrob Agents Chemother 1988; 32(1):150-152. [PubMed]
14. Bassetti M, Di Biagio A, Cenderello G, Del Bono V, Palermo A, Cruciani M, Bassetti D. Linezolid treatment of prosthetic hip Infections due to methicillin-resistant Staphylococcus aureus (MRSA). J Infect 2001; 43(2):148-149. [PubMed]
15. Baum SE, Crawford SA, McElmeel ML, Whitney CG, Jorgensen JH. Comparative activities of the oxazolidinone AZD2563 and linezolid against selected recent North American isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother 2002; 46(9):3094-3095. [PubMed]
16. Behra-Miellet J, Calvet L, Dubreuil L. Activity of linezolid against anaerobic bacteria. Int J Antimicrob Agents 2003; 22(1):28-34. [PubMed]
17. Betriu C, Redondo M, Palau ML, Sanchez A, Gomez M, Culebras E, Boloix A, Picazo JJ. Comparative in vitro activities of linezolid, quinupristin-dalfopristin, moxifloxacin, and trovafloxacin against erythromycin-susceptible and -resistant streptococci. Antimicrob Agents Chemother 2000; 44(7):1838-1841. [PubMed]
18. Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH, Schentag JJ. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate- use program. Clin Infect Dis 2003; 36(2):159-168. [PubMed]
19. Bostic GD, Perri MB, Thal LA, Zervos MJ. Comparative in vitro and bactericidal activity of oxazolidinone antibiotics against multidrug-resistant enterococci. Diagn Microbiol Infect Dis 1998; 30(2):109-112. [PubMed]
20. Brickner SJ, Hutchinson DK, Barbachyn MR, Manninen PR, Ulanowicz DA, Garmon SA et al. Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections. J Med Chem 1996; 39(3):673-679. [PubMed]
21. Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O Grady MA et al. Pharmacokinetics of linezolid in subjects with varying degrees of renal function and on dialysis. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 24-27, 1998; San Diego.Abstract A-54 . 2003. [PubMed]
22. Brumfitt W, Hamilton-Miller JM. In-vitro microbiological activities of DuP 105 and DuP 721, novel synthetic oxazolidinones. J Antimicrob Chemother 1988; 21(6):711-720.[PubMed]
23. Cercenado E, Garcia-Garrote F, Bouza E. In vitro activity of linezolid against multiply resistant Gram-positive clinical isolates. J Antimicrob Chemother 2001; 47(1):77-81. [PubMed]
24. Cha R, Rybak MJ. Linezolid and Vancomycin, Alone and in Combination with Rifampin, Compared with Moxifloxacin against a Multidrug-Resistant and a Vancomycin-Tolerant Streptococcus pneumoniae Strain in an In Vitro Pharmacodynamic Model. Antimicrob Agents Chemother 2003; 47(6):1984-1987. [PubMed]
25. Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 2003; 348(14):1342-1347. [PubMed]
26. Chiang FY, Climo M. Efficacy of Linezolid Alone or in Combination with Vancomycin for Treatment of Experimental Endocarditis Due to Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother 2003; 47:3002-3004. [PubMed]
27. Chien JW, Kucia ML, Salata RA. Use of linezolid, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clin Infect Dis 2000; 30(1):146- 151.[PubMed]
28. Cirincione b, Phillips L, Grasela T, Sardella S, Ludwig E, Bruss J. Population pharmacokinetics (pK) of linezolid in patients with community acquired pneumonia and skin and soft tissue infection. Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 2000.Page 36, F-1205 . 2000.
29. Citron DM, Merriam CV, Tyrrell KL, Warren YA, Fernandez H, Goldstein EJ. In vitro activities of ramoplanin, teicoplanin, vancomycin, linezolid, bacitracin, and four other antimicrobials against intestinal anaerobic bacteria. Antimicrob Agents Chemother 2003; 47(7):2334-2338. [PubMed]
30. Coyle EA. Targeting bacterial virulence: the role of protein synthesis inhibitors in severe infections. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2003; 23(5):638-642. [PubMed]
31. Coyle EA, Cha R, Rybak MJ. Influences of linezolid, penicillin, and clindamycin, alone and in combination, on streptococcal pyrogenic exotoxin a release. Antimicrob Agents Chemother 2003; 47(5):1752-1755. [PubMed]
32. Craig W.A, Andes D.R. Pharmacodynamic Characteristics of AZD2563, the New Oxazolidinones, in a Murine Thigh-Infection Model. Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, September and December 2001.Page 226, F-1037. 2001.
33. Dailey CF, Pagano PJ, Buchanan LV, Paquette JA, Haas JV, Gibson JK. Efficacy of linezolid plus rifampin in an experimental model of methicillin-susceptible Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 2003; 47(8):2655- 2658. [PubMed]
34. Daly JS, Eliopoulos GM, Reiszner E, Moellering RC Jr. Activity and mechanism of action of DuP 105 and DuP 721, new oxazolidinone compounds. J Antimicrob Chemother 1988; 21(6):721-730. [PubMed]
35. Di Pentima MC, Mason EO Jr, Kaplan SL. In vitro antibiotic synergy against Flavobacterium meningosepticum: implications for therapeutic options. Clin Infect Dis 1998; 26(5):1169-1176. [PubMed]
36. Ednie LM, Jacobs MR, Appelbaum PC. Anti-anaerobic activity of AZD2563, a new oxazolidinone, compared with eight other agents. J Antimicrob Chemother 2002; 50(1):101-105.[PubMed]
37. Ednie LM, Rattan A, Jacobs MR, Appelbaum PC. Antianaerobe Activity of RBX 7644 (Ranbezolid), a New Oxazolidinone, Compared with Those of Eight Other Agents. Antimicrob Agents Chemother 2003; 47(3):1143-1147. [PubMed]
38. Egebjerg J, Larsen N, Garrett RA. Structural map of 23S rRNA. In: Hill WE DAGRMPSDWJEASfM, editor. The Ribosome: Structure, Function, and Evolution. Washington, DC: 1990: 168-179.
39. Eliopoulos GM, Wennersten CB, Gold HS, Moellering RC Jr. In vitro activities in new oxazolidinone antimicrobial agents against enterococci. Antimicrob Agents Chemother 1996; 40(7):1745-1747. [PubMed]
40. Eliopoulos GM, Wennersten CB, Moellering RC Jr. In vitro activity of the new oxazolidinone AZD2563 against Enterococci. Antimicrob Agents Chemother 2002; 46(10):3273-3275.[PubMed]
41. Eustice DC, Feldman PA, Zajac I, Slee AM. Mechanism of action of DuP 721: inhibition of an early event during initiation of protein synthesis. Antimicrob Agents Chemother 1988; 32(8):1218-1222. [PubMed]
42. Fernandez-Munoz R, Monro RE, Torres-Pinedo R, Vazquez D. Substrate- and antibiotic- binding sites at the peptidyl-transferase centre of Escherichia coli ribosomes. Studies on the chloramphenicol. lincomycin and erythromycin sites. Eur J Biochem 1971; 23(1):185-193. [PubMed]
43. Fines M, Leclercq R. Activity of linezolid against Gram-positive cocci possessing genes conferring resistance to protein synthesis inhibitors. J Antimicrob Chemother 2000; 45(6):797-802. [PubMed]
44. Fluit AC, Schmitz FJ, Verhoef J, Milatovic D. In vitro activity of AZD2563, a novel oxazolidinone, against European Gram-positive cocci. J Antimicrob Chemother 2002; 50(2):271-276. [PubMed]
45. Ford CW, Hamel JC, Wilson DM, Moerman JK, Stapert D, Yancey RJ Jr, Hutchinson DK, Barbachyn MR, Brickner SJ. In vivo activities of U-100592 and U-100766, novel oxazolidinone antimicrobial agents, against experimental bacterial infections. Antimicrob Agents Chemother 1996; 40(6):1508-1513. [PubMed]
46. French G. Safety and tolerability of linezolid. J Antimicrob Chemother 2003; 51 Suppl 2:II45- II53. [PubMed]
47. Gee T, Ellis R, Marshall G, Andrews J, Ashby J, Wise R. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob Agents Chemother 2001; 45(6):1843-1846. [PubMed]
48. Gemmell CG. Susceptibility of a variety of clinical isolates to linezolid: a European inter- country comparison. J Antimicrob Chemother 2001; 48(1):47-52. [PubMed]
49. Gemmell CG, Ford CW. Virulence factor expression by Gram-positive cocci exposed to subinhibitory concentrations of linezolid. J Antimicrob Chemother 2002; 50(5):665-672.[PubMed]
50. Gentry-Nielsen MJ, Olsen KM, Preheim LC. Pharmacodynamic activity and efficacy of linezolid in a rat model of pneumococcal pneumonia. Antimicrob Agents Chemother 2002; 46(5):1345-1351. [PubMed]
51. Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother 2002; 46(8):2723-2726. [PubMed]
52. Gill CJ, Murphy MA, Hamer DH. Treatment of Staphylococcus epidermidis ventriculo- peritoneal shunt infection with linezolid. J Infect 2002; 45(2):129-132. [PubMed]
53. Goldstein EJ, Citron DM, Merriam CV. Linezolid activity compared to those of selected macrolides and other agents against aerobic and anaerobic pathogens isolated from soft tissue bite infections in humans. Antimicrob Agents Chemother 1999; 43(6):1469-1474. [PubMed]
54. Goldstein EJ, Citron DM, Merriam CV, Warren Y, Tyrrell K, Fernandez HT. In Vitro Activities of Dalbavancin and Nine Comparator Agents against Anaerobic Gram-Positive Species and Corynebacteria. Antimicrob Agents Chemother 2003; 47(6):1968-1971. [PubMed]
55. Goldstein EJ, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT. In vitro activities of daptomycin, vancomycin, quinupristin- dalfopristin, linezolid, and five other antimicrobials against 307 gram-positive anaerobic and 31 Corynebacterium clinical isolates. Antimicrob Agents Chemother 2003; 47(1):337-341. [PubMed]
56. Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357(9263):1179. [PubMed]
57. Graham PL, Ampofo K, Saiman L. Linezolid treatment of vancomycin-resistant Enterococcus faecium ventriculitis. Pediatr Infect Dis J 2002; 21(8):798-800. [PubMed]
58. Gravestock MB, Betts MJ, Chawner E, Dawson L, Mcgregor A, Mills SD et al. In Vivo Studies of Novel Oxazolidinones with O- and N-linked C-5 Heterocyclic Side-Chains, Including AZD2563. Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, September and December 2001.Page 222, F-1023. 2001.
59. Green SL, Maddox JC, Huttenbach ED. Linezolid and reversible myelosuppression. JAMA 2001; 285(10):1291. [PubMed]
60. Grohs P, Kitzis MD, Gutmann L. In vitro bactericidal activities of linezolid in combination with vancomycin, gentamicin, ciprofloxacin, fusidic acid, and rifampin against Staphylococcus aureus. Antimicrob Agents Chemother 2003; 47(1):418-420. [PubMed]
61. Hachem R, Afif C, Gokaslan Z, Raad I. Successful treatment of vancomycin-resistant Enterococcus meningitis with linezolid. Eur J Clin Microbiol Infect Dis 2001; 20(6):432- 434.[PubMed]
62. Halpern M. Linezolid-induced pancytopenia. Clin Infect Dis 2002; 35(3):347-348. [PubMed]
63. Hendershot PE, Antal EJ, Welshman IR, Batts DH, Hopkins NK. Linezolid: pharmacokinetic and pharmacodynamic evaluation of coadministration with pseudoephedrine HCl, phenylpropanolamine HCl, and dextromethorpan HBr. J Clin Pharmacol 2001; 41(5):563-572. [PubMed]
64. Hendershot PE, Jungcluth GL, Cammarata SK, Hopkins NK. Pharmacokinetics of linezolid in patients with liver diseases. Journal of Antimicrobial Chemotherapy 44, Suppl.A, 55. 1999.
65. Herrero IA, Issa NC, Patel R. Nosocomial spread of linezolid-resistant, vancomycin- resistant Enterococcus faecium. N Engl J Med 2002; 346(11):867-869. [PubMed]
66. Hoppe JE. In vitro susceptibilities of Bordetella pertussis and Bordetella parapertussis to the novel oxazolidinones eperezolid (PNU-100592) and linezolid (PNU-100766). J Chemother 1999; 11(3):220-221. [PubMed]
67. Jacqueline C, Caillon J, Le Mabecque V, Miegeville AF, Donnio PY, Bugnon D, Potel G. In vitro activity of linezolid alone and in combination with gentamicin, vancomycin or rifampicin against methicillin-resistant Staphylococcus aureus by time-kill curve methods. J Antimicrob Chemother 2003; 51(4):857-864. [PubMed]
68. Johnson AP. AZD-2563 AstraZeneca. Curr Opin Investig Drugs 2002; 3(6):848-852. [PubMed]
69. Jones RN, Anderegg TR, Deshpande LM. AZD2563, a new oxazolidinone: bactericidal activity and synergy studies combined with gentamicin or vancomycin against staphylococci and streptococcal strains. Diagn Microbiol Infect Dis 2002; 43(1):87-90. [PubMed]
70. Jones RN, Biedenbach DJ, Anderegg TR. In vitro evaluation of AZD2563, a new oxazolidinone, tested against unusual gram-positive species. Diagn Microbiol Infect Dis 2002; 42(2):119-122. [PubMed]
71. Jones RN, Della-Latta P, Lee LV, Biedenbach DJ. Linezolid-resistant Enterococcus faecium isolated from a patient without prior exposure to an oxazolidinone: report from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis 2002; 42(2):137-139. [PubMed]
72. Jones RN, Johnson DM, Erwin ME. In vitro antimicrobial activities and spectra of U-100592 and U-100766, two novel fluorinated oxazolidinones. Antimicrob Agents Chemother 1996; 40(3):720-726. [PubMed]
73. Kaatz GW, Seo SM. In vitro activities of oxazolidinone compounds U100592 and U100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother 1996; 40(3):799-801. [PubMed]
74. Kaplan SL, Patterson L, Edwards KM, Azimi PH, Bradley JS, Blumer JL, Tan TQ, Lobeck FG, Anderson DC; Linezolid Pediatric Pheumonia Study Group. Pharmacia and Upjohn. Linezolid for the treatment of community-acquired pneumonia in hospitalized children. Linezolid Pediatric Pneumonia Study Group. Pediatr Infect Dis J 2001; 20(5):488-494. [PubMed]
75. Kearns GL, Abdel-Rahman SM, Blumer JL, Reed MD, James LP, Jacobs RF, Bradley JA, Welshman IR, Jungbluth GL, Stalker DJ; Pediatric Pharmacology Research Unit Network. Single dose pharmacokinetics of linezolid in infants and children. Pediatr Infect Dis J 2000;9(12):1178-1184. [PubMed]
76. Kloss P, Xiong L, Shinabarger DL, Mankin AS. Resistance mutations in 23 S rRNA identify the site of action of the protein synthesis inhibitor linezolid in the ribosomal peptidyl transferase center. J Mol Biol 1999; 294(1):93-101. [PubMed]
77. Kuter DJ, Tillotson GS. Hematologic effects of antimicrobials: focus on the oxazolidinone linezolid. Pharmacotherapy 2001; 21(8):1010-1013. [PubMed]
78. Li JZ, Willke RJ, Rittenhouse BE, Rybak MJ. Effect of linezolid versus vancomycin on length of hospital stay in patients with complicated skin and soft tissue infections caused by known or suspected methicillin-resistant staphylococci: results from a randomized clinical trial. Surg Infect (Larchmt ) 2003; 4(1):57-70. [PubMed]
79. Li Z, Willke RJ, Pinto LA, Rittenhouse BE, Rybak MJ, Pleil AM, Crouch CW, Hafkin B, Glick HA. Comparison of length of hospital stay for patients with known or suspected methicillin-resistant Staphylococcus species infections treated with linezolid or vancomycin: a randomized, multicenter trial. Pharmacotherapy 2001; 21(3):263-274. [PubMed]
80. Lin AH, Murray RW, Vidmar TJ, Marotti KR. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob Agents Chemother 1997; 41(10):2127-2131. [PubMed]
81. Livermore DM. Linezolid in vitro: mechanism and antibacterial spectrum. J Antimicrob Chemother 2003; 51 Suppl 2:II9-II16. [PubMed]
82. Lopez H, Li JZ, Balan DA, Willke RJ, Rittenhouse BE, Mozaffari E, Vidal G, Zitto T, Tang T. Hospital resource use and cost of treatment with linezolid versus teicoplanin for treatment of serious gram-positive bacterial infections among hospitalized patients from South America and Mexico: results from a multicenter trial. Clin Ther 2003; 25(6):1846-1871. [PubMed]
83. Lovering AM, Zhang J, Bannister GC, Lankester BJ, Brown JH, Narendra G, MacGowan AP. Penetration of linezolid into bone, fat, muscle and haematoma of patients undergoing routine hip replacement. J Antimicrob Chemother 2002; 50(1):73-77. [PubMed]
84. MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother 2003; 51 Suppl 2:II17-II25. [PubMed]
85. Manzor O, Pawlak J, Saravolatz L. In-vitro activity of 29 antimicrobial agents against penicillin-resistant and -intermediate isolates of Streptococcus pneumoniae. J Antimicrob Chemother 1999; 43(1):31-36. [PubMed]
86. Moellering RC Jr. A novel antimicrobial agent joins the battle against resistant bacteria. Ann Intern Med 1999; 130(2):155-157. [PubMed]
87. Moellering RC. Linezolid: the first oxazolidinone antimicrobial. Ann Intern Med 2003; 138(2):135-142. [PubMed]
88. Mulazimoglu L, Drenning SD, Yu VL. In vitro activities of two novel oxazolidinones (U100592 and U100766), a new fluoroquinolone (trovafloxacin), and dalfopristin-quinupristin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother 1996; 40(10):2428-2430. [PubMed]
89. Mutnick AH, Enne V, Jones RN. Linezolid resistance since 2001: SENTRY Antimicrobial Surveillance Program. Ann Pharmacother 2003; 37(6):769-774. [PubMed]
90. Neu HC, Novelli A, Saha G, Chin NX. In vitro activities of two oxazolidinone antimicrobial agents, DuP 721 and DuP 105. Antimicrob Agents Chemother 1988; 32(4):580-583.[PubMed]
91. Noskin GA, Siddiqui F, Stosor V, Hacek D, Peterson LR. In vitro activities of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob Agents Chemother 1999; 43(8):2059-2062. [PubMed]
92. Orrick JJ, Johns T, Janelle J, Ramphal R. Thrombocytopenia secondary to linezolid administration: what is the risk? Clin Infect Dis 2002; 35(3):348-349. [PubMed]
93. Pai MP, Rodvold KA, Schreckenberger PC, Gonzales RD, Petrolatti JM, Quinn JP. Risk factors associated with the development of infection with linezolid- and vancomycin-resistant Enterococcus faecium. Clin Infect Dis 2002; 35(10):1269-1272. [PubMed]
94. Pelaez T, Alonso R, Perez C, Alcala L, Cuevas O, Bouza E. In vitro activity of linezolid against Clostridium difficile. Antimicrob Agents Chemother 2002; 46(5):1617-1618. [PubMed]
95. Peric M, Lin G, Clark CL, Jacobs MR, Appelbaum PC. Antipneumococcal activity of AZD2563, a new oxazolidinone, compared with nine other agents. J Antimicrob Chemother 2002; 50(1):95-100. [PubMed]
96. Peters J, Kondo KL, Lee RK, Lin CK, Inderlied CB. In-vitro activity of oxazolidinones against Mycobacterium avium complex. J Antimicrob Chemother 1995; 35(5):675-679.[PubMed]
97. Phillips OA, Rotimi VO, Jamal WY, Shahin M, Verghese TL. Comparative in vitro activity of PH-027 versus linezolid and other anti-anaerobic antimicrobials against clinical isolates of Clostridium difficile and other anaerobic bacteria. J Chemother 2003; 15(2):113-117. [PubMed]
98. Prystowsky J, Siddiqui F, Chosay J, Shinabarger DL, Millichap J, Peterson LR, Noskin GA Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob Agents Chemother 2001; 45(7):2154-2156. [PubMed]
99. Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis 2001; 32(3):402-412. [PubMed]
100. Rubinstein E, Isturiz R, Standiford HC, Smith LG, Oliphant TH, Cammarata S, Hafkin B, Le V, Remington J. Worldwide assessment of linezolid's clinical safety and tolerability: comparator-controlled phase III studies. Antimicrob Agents Chemother 2003; 47(6):1824-1831. [PubMed]
101. Ruiz ME, Guerrero IC, Tuazon CU. Endocarditis caused by methicillin-resistant Staphylococcus aureus: treatment failure with linezolid. Clin Infect Dis 2002; 35(8):1018-1020.[PubMed]
102. Rybak MJ, Cappelletty DM, Moldovan T, Aeschlimann JR, Kaatz GW. Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob Agents Chemother 1998; 42(3):721-724. [PubMed]
103. Rybak MJ, Hershberger E, Moldovan T, Grucz RG. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against Staphylococci and Enterococci, including vancomycin- intermediate and -resistant strains. Antimicrob Agents Chemother 2000; 44(4):1062-1066. [PubMed]
104. San Pedro GS, Cammarata SK, Oliphant TH, Todisco T. Linezolid versus ceftriaxone/cefpodoxime in patients hospitalized for the treatment of Streptococcus pneumoniae pneumonia. Scand J Infect Dis 2002; 34(10):720-728. [PubMed]
105. Schulin T, Wennersten CB, Ferraro MJ, Moellering RC Jr., Eliopoulos GM. Susceptibilities of Legionella spp. to newer antimicrobials in vitro. Antimicrob Agents Chemother 1998; 42(6):1520-1523. [PubMed]
106. Shaikh ZH, Peloquin CA, Ericsson CD. Successful treatment of vancomycin-resistant Enterococcus faecium meningitis with linezolid: case report and literature review. Scand J Infect Dis 2001; 33(5):375-379. [PubMed]
107. Shinabarger DL, Marotti KR, Murray RW, Lin AH, Melchior EP, Swaney SM, Dunyak DS, Demyan WF, Buysse JM. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob Agents Chemother 1997; 41(10):2132-2136. [PubMed]
108. Slatter JG, Stalker DJ, Feenstra KL, Welshman IR, Bruss JB, Sams JP, Johnson MG, Sanders PE, Hauer MJ, Fagerness PE, Stryd RP, Peng GW, Shobe EM. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab Dispos 2001; 29(8):1136-1145. [PubMed]
109. Slee AM, Wuonola MA, McRipley RJ, Zajac I, Zawada MJ, Bartholomew PT, Gregory WA, Forbes M. Oxazolidinones, a new class of synthetic antibacterial agents: in vitro and in vivo activities of DuP 105 and DuP 721. Antimicrob Agents Chemother 1987; 31(11):1791-1797. [PubMed]
110. Spangler SK, Jacobs MR, Appelbaum PC. Activities of RPR 106972 (a new oral streptogramin), cefditoren (a new oral cephalosporin), two new oxazolidinones (U-100592 and U-100766), and other oral and parenteral agents against 203 penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother 1996; 40(2):481-484. [PubMed]
111. Stalker DJ, Jungbluth GL, Hopkins NK, Batts DH. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother 2003; 51(5):1239-1246. [PubMed]
112. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2002; 34(11):1481-1490. [PubMed]
113. Stevens DL, Smith LG, Bruss JB, McConnell-Martin MA, Duvall SE, Todd WM, Hafkin B. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 2000; 44(12):3408-3413. [PubMed]
114. Struwig MC, Botha PL, Chalkley LJ. In vitro activities of 15 antimicrobial agents against clinical isolates of South African enterococci. Antimicrob Agents Chemother 1998; 42(10):2752-2755. [PubMed]
115. Swaney SM, Aoki H, Ganoza MC, Shinabarger DL. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob Agents Chemother 1998; 42(12):3251-3255. [PubMed]
116. Sweeney MT, Zurenko GE. In Vitro Activities of Linezolid Combined with Other Antimicrobial Agents against Staphylococci, Enterococci, Pneumococci, and Selected Gram-Negative Organisms. Antimicrob Agents Chemother 2003; 47(6):1902-1906. [PubMed]
117. Till M, Wixson RL, Pertel PE. Linezolid treatment for osteomyelitis due to vancomycin-resistant Enterococcus faecium. Clin Infect Dis 2002; 34(10):1412-1414. [PubMed]
118. Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, Moellering RC, Ferraro MJ. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 2001; 358(9277):207-208. [PubMed]
119. US Food and Drug Administration approved labeling for linezolid. see www.fda.gov/cder/foi/label/2000/21130lbl.pdf. 2003.
120. Viale P, Pagani L, Cristini F, Stefini R, Bergomi R, Colombini P, Carosi G. Linezolid for the treatment of central nervous system infections in neurosurgical patients. Scand J Infect Dis 2002; 34(6):456-459. [PubMed]
121. Villani P, Regazzi MB, Marubbi F, Viale P, Pagani L, Cristini F, Cadeo B, Carosi G, Bergomi R. Cerebrospinal fluid linezolid concentrations in postneurosurgical central nervous system infections. Antimicrob Agents Chemother 2002; 46(3):936-937. [PubMed]
122. von Eiff C, Peters G. Comparative in-vitro activities of moxifloxacin, trovafloxacin, quinupristin/dalfopristin and linezolid against staphylococci. J Antimicrob Chemother 1999; 43(4):569-573. [PubMed]
123. Walker SE, Shulman KI, Tailor SA, Gardner D. Tyramine content of previously restricted foods in monoamine oxidase inhibitor diets. J Clin Psychopharmacol 1996; 16(5):383-388.[PubMed]
124. Wallace RJ Jr., Brown-Elliott BA, Ward SC, Crist CJ, Mann LB, Wilson RW. Activities of linezolid against rapidly growing mycobacteria. Antimicrob Agents Chemother 2001; 45(3):764-767. [PubMed]
125. Wilcox MH. Efficacy of linezolid versus comparator therapies in Gram-positive infections. J Antimicrob Chemother 2003; 51 Suppl 2:II27-II35. [PubMed]
126. Wilson P, Andrews JA, Charlesworth R, Walesby R, Singer M, Farrell DJ, Robbins M. Linezolid resistance in clinical isolates of Staphylococcus aureus. J Antimicrob Chemother 2003; 51(1):186-188. [PubMed]
127. Wise R, Andrews JM, Boswell FJ, Ashby JP. The in-vitro activity of linezolid (U-100766) and tentative breakpoints. J Antimicrob Chemother 1998; 42(6):721-728. [PubMed]
128. Wunderink RG, Cammarata SK, Oliphant TH, Kollef MH. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clin Ther 2003; 25(3):980-992. [PubMed]
129. Xiong L, Kloss P, Douthwaite S, Andersen NM, Swaney S, Shinabarger DL, Mankin AS. Oxazolidinone resistance mutations in 23S rRNA of Escherichia coli reveal the central region of domain V as the primary site of drug action. J Bacteriol 2000; 182(19):5325-5331. [PubMed]
130. Zhanel GG, Karlowsky JA, Low DE, Hoban DJ. Antibiotic resistance in respiratory tract isolates of Haemophilus influenzae and Moraxella catarrhalis collected from across Canada in 1997-1998. J Antimicrob Chemother 2000; 45(5):655-662. [PubMed]
131. Zhanel GG, Laing NM, Nichol KA, Palatnick LP, Noreddin A, Hisanaga T, Johnson JL, Hoban DJ; NAVRESS Group. Antibiotic activity against urinary tract infection (UTI) isolates of vancomycin-resistant enterococci (VRE): results from the 2002 North American Vancomycin Resistant Enterococci Susceptibility Study (NAVRESS). J Antimicrob Chemother 2003;52(3):382-8. [PubMed]
132. Zhanel GG, Palatnick L, Nichol KA, Bellyou T, Low DE, Hoban DJ. Antimicrobial Resistance in Respiratory Tract Streptococcus pneumoniae Isolates: Results of the Canadian Respiratory Organism Susceptibility Study, 1997 to 2002. Antimicrob Agents Chemother 2003; 47(6):1867-1874. [PubMed]
133. Zimmer SM, Caliendo AM, Thigpen MC, Somani J. Failure of linezolid treatment for enterococcal endocarditis. Clin Infect Dis 2003; 37(3):e29-e30. [PubMed]
134. Zurenko GE, Gibson JK, Shinabarger DL, Aristoff PA, Ford CW, Tarpley WG. Oxazolidinones: a new class of antibacterials. Curr Opin Pharmacol 2001; 1(5):470-476. [PubMed]
135. Zurenko GE, Todd WM, Hafkin BA. Development of linezolid-resistant Enterococcus faecium in two compassionate use programs related to linezolid. Program and Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy.San Francisco, USA.Abstract 848. 1999.
136. Zurenko GE, Yagi BH, Schaadt RD, Allison JW, Kilburn JO, Glickman SE, Hutchinson DK, Barbachyn MR, Brickner SJ. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother 1996; 40(4):839-845. [PubMed]
137. Zyvox [package insert]. Kalamazoo, MI: Pharmacia & Upjohn. 2003.
Tables
Table 1. In Vitro Susceptibility of Multi-Drug Resistant and Susceptible Gram-Positive, Gram Negative and Anaerobic Pathogens to Linezolid and AZD-2563.
| Organism | Linezolid MIC90 | AZD-2563 MIC90 | Reference |
|---|---|---|---|
| Gram-positive bacteria | |||
| Bacillus spp. | 1 | 1 | (72,136) |
| Corynebacterium spp. | 0.5-2 | 0.25 | (70,72,136) |
| Listeria monocytogenes | 2 | 2 | (70,136) |
| Micrococcus spp. | 2 | 2 | (70) |
| Enterococcus spp. | |||
| E. avium | 2-4 | 1 | (19,39,40) |
| E. casselliflavus | 2-4 | 2 | (19,39,40) |
| E. gallinarum | 2-4 | 2 | (19,40) |
| E. raffinosus | 2-4 | 2 | (39,40) |
| Enterococcus faecalis | 1-4 | 2 | (19,44,91,102,103,127) |
| Vancomycin – Susceptible | 1-4 | 2 | (23,39,40,72,114,131,136) |
| Vancomycin - Resistant | 2-4 | 1 | (23,39,40,131,136) |
| Enterococcus faecium | 1-4 | 4 | (19,44,91,102,103,127) |
| Vancomycin – Susceptible | 1-2 | 2 | (23,39,40,72,114,131) |
| Vancomycin - Resistant | 2-4 | 2 | (23,39,40,131,136) |
| Staphylococcus aureus | |||
| Methicillin – Resistant | 1-4 | 2 | (6,23,44,48,72,88,91,102,103,122,127,136) |
| Methicillin – Susceptible | 1-4 | 2 | (6,23,44,48,72;88,91,102,103,122,127,136) |
| Vancomycin – Intermediate | 1-2a | -- | (4,103) |
| Vancomycin – Resistant | 2a | -- | (25) |
| Staphylococcus epidermidis | |||
| Methicillin - Resistant | 1-4 | 1 | (44,48,72,73,91,102,103,122,127,136) |
| Methicillin - Susceptible | 0.5-4 | 1 | (44,48,72,73,91,102,122,127,136) |
| Streptococcus spp. | |||
| ß-Hemolytic | 2 | 2 | (5) |
| S. agalactiae | 1-2 | 2 | (17,44,72) |
| S. pyogenes | 1-4 | 2 | (17,44,72,127,136) |
| Viridans Group | 1-2 | 1 | (5,23) |
| Streptococcus pneumoniae | 1-2 | 1 | (1,17,44,72,85,91,127,132) |
| Penicillin - Susceptible | 1-2 | 1 | (15,23,95,110,136) |
| Penicillin - Intermediate | 1 | 1 | (15,23,95,110,136) |
| Penicillin - Resistant | 1-2 | 1-2 | (15,23,95,110,136) |
| Gram-negative bacteria | |||
| Bordetella pertussis | 4 | -- | (66) |
| Flavobacterium menigosepticum | 2 | -- | (35) |
| Haemophilus influenzae | 16 | -- | (72,130) |
| Leigionella spp. | 4 | -- | (105) |
| Moraxella catarrhalis | 4 | -- | (130,136) |
| Neisseria gonorrhoeae | 16 | -- | (72) |
| Pasteurella multocida | 4 | -- | (53) |
| Anaerobic bacteria | |||
| Bacteroides fragilis group | 4 | >16 | (16,29,36,97,127,136) |
| Clostridium difficile | 2-16 | 2 | (29,36,54,55,94,97,127,136) |
| Clostridium perfringens | 2 | 2 | (29,36,54,55,127,136) |
| Fusobacterium spp. | 0.5-2 | >16 | (16,29,36,97,136) |
| Peptostreptococcus spp. | 0.5-4 | 2 | (29,36,54,55,72,97,136) |
| Prevotella spp. | 1-4 | 16 | (16,29,36,72,97,136) |
a = reported as MIC
Table 2. Susceptibility Breakpoints for Linezolid Approved by the National Committee for Clinical Laboratory Standards (NCCLS)(137).
| SUSCEPTIBILITY BREAKPOINTS | Minimum Inhibitory Concentration (µg/mL) | ||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| Enterococcus spp. | ≤2 | 4 | ≥8 |
| Staphylococcus spp. | ≤4 | -- | -- |
| Streptococcus pneumoniae | ≤2 | -- | -- |
| Streptococcus spp. (other than Streptococcus pneumoniae) | ≤2 | -- | -- |
Table 3. Mean Pharmacokinetic Values for Linezolid After Intravenous and Oral Administration to Healthy Volunteers (137).
| DOSE OF LINEZOLID | Cmax µg/mL | Cmin µg/mL | Tmax H | AUC µg•h/mL | t1/2 h | CL mL/min |
|---|---|---|---|---|---|---|
| 400 mg tablet q 12h | 11.00 ± 4.37 | 3.08 ± 2.25 | 1.12 ± 0.47 | 73.40 ± 33.50 | 4.69 ± 1.70 | 110 ± 49 |
| 600 mg tablet q 12h | 21.20 ±5.78 | 6.15 ±2.94 | 1.03 ± 0.62 | 138.00 ± 42.10 | 5.40 ± 2.06 | 80 ± 29 |
| 600 mg IV injection | 15.10 ±2.52 | 3.68 ±2.36 | 0.51 ±0.03 | 89.70 ±31.00 | 4.80 ±1.70 | 123 ±40 |
± = standard deviation Cmax = Maximum plasma concentration; Cmin = Minimum plasma concentration; Tmax = Time to maximum plasma concentration; AUC = Area under concentration-time curve; t½= Elimination half-life; CL = Systemic clearance
Table 4. Adverse Events Summary Data In Phase III Comparator-Controlled Trials (46, 100).
| Adverse Event Summary | Linezolid (n=2046) | Comparator (n=2001) | P value |
|---|---|---|---|
| Patients with one or more drug-related adverse event | 21.7 | 15.7 | 0.001 |
| Treatment discontinued due to drug-related event | 2.4 | 5.2 | 0.230 |
| Patients who died | 4.8 | 4.9 | 0.596 |
| Diarrhea | 4.3 | 3.2 | 0.074 |
| Nausea | 3.4 | 2.3 | 0.036 |
| Headache | 2.2 | 1.3 | 0.047 |
| Taste alteration | 1.2 | 0.7 | 0.117 |
| Vaginal moniliasis | 1.2 | 0.6 | 0.85 |
| Vomiting | 1.1 | 0.4 | 0.008 |
| Abnormal Liver Function Tests | 1.0 | 0.3 | 0.010 |
Table 5. Dosing during Continuous Renal Replacement Therapy.
| Renal Replacement Therapy | Dose Adjustment |
|---|---|
| CVVH (Continuous venovenous hemofiltration): | 600mg q12h |
| CVVHD (Continuous venovenous hemodialysis): | 600mg q12h |
| CVVHDF (Continuous venovenous hemodiafiltration) | 600mg q12h |
Note: CVVH is mainly for fluid removal alone. Many institutions will employ more CVVHD or CVVHDF which combine dialysis with fluid removal.
Figure 1. Structures of Extensively Evaluated Oxazolidinone Antimicrobial Agents.

Figure 2. Relationship Between the Percentage of Time that Drug Concentrations Remain Above the MIC for the Dosing Interval (%T>MIC), Peak Drug Concentration Level in Serum To MIC Ratio (Peak/MIC), and the 24 H Area Under the Concentration Time Curve to MIC Ratio (AUC/MIC) and the Log10 Number Of CFU/Thigh After 24 H of Linezolid Therapy in a Murine-Thigh S. Aureus Infection Model (7).
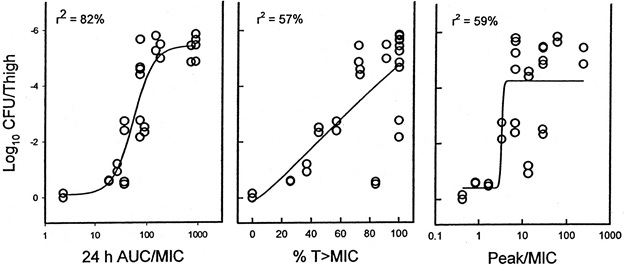
R2 = Coefficient Of Determination.
Figure 3. Scanning Electron Micrographs of a Methicillin-Resistant S. Aureus Strain Exposed to Linezolid Alone and in Combination with Gentamicin, Vancomycin Or Rifampicin. (a) Control (b) Linezolid (c) Gentamicin (d) Linezolid And Gentamicin (e) Vancomycin (f) Linezolid And Vancomycin (g) Rifampicin (h) Linezolid And Rifampicin (67).
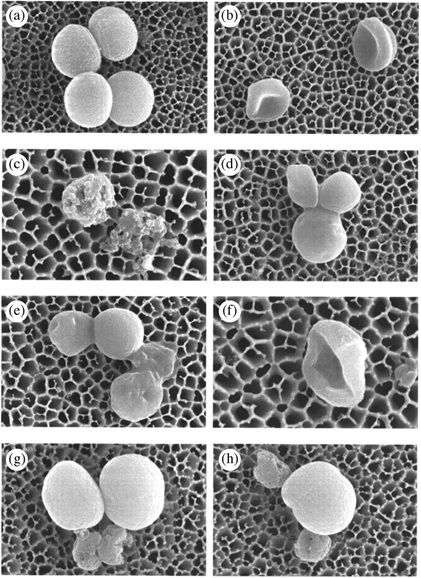
Figure 4. Mechanism of Action of the Oxazolidinone Antimicrobial Agents.
Steps At Which Other Protein Synthesis Inhibitors Act Are Shown for Comparison.
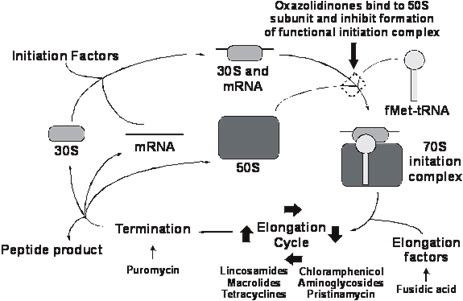
What's New
Boak LM, et al. Clinical Population Pharmacokinetics and Toxicodynamics of Linezolid. Antimicrobial Agents Chemother 2014;58:2334-43.
Gu B, et al. The Emerging Problem of Linezolid-resistant Staphylococcus. J Antimicrob Chemother 2013;68:4-11.
Nukui Y, et al. High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J Antimicrob Chemother 2013: April [Epub ahead of publication].
Cattaneo D, et al. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with Gram-positive infections. Int J Antimicrob Agents 2013;41:586-589.
Deresinski SC, In The Literature. What is the Linezolid Concentration? Clin Infect Dis 2013;57 (1 December):iii.
Butterfield JM, et al. Can Linezolid be Safely Used in Patients Receiving Serotonergic Drugs? In The Literature Section, Clin Infect Dis 2012;55:iii.
Long KS and Birte Vester. Resistance to Linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother. 2012;56(2):603-12.
Nambiar S, et al. Linezolid-Associated Peripheral and Optic Neuropathy in Children.Pediatrics. 2011 May 9. [Epub ahead of print]
GUIDED MEDLINE SEARCH FOR:
Linezolid
Therapeutic Effects
Dosage
Adverse Effects
Drug Interactions
Pharmacokinetics/Pharmacodynamics
Pharmacoeconomics
Oxazolidinones
Therapeutic Effects
Dosage
Adverse Effects
Drug Interactions
Pharmacokinetics/Pharmacodynamics
Pharmacoeconomics
Reviews
Lentino JR, Narita M, Yu VL. New Antimicrobial Agents as Therapy for Resistant Gram-Positive Cocci.
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS:
Linezolid
Therapeutic Effects
Dosage
Adverse Effects
Drug Interactions
Pharmacokinetics/Pharmacodynamics
Pharmacoeconomics
Oxazolidinones
Therapeutic Effects
Dosage
Adverse Effects
Drug Interactions
Pharmacokinetics/Pharmacodynamics
Pharmacoeconomics
