Table 1. Antibacterial Activity (MIC90/MIC90) of Selected Carbapenems
|
ORGANISM |
Imipenema |
Meropenema |
Ertapenema |
Biapenem |
Panipenem |
Faropenem |
|
GRAM-POSITIVE AEROBES |
|
|
|
|
|
|
|
Staphylococcus aureus (MS) |
0.06/0.06 |
0.12/0.25 |
0.12/0.25 |
0.12/0.12 |
-/0.25 |
0.06/0.12 |
|
Staphylococcus epidermidis (MS) |
0.01/0.12 |
0.06/0.5 |
0.12/0.5 |
0.12/1 |
-/2 |
0.06/0.5 |
|
Staphylococcus saprophyticus |
0.06/0.06 |
0.25/0.5 |
|
|
|
0.5/0.5 |
|
Streptococcus pyogenes |
<0.015/<0.015 |
0.008/0.008 |
0.008/0.015 |
0.008/0.015 |
|
0.015/0.015 |
|
Streptococcus pneumoniae (PS)b |
<0.015/<0.015 |
0.015/<0.015 |
0.015/<0.015 |
0.06/0.06 |
-/0.06 |
0.008/0.25 |
|
Streptococcus pneumoniae (PR)b |
0.03/0.12 |
0.12/0.25 |
0.5/2 |
0.5/1 |
-/0.25 |
0.06/0.5 |
|
Enterococcus faecalis |
2/4 |
4/4 |
8/>16 |
4/16 |
-/1 |
1/2 |
|
Enterococcus faecium |
32/>128 |
32/128 |
>16/>16 |
32/>128 |
-/>128 |
64/>128 |
|
GRAM-NEGATIVE AEROBES |
|
|
|
|
|
|
|
Escherichia coli |
0.12/0.25 |
0.01/0.03 |
<0.008/0.016 |
0.06/0.12 |
-/0.12 |
0.5/1 |
|
Citrobacter freundii |
0.25/2 |
0.03/0.06 |
<0.008/0.25 |
0.12/0.25 |
-/0.5 |
0.5/4 |
|
Salmonella spp. |
0.12/0.12 |
0.03/0.03 |
<0.008/0.016 |
|
|
0.5/0.5 |
|
Shigella spp. |
0.12/0.25 |
0.03/0.06 |
0.008/0.016 |
|
|
0.5/0.5 |
|
Klebsiella pneumoniae |
0.12/0.25 |
0.03/0.06 |
<0.008/0.03 |
0.25/0.5 |
-/0.12 |
0.5/2 |
|
Klebsiella oxytoca |
0.25/0.5 |
0.03/0.03 |
0.08/0.03 |
|
|
0.5/2 |
|
Enterobacter cloacae |
0.5/0.5 |
0.03/0.06 |
0.06/1 |
0.25/0.5 |
-/0.5 |
2/4 |
|
Enterobacter aerogenes |
0.5/0.5 |
0.03/0.06 |
0.06/1 |
|
|
2/4 |
|
Serratia marcescens |
0.5/1 |
0.06/0.25 |
0.03/0.12 |
0.5/4 |
-/16 |
2/32 |
|
Proteus mirabilis |
0.5/2 |
0.06/0.06 |
0.016/0.06 |
1/2 |
-/2 |
1/2 |
|
Proteus vulgaris |
2/4 |
0.06/0.06 |
0.016/0.25 |
|
|
1/4 |
|
Morganella morganii |
2/2 |
0.12/0.25 |
0.03/0.06 |
1/ 2 |
0.5/2 |
4/8 |
|
Providencia rettgeri |
1/1 |
0.03/0.12 |
0.03/0.25 |
2/>8 |
|
1/ 2 |
|
Providencia stuartii |
1/ 2 |
0.06/0.06 |
|
|
|
|
|
Acinetobacter baumannii |
1/ 2 |
1/ 2 |
4/16 |
0.5/0.5 |
|
4/8 |
|
Pseudomonas aeruginosa |
4/16 |
2/8 |
8/>16 |
4/16 |
-/32 |
>128/>128 |
|
Burkholderia cepacia |
8/8 |
2/2 |
|
|
|
16/>32 |
|
Haemophilus influenzaec,d,e |
0.12/1 |
0.12/0.5 |
0.03/0.06 |
0.5/2 |
0.03/2 |
0.5/1 |
|
Moraxella catarrhalis |
<0.015/0.03 |
<0.03/<0.03 |
<0.008/0.016 |
0.12/0.12 |
|
0.06/0.5 |
|
Neisseria gonorrhoeae (PS) |
0.12/0.5 |
0.015/0.03 |
|
|
|
0.03/0.06 |
|
Neusseria meningitides |
0.03/0.03 |
<0.004/0.016 |
|
|
|
0.008/0.008 |
|
ANAEROBES |
|
|
|
|
|
|
|
Peptococcus magnus |
0.25/1 |
0.12/0.25 |
0.5/1 |
0.5/1 |
|
0.06/0.5 |
|
Clostridium perfringens |
0.03/0.03 |
=0.008/<0.06 |
0.06/0.06 |
0.06/0.06 |
|
0.5/1 |
|
Clostridium difficile |
4/8 |
1/ 2 |
4/8 |
|
-/4 |
8/16 |
|
Bacteroides fragilis |
0.06/0.5 |
0.12/1 |
0.25/1 |
0.06/1 |
-/1 |
0.25/1 |
|
Bacteroides vulgatus |
0.5/1 |
0.5/0.5 |
|
|
|
2/4 |
|
Bacteroides distasonis |
0.12/1 |
0.25/1 |
0.5/2 |
|
|
2/2 |
|
Bacteroides thetaiotaomicron |
0.25/0.5 |
0.5/0.5 |
0.5/1 |
|
|
0.5/2 |
|
Bacteroides ovatus |
0.25/1 |
0.5/0.5 |
0.5/1 |
|
|
2/2 |
|
Bacteroides uniformis |
0.12/1 |
0.25/0.5 |
0.5/2 |
|
|
1/2 |
|
Prevotella bivia |
0.25/0.5 |
0.25/0.5 |
0.25/0.5 |
0.25/1 |
|
0.25/0.5 |
|
Fusobacterium nucleatum |
0.03/0.06 |
0.008/0.016 |
0.015/0.12 |
0.03/0.12 |
|
0.12/0.12 |
|
|
|
|
|
|
|
|
a NCCLS-recommended susceptibility breakpoints for most gram-negative and gram-positive organisms for imipenem and meropenem are: susceptible = MIC <4 mg/L, intermediate MIC >4 to <16 mg/L, resistant >16 mg/L; for ertapenem: susceptible = MIC < 2mg/L, intermediate MIC = 4, resistant > 8 mg/L.
b NCCLS-recommended susceptibility breakpoints for meropenem and ertapenem for Streptococcus pneumoniae are < 0.12 mg/L and < 1.0 mg/L, respectively.
c Includes b-lactamase positive and ampicillin-resistant non-b-lactamase positive strains.
d NCCLS-recommended meropenem susceptibility breakpoints for Haemophilus spp. and viridans streptococci are < 0.5 mg/ L.
e NCCLS-recommended ertapenem susceptibility breakpoints for Haemophilus spp. are < 0.5 mg/ L.
MS = methicillin-susceptible; PS = penicillin-susceptible; PR = penicillin-resistant.
Table 2. Mean Pharmacokinetics Parameters of Carbapenems in Healthy Volunteers at Steady State After Intravenous Infusions.
|
Drug |
Imipenem |
Meropenem |
Ertapenem |
Biapenem |
Panipenem |
Faropenem |
|
Dose |
1.0 gram |
1.0 gram |
1.0 gram |
0.3 gram |
1.0 gram |
0.3 gram (single dose) |
|
Cmax (mg/L) |
69.9 |
61.6 |
155 |
14.7 |
51.4 |
13.5 |
|
T1/2 (hour) |
1.11 |
0.98 |
3.8 |
0.96 |
1.16 |
0.88 |
|
T1/2 renal failure (hours) |
3 4 |
7 10 |
11 14 |
5 6 |
4 5 |
NR |
|
% serum protein binding |
15 |
2 |
95 |
4 |
6 |
NR |
|
V (L) |
14.4 |
12.5 |
8.2 |
9.2 |
20.0 |
NR |
|
AUC (mghr/L) |
92.5 |
90.8 |
501.4 |
29.2 |
84.8 |
26.0 |
|
Renal clearance (ml/min/1.73m2) |
130 |
176 |
13 |
110 |
105 |
NR |
|
Urinary excretion (%) |
70 |
75 |
80 |
64 |
30 |
NR |
|
|
|
|
|
|
|
|
NR = Not reported
Table 3.
Concentrations of Imipenem annd Meropenem In Body Fluids and Tissues After Single
500 mg Intravenous Doses
|
|
|
|
|
|
|
|
Body Tissue or Fluid |
Sample Times (hrs) |
Meropenem Concentration (mg/L)a |
Tissue/Fluid: Serum Ratio |
Imipenem Concentration (mg/L)a |
Tissue/Fluid Serum Ratio |
|
|
|
|
|
|
|
|
CSF (infected) |
1.5-3.5 |
0.33-6.5b |
2-52% |
1.1-2.3b |
4-16% |
|
CSF (uninfected) |
1.5-3.5 |
0.10-0.18b |
-- |
0.62-0.9b |
2-5% |
|
Tonsil |
0.5-1.5 |
0.6 |
5.3% |
2.2 |
15% |
|
Maxillary sinus mucosa |
0.5-2.5 |
-- |
7-40% |
10.7 |
32% |
|
Aqueous humor |
5-35 min |
0.52 |
3% |
1.6 |
6% |
|
Sputum |
1-1.5 |
2.2 |
8% |
1.6-2.7b |
6-11% |
|
Lung |
0.5-3.5 |
2.86-4.83b |
40% |
13.0 |
29% |
|
Peritoneal exudate |
2.5-3.5 |
6.84 |
-- |
12.9 |
43% |
|
Peritoneal fluid |
1.5-2.5 |
14.3 |
-- |
6.0 |
-- |
|
Bile |
0.5-2.5 |
-- |
0.2-16% |
3.0-5.3b |
10-26% |
|
Gall bladder |
0.5-3.5 |
0.92-3.93b |
-- |
1.8 |
11% |
|
Colon |
0.5-3.5 |
2.07-2.57 b |
-- |
1.8 |
11% |
|
Prostate |
0.5-2.5 |
2.3 |
16% |
5.3 |
28% |
|
Ovary |
0.5-4.5 |
1.23-2.76b |
-- |
13.2 |
20% |
|
Myometrium |
0.5-3.5 |
0.99-4.16b |
-- |
2.2-3.8b |
7-13% |
|
Endometrium |
0.5-3.5 |
0.99-4.16b |
-- |
2.2-3.8b |
7-13% |
|
Joint tissue & fluid |
1-2.5 |
-- |
>50% |
7.9-20.4b |
64-105% |
|
Skin |
1-2 |
3.97 |
10% |
4.3 |
7% |
|
Blister fluid |
3.5-4.5 |
1.36 |
85% |
>1.0 |
>40%
|
a = Mean concentration
b = Reported as range of actual concentrations rather than mean value
Table 4. Dosage in Patients with Normal Renal Function and Renal Insufficiency
| Drug | Caculated Creatinine Clearance | |||
|
|
>70 ml/min |
50-70 ml/min |
25-50 ml/min |
<25 ml/min |
|
|
|
|
|
|
|
Imipenem Adult |
0.25-1.0 gram q 6 h |
0.25-0.5 gram q 6-8 h |
0.25-0.5 gram q 8-12 h |
0.25-0.5 gram q 12 h |
|
Meropenem Adult Pediatrica |
1.0 gram q 8 h 20-40 mg/kg q 8 h |
1.0 gram q 8 h 20-40 mg/kg q 8 h |
1.0 gram q 12 h 20-40 mg/kg q 12 h |
0.5 gram q 12-24 h 10-20 mg/kg q 12-24 h |
|
Ertapenem Adult |
1.0 gram q 24 h |
1.0 gram q 24 h |
1.0 gram q 24 hb |
0.5 gram q 24 h |
|
Biapenem Adult |
0.3 gram q 12 h |
0.5-1.0 gram q 12 h |
0.5-1.0 gram q 24 hb |
0.15-0.3 gram q 24 hb |
|
Pamipenem Adult Pediatric |
0.5-1.0 gram q 12 h 10-25 mg/kg q 8 h |
0.5-1.0 gram q 12 h 10-25 mg/kg q 8 h |
0.5-1.0 gram q 24 hb 10-25 mg/kg q 12 hb |
0.25-0.5 gram q 24 hb 10-25 mg/kg q 24 hb
|
a = Recommended for children > 3 months of age.
b = Estimated doses based on pharmacokinetic alterations; no formal recommendations available.
Table 5. Dosing During Continuous Renal Replacement Therapy (Imipenem)
|
CVVH (Continuous venovenous hemofiltration): 250mg IV q6h OR 500mg IV q8h |
|
CVVHD (Continuous venovenous hemodialysis): 250mg IV q6h OR 500mg IV q6-8h |
|
CVVHDF (Continuous venovenous hemodiafiltration) 250mg IV q6h OR 500mg IV q6-8h |
Note: CVVH is mainly for fluid removal alone. Many institutions will employ more CVVHD or CVVHDF which
combine dialysis with fluid removal.
Table 6. Dosing During Continuous Renal Replacement Therapy (Meropenem)
|
CVVH (Continuous venovenous hemofiltration): 1g IV q12h |
|
CVVHD (Continuous venovenous hemodialysis): 1g IV q12h |
|
CVVHDF (Continuous venovenous hemodiafiltration) 1g IV q12h |
Note: CVVH is mainly for fluid removal alone. Many institutions will employ more CVVHD or
CVVHDF which combine dialysis with fluid removal.
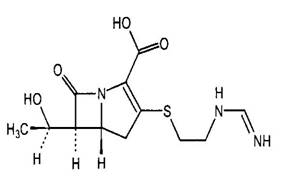
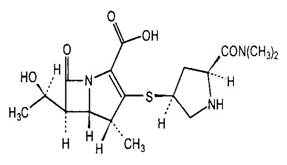
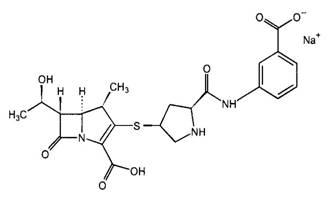
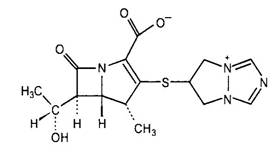
Figure 1. Chemical structures of selected carbapenems.
 |
 |
| Imipenem | Meropenem |
 |
 |
| Ertapenem | Biapenem |
 |
 |
``````````````````````````````````````````````````````````````````````