Trichosporon species and Blastoschizomyces capitatus
Authors:Nikolaos V. Sipsas, M.D., Dimitrios P. Kontoyiannis, M.D, ScD
Microbiology
Since its first description in 1865 as a peculiar gritty nodules on the hair of a wig, the so-called white piedra (6,8), for the last 3 decades Trichosporon spp have been increasingly recognized as opportunistic pathogens capable of causing invasive disease, especially in immunosuppressed patients (102).
The members of the genus Trichosporon belong to the basidiomycetous yeasts. Ultrastructural and molecular studies have shown that basidiomycetous yeasts are distributed among the phylogenetic lines of the Basidiomycota, the Hymenomycetes, Urediniomycetes, and Ustilaginomycetes (92). Analysis of the D1/D2 region of rRNA genes categorized the Hymenomycetes into four major clades: the Tremellales, the Filobasidiales, the Trichosporonales and the Cystofilobasidiales. With the exception of T. pullulans, which is classified in the Cystofilobasidiales, all other Trichosporon species are grouped in the order Trichosporonales (19).
Multiple species of the genus Trichosporon used to be called collectively T. beigelii, although this designation referred to a heterogeneous group of organisms. This misclassification was the result of the absence of simple methods to distinguish among the species in the microbiology laboratory. Guého and colleagues (29,30), using the characteristics of morphology, ultrastructure, physiology, the ubiquinone system, as well as molecular studies, performed an extensive taxonomic revaluation of the genus Trichosporon. Using strains recovered from humans, animals and environmental sources, he showed that the formerly used designation T. beigelli includes several genetically different species. A total of 19 taxa were distinguished within the genus. Subsequent studies using DNA sequence analysis of the internally transcribed spacer regions (89) and the D1/D2 region of the large subunit (26) rRNA gene, described 15 additional species (58).
Today, the genus Trichosporon comprises 34 species or varieties (19), six of which, theT. asahii, T. asteroides, T. cutaneum, T. inkin, T. mucoides, and T. ovoides are human pathogens causing superficial and disseminated infections (74). Recently T. loubieri (50), and T. pullulans(25) have also been reported as a causes of invasive human disease. It has been suggested that each species is associated with a different type of infection. More specifically:
- T. asahii, and T. mucoides appears to be much more common in cases of systemic mycosis, in immunocompromised patients.
- T. inkin is associated with pubic white piedra and T. ovoides is associated with white piedra of the head.
- T. asteroides and T. cutaneum are associated with superficial skin lesions
However, these correlations are rather arbitrary, since in the majority of published reports, isolates were identified with the older, inclusive terms T. beigelii or T. cutaneum. More recently, T. asahii, T. mucoides T. ovoides, T. montevideense, and T. domesticum were implicated in hypersensitivity pneumonia cases (88).
Trichosporon spp. are characterized by the production of true hyphae, pseudohyphae, arthroconidia and blastoconidia. The genus Trichosporon is morphologically similar toCryptococcus and the cell wall antigen of Trichosporon species cross-reacts with the capsular polysaccharide of C. neoformans; therefore the assay for the cryptococcal polysaccharide antigen may be positive in patients with trichosporonosis (52).
Trichosporon species do not ferment carbohydrates but do assimilate glucose, galactose, sucrose, maltose, and lactose and hydrolyse urea (41). There is no sexual state known for the genus.
Trichosporon species can be easily isolated from clinical specimens since it grows rapidly on almost all standard fungal media. Colonies usually grow within 10 days at 28oC and 35oC, are cream- or beige-colored and generally appear flat with a farinose covering (2). Microscopy shows disarticulating, nonbranching, septate, hyaline hyphae, and abundant, rectangular arthroconidia. Pseudohyphae and blastoconidia may also be present.
In clinical practice, identification of Trichosporon spp depends on cell and colony morphology and biochemical characteristics. Practically, a yeast-like organism that forms arthroconidia and is urease positive can be presumptively identified as Trichosporon spp. Additional tests of carbohydrate assimilation, nitrate assimilation, and phenol oxidase as well as temperature studies can confirm the diagnosis. Distinguishing between the Trichosporon species requires the technician to have experience, is time consuming and sometimes the available methods are not discriminative. Therefore, molecular methods such as sequencing of the ITS region located between the 18S and 26S rRNA genes and comprising ITS-1 and ITS2 (69) and the intergenic spacer (IGS) region located between the 26S and 5S rRNA genes, have been used successfully for the identification of Trichosporon to the species level (89). It appears that IGS is superior to ITS for identifying the different species in the Trichosporon genera, especially, when these species are phylogenetically closely related (74).
Blastoschizomyces capitatus was described in 1945 as T. capitatum (15) and it was placed in the genus Trichosporon. Later it was transferred to the genus Geotrichum (98), because of its physiologic characteristics suggesting a closer affinity to ascomycetous rather than basidiomycetous yeasts. Finally, it was reclassified to the new genus Blastoschizomyces, which was established to accommodate this fungus (76). However, from a taxonomic point of view, some authors maintain that G. capitatum is the correct anamorphic name (13). B. capitatus is implicated in disseminated infections in immunocompromised patients (25), although cases of systemic infections in immunocompetent patients have been described (105).
Blastoschizomyces capitatus grows quickly as cream-colored, smooth or wrinkled, round to oval colonies on blood agar, Sabouraud glucose agar, and on most mycologic media (47). Microscopically, the morphologic characteristics of B. capitatus include septate, branching hyphae, with narrow angle branching, that disarticulate to form arthroconidia with tapered ends at the tip of proliferating cells, the so-called annelloconidia.
B. capitatus can easily be mistaken for other morphologically similar yeast-like pathogens, namely Trichosporon species and Geotrichum candidum. The characteristics that help to distinguish B. capitatus include a) formation of anneloconidia at the tip of a conidiogenous cell, b) the urease test; all Trichosporon species are urease positive, B. capitatus is always urease negative, c) growth at 45o C on standard media d) resistance to cycloheximide (68).
Epidemiology
Trichosporon species have various habitats in nature and they occupy narrow, circumscribed, ecological niches (28). They can be found, predominantly in soil, but also in water, air, and on plants (90). Some Trichosporon species are associated with animals, as they have been cultured from animal droppings (30). Human white piedra can result from contact with infected horses, monkeys, dogs, or other animals. (42). Regarding the hospital environment, there are no hospital environment sampling studies reporting any isolates of these fungi (79). Yet, in a recent study from 3 different ICUs, reporting 6 patients with invasive Trichosporon infection, the isolates of T. asahii shared a similar phenotype and genotype, suggesting a common nosocomial origin (106). Contamination of endoscopes with Trichosporon spp has also been reported (85,43).
Humans can be colonized with Trichosporon species. Gastrointestinal tract, skin, mucosal surfaces, stools, central venous catheters, sputum and hair have all been reported as sites of colonization (30).
Colonization on human hair may occur as a consequence of poor personal hygiene, washing of hair in stagnant water, persistence of warm and moist conditions on the scalp, excessive use of hair oil, and irregular combing habits (38) According to published studies, the rates of colonization vary from 1 to 3 percent in patients admitted to general hospital wards (75,33), although skin colonization rates as high as 12.4 percent have been reported among asymptomatic outpatient volunteers (18). A Danish study reported rectal colonization rates of 13 percent among male homosexuals and 2.5 percent among male heterosexuals, who attended a sexually transmitted diseases clinic (96).
Geographical distribution of infections due to Trichosporon species varies. White piedra is most prevalent in temperate and semitropical climates, such as South America, the Middle East, India, Southeast Asia, Africa, Europe, Japan, and parts of southern USA (80). In a report from a clinic at Houston, Texas, genital white piedra was more typical in black men compared with white men or those of other ethnic origins, and it was found in 40% of young men who presented with various genital symptoms (34). A study, carried out in Libreville (Gabon), an equatorial region of Africa (95), showed that the incidence of this infection is 18% of inguinal specimens in a population of 449 women aged 15-60 years, with predominance in young patients (15-44 years). Another study reporting 8 cases of scalp white piedra in Northeastern United States suggested resurgence and underreporting of this superficial mucosis (38). The majority of cases of white piedra, occur in children and young adults, who are most often women.
The mode of transmission of superficial Trichosporon spp infections is not clear. Close contact is a risk factor, since two cases of sibling pairs with white piedra have been reported (108,38). Other reported risk factors include poor hygienic habits, such as bathing in stagnant waters, long hair, and humidity (37). Sexual transmission has been reported in cases of pubic white piedra (34,87). Finally, immigration from tropic endemic areas might be associated with the occurrence of the disease in temperate regions (80).
In the past 3 decades Trichosporon spp have been implicated in invasive infections in the immunocompromised host, even though, there are rare reports of trichosporonosis in immunocompetent patients (71, 62). Most cases have been reported among neutropenic patients with hematological or solid organ malignancies, and bone marrow or solid organ transplantation. In a recent series, the overall incidence of trichosporonosis was 8 cases per 100.000 admitted patients with cancer, and it was significantly higher among patients with hematological malignancies (81/100.000) than patients with solid tumors (3/100.000) (39). An Italian retrospective study reported an incidence of 4 cases per 1.000 adult patients with leukemia (25). The incidence of trichosporonosis may be underestimated as an autopsy study from Japan found the disease in 7 of 203 autopsy patients with malignancy, and only 2 of them have been diagnosed correctly ante mortem (93).Other patients at risk for invasive disease include premature infants with a low birth weight (21), patients with AIDS (7,40), extensive burns (32), intravascular catheters (20,39) and patients receiving corticosteroids (86) or undergoing heart valve surgery (70,11), liver transplantation (1), and patients on dialysis (44). Recently, there has been a report of disseminated disease among non-neutropenic, ICU patients (106). The reported risk factors for these patients include mechanical ventilation, trauma, intravascular catheters, and prolonged use of broad-spectrum antibiotics.
The geographical distribution of invasive Trichosporon infections is also interesting. A review of the literature through 1988 noted concentration of these infections in the USA (47). However, a recent review (25) and data collected during the ARTEMIS DISK Surveillance Study contacted between 1997 and 2005 in more than 30 countries (67) did not confirmed this finding, as Trichosporon species strains were recovered with equal frequencies in Europe, The Americas, and Asia.
Blastoschizomyces capitatus is a commonly found soil saprophyte, and is a normal constituent of the flora of the skin, the gastrointestinal and respiratory tract (68). The 2 largest published series (25,48) of patients with infection caused by this organism failed to reveal a common environmental source (nosocomial or not). It is an opportunistic pathogen causing systemic infection in a variety of immunocompromised hosts, although cases in immunocompetent patients have been described. The vast majority of cases (>85%) have been reported among neutropenic patients with hematological malignancies. Cardiac valve replacement in immunocompromised patients has also led to disseminated infections with this fungus (68).
Regarding the geographical distribution, early and recent studies have shown a significantly higher frequency of B. capitatus infections in Europe, which accounts for almost 80% of the reported cases (47,25). Furthermore, 87% of the European cases have occurred in the Southern Mediterranean regions. A recent study noted clustering of the Italian cases in central and southern Italy (25). All these geographical distribution data taken together suggest that climatic factors might play a role in the epidemiology of this infection, which is favored in warmer climates.
Clinical Manifestations
Trichosporon spp are causing both superficial and systemic infections and are implicated as a cause of hypersensitivity pneumonia in Japan. The disseminated disease is called trichosporonosis, whereas the innocuous superficial hair infection is called trichosporosis by some authors.
Superficial Infection
White piedra (Spanish for stone) is an infection of the hair shaft caused by members of the genous Trichosporon. The disease presents as soft, pasty, white to tan, or even reddish-green, irregular nodules along the hair shaft. The nodules are easily detached but, when left in place, the hair shaft becomes weakened and breaks at the site of the nodule, while the affected hair can become rough and brittle. Hyperkeratosis of the scalp is occasionally observed. Skin irritation and pruritus are the main symptoms, although patients are usually asymptomatic and seek medical attention only when they notice the nodules on the affected hair. White piedra can be found in all hairy areas of the body, including the scalp, facial hair (beard, moustache, eyebrows, and eyelashes), genital hair and even axilla. The species implicated in this cutaneous infection are mainly T. asahii, T. cutaneum, and T. mucoides, for scalp infections and T. inkin for genital infections.
Invasive Infection
Invasive infections caused by Trichosporon spp are collectively referred as trichosporonosis and can be divided into 3 forms: disseminated disease, disease localized to major organs and catheter-related infections without tissue invasion. The disseminated form is more common, affecting mainly neutropenic patients with hematological malignancies, although a shift to a predominance of catheter-related fungemia, without evidence of organ involvement, has been reported recently in a US hospital. The proposed explanation for this change is the increased use of CVCs and the widespread use of fluconazole prophylaxis, which prevents tissue invasion among hematological and BMT patients (39). In a recent review of the literature 50% of all reported Trichosporon spp infections were classified as disseminated; 16% of the cases had disease restricted to the lung and 3.2% had focal hepatosplenic involvement (25).
In the majority of the reported older cases T. beigelli was reported as the causative species. After the revised classification in 1995, and the introduction in the clinical practice of methods to distinguish between species, it seems that T. asahii and less frequently T. mucoides, and T. inkin, are the main causative agents of serious systemic infections in immunocompromised patients.
The clinical picture of trichosporonosis resembles that of invasive candidiasis, i.e. a neutropenic patient with an acute febrile illness not responding to empirical broad- spectrum antibiotics or even to empirical antifungal agents. The patient may develop rapidly multiorgan failure, and become septic. Fungemia is present in the vast majority of cases (>70%). The prognosis is dismal as the crude mortality rate is as high as 77%, in the published cases (25)
Pulmonary involvement has the signs and symptoms of a fungal pneumonia, such as rapidly progressive dyspnea, cough, and production of scant, bloody sputum (94). Chest radiographs usually show diffuse alveolar infiltrates. Other observed patterns include focal infiltrates, diffuse interstitial infiltrates, patchy reticulonodular infiltrates, and cavitation (61). In a published series, the infiltrates appeared 8 to 20 days before death and progressed rapidly in both lungs (94).
Chronic hepatosplenic trichosporonosis, a disease mimicking chronic hepatosplenic candidiasis, has been described in few patients recovering from neutropenia (99). This entity is characterized by persisting fever, multiple low-attenuation lesions in the liver and spleen, and elevated serum alkaline phosphatase concentrations (39). Liver and spleen can also be involved acutely, during disseminated trichosporonosis and the patient presents with fever, nausea, vomiting and right-quadrant discomfort (64,9). The abdominal CT shows multiple hypodense lesions in the liver and splenic infracts (97).
Renal involvement has been found in the majority of autopsies of patients with disseminated trichosporonosis (93). The patients usually have microscopic hematuria and proteinuria and may progress to renal failure.
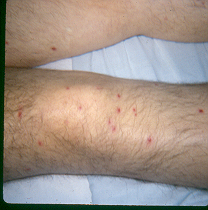
Skin lesions are a common clinical finding as they are present in about one-third of patients with disseminated trichosporonosis. The cutaneous manifestations are similar to those of disseminated candidiasis and include macules, papules which can develop central necrosis and appear as eschars, vesicopustules, and nodules on the trunk, extremities, or over the entire body (66) (Figures 1 and 2). Skin biopsy and culture are necessary to establish the diagnosis (60).
CNS infection due to Trichosporon spp, have been described among patient with hematological malignancies receiving chemotherapy. Intrathecal administration of cytotoxic agents may cause direct inoculation of the pathogen and CNS invasion. The predominant symptoms are headache, fever, nausea, and vomiting (91). Endophthalmitis, brain abscess and myositis after disseminated trichosporonosis have been reported (31,102, 56).
Trichosporon infections of a single organ without disseminated disease have been described among non-neutopenic patients. The reported cases of localized infections involved the heart valves, central nervous system, peritoneum, and surgical wounds. Endocarditis due toTrichosporon spp affects mainly prosthetic valves (49,70), although cases of native valve endocarditis have been described among intravenous drug users. Trichosporon endocarditis is characterized by large vegetations, which tend embolize the lower limbs and the CNS. In a review of 11 published cases, clinical symptoms developed 2 months to 7 years after surgical valve replacement, embolic complications have been reported in 27% of the patients, and the mortality rate was 82% (59). Cases of peritonitis due to Trichosporon spp were described as a complication of chronic ambulatory peritoneal dialysis (44). Sternal wound infection following cardiac surgery (11), pneumonia (57), meningitis (51), post-operative endophthalmitis (84), and subcutaneous infection (86) due to Trichosporon spp have all been described in immunocompetent patients. A case of breast implant infection caused by Trichosporon beigelii has also been reported (72)
In addition to infection Trichosporon species have also been implicated in the development of summer-type hypersensitivity pneumonitis in Japan (88) This form of pneumonitis affects individuals possibly with an underlying genetic predisposition, and follows the development of type III or IV allergies by repeated inhalation of Trichosporon arthroconidia, which contaminate home environments during the summer season. After decontamination of the homes of affected patients, the incidence of the disease decreases (104). The strains that play the most significant role in the development of summer-type hypersensitivity pneumonitis are T. dermatis, T.asahii, and T. montevideense (88).
The clinical presentation of disseminated infections due to B. capitatus is similar to those of trichosporonosis and invasive candidiasis. Affected patients are typically neutropenic with hematological malignancies who present with fever not responding to broad-spectrum antibiotics and develop rapidly multiorgan failure and signs of sepsis. The prognosis is poor as the reported mortality rates ranged from 60% to 80% (48,25). More than 70% of patients have fungemia and 60% to 80% develop deep organ involvement. In a literature review, out of 88 cases of B. capitatus infections among patients with hematological malignancies 46.6% had disseminated infection, 15.9% had fungemia without tissue invasion and 19.3% had lung involvement (25). Pulmonary disease is manifested with cough, dyspnea and infiltrates or nodules on the chest x-ray (10). Skin lesions are rather infrequent and consist of necrotic papules. CNS involvement is characterized by meningitis and abscesses formation (24). Hepatosplenic involvement is associated with abnormal liver function tests and nodular lesions in the imaging studies (48). Other organs affected include bone and joints, esophagus, kidney, and palate (25).
Although infections caused by B. capitatus affects almost exclusively patients with some form of immunosuppression, there are cases in immunocompetent patients such as pneumonia (105) and endocarditis of prosthetic cardiac valves (68).
Laboratory Diagnosis
The diagnosis of white piedra is based on clinical findings and confirmed by microscopy and culture. The characteristic nodules on the shafts of affected hairs are visible to the naked eye and they do not fluoresce on Wood’s light examination. Examination of an affected hair mounted on a slide with 10 percent potassium hydroxide reveals sleeve-like concretions that are composed of arthrospores and blastospores. Hair-shaft nodules are outlined with hyaline arthroconidia, 2–4 septate hyphae, and differentiated blastoconidia that arise from loosely packed hyphae (12). A fungal stain, such as chlorazol black E stain or Parker blue-black ink, is added to delineate the hyphae. Culture on Sabouraud’s agar reveals the characteristic creamy colonies of Trichosporon in a few days (38).
In patients with disseminated trichosporonosis, cultures of blood, urine, spinal fluid and tissue can yield the fungus. Trichosporon spp are isolated from blood in over 70% of invasive infections (25). The commercial fungus identification system API is capable of identifying T. asahii and T. inkin, but not effective for the other species (Li HM). Biochemical tests are also of little value to identify Trichosporon to the species level (74). Therefore, molecular studies, such as sequencing of the IGS1 region of the rRNA gene, may be necessary for the final diagnosis.
If the patient has skin lesions biopsy and subsequent tissue culture and histopathology may be helpful in establishing the diagnosis in disseminated infection. Pathology of the lesions usually reveal invasion of the skin by hyphae and arthroconidia, and sometimes vasculitis (60).
There is no consensus on the significance of recovery of Trichosporon spp from respiratory tract specimens such as sputum (94), and alveolar lavage during bronchoscopy. Since the fungus is potentially part of normal flora of the respiratory tract it is difficult to distinguish between infection and colonization. However, a study from Houston showed than 6 out of 9 patients whose sputum and bronchial lavage specimens had repeatedly grown Trichosporon spp developed ultimately invasive pulmonary infections (39). Therefore, investigators believe that in a neutropenic host with pneumonia, in the absence of other identifiable pathogens, the culture of this organism from respiratory tract specimens is indicative of pulmonary trichosporonosis.
As it was mentioned above, patients with disseminated trichosporonosis may have a positive Cryptococcus neoformans polysaccharide antigen assay due to cross-reactivity (55). Yet, this is not always true; therefore a negative cryptococcal polysaccharide antigen assay does not exclude trichosporonosis (32). The same applies to cerebrospinal fluid cryptococcal antigen assay, which may be positive in patients with meningitis due to Trichosporon spp. (91) It should be noted the lack of available serologic tests for the detection of Trichosporon spp, while PCR is a promising, yet investigational method (54,77,61).
B. capitatus infection is characterized by a high percentage (>70%) of positive blood cultures (48). In cases with CNS involvement CSF culture may be positive (24). Positive cultures of urine, sputum, stool, and oral swab may represent transient colonization or infection. In an epidemiological survey from Italy (25), among 26 patients with such positive cultures 13 (50%) developed invasive infections, while 5 patients with pulmonary infiltrates had sputum yielding B. capitatus. These data suggest that in the context of neutropenia and an organ disease such as pneumonia, a positive mucosal culture for B. capitatus should be taken seriously. There are no specific serologic tests for this fungus, and there is no cross-reactivity with cryptococcal polysaccharide antigens. Yet, B. capitatus produces a soluble antigen that cross-reacts withAspergillus galactomannan (23). Therefore, a positive galactomannan assay in a neutropenic patient with a clinical picture suggestive of disseminated candidiasis should raise the suspicion forB. capitatus infection.
As a general rule, in the neutropenic patient isolation of a Trichosporon species or B. capitatus from a sterile body site should be taken into account and prompt further evaluation, while mucosal cultures growing these fungi should interpreted in the clinical context, because they may represent colonization.
Pathogenesis
In white piedra, patients have scalp colonization with Trichosporon, which begins to grow just beneath the cuticle of hair shafts and leads to nodule formation along the shaft (38). The fungus can also affect the skin of the scalp causing occasionally hyperkeratosis. It is possible that the colonization involves the follicular orifices, resulting in low clearance and high relapse rates when topical treatment is used alone (80). There is limited evidence that white piedra is a mixed infection caused by the synergistic action between Trichosporon spp and specific strains ofCorynebacterium, which enhance the growth of the fungus resulting in invasion of the hair cuticle and cortex (108) In genital white piedra, contamination of the patient’s underwear may result in re-infection (63).
As it happens with most other invasive mycoses, trichosporonosis begins with colonization of mucosal surfaces by Trichosporon spp. Concurrent use of broad-spectrum antibiotics increases the extent of colonization by compromising the normal flora. Seeding ofTrichosporon in the blood stream occurs after breaks of the integrity of mucosa, such as chemotherapy-induced epithelial damage, insertion of intravascular catheters, skin lesions, or burns. The ability of T. asahii to form a biofilm on catheters may be a major pathogenetic factor, as it protects from antifungal agents and host defences (14). Direct inoculation of the fungus is another pathogenetic mechanism, resulting in invasive localized infection. Examples of this mode of infection include prosthetic valve endocarditis due to Trichosporon spp or B. capitatus, as a result of contamination of the prosthesis during the operative procedure, native valve endocarditis in intravenous drug users, endophthalmitis after ocular surgery, peritonitis in chronic ambulatory peritoneal dialysis, meningitis after lumbar puncture or intrathecal drug administration, and post-operative wound infection. Invasive trichosporonosis due to direct inoculation may happen in both the immunocompetent and immunocompromised host.
In vitro studies investigated the response polymorphonuclear leukocytes (PMNs) and human monocytes (HM) to Trichosporon spp. and showed that this fungus is more resistant to phagocytosis by PMNs and monocytes than are isolates of C. albicans (46,78). Pretreatment with colony-stimulating factors and gamma-interferon did not enhance fungal killing by PMNs but did increase killing by HM (46). These data suggest that Trichosporon is more pathogenic than Candida and that human monocytes are more important in the control of trichosporonosis.
Trichosporon spp are capable of producing glucuronoxylomannan (GXM) antigen, an extractable, heat-stable antigen that shares antigenic determinants with glucuronoxylomannan of the capsular polysaccharide of C. neoformans (52). Several studies suggested that GXM may be a virulence factor of Trichosporon. It has been shown that pathogenic Trichosporon isolates produced significantly more antigen than superficial or environmental isolates (45) and that after repeated subculture or passages of Trichosporon species in vivo, in mice, their phenotype undergo changes in order to produce more GXM. This process may allow a part of the fungal population to escape eradication by the host immune system, as GXM antigen is considered to protect the fungi against phagocytosis by polymorphonuclear leukocytes (35).
Several murine models of experimental disseminated trichosporonosis have been developed (26,107,3). It has been shown that after inoculation of the neutropenic animal withTrichosporon arthroconidia the pathogen disseminated rapidly, involving numerous organs including the heart, brain, kidneys, lungs, and liver. The heart and kidneys of the infected animals showed evidence of infection as early as 6 hours following inoculation. Histologic investigation showed necrotizing abscesses with conidial and hyphal elements and neutrophil and macrophage infiltration in all major organs examined. Another finding was multiple emboli consisting of hyphae and occluding small blood vessels; therefore the lesions observed in major organs of patients with trichosporonosis may be a result of angioinvasion.
SUSCEPTIBILITY IN VITRO AND IN VIVO
Single Drug
Isolates of Trichosporon spp (n=443) and B. capitatus (n=86) were collected during the ARTEMIS DISK Global Antifungal Surveillance Study contacted between 1997 and 2005 from 134 study sites in 40 countries (67), and were analyzed for susceptibility to fluconazole and voriconazole, using the Clinical Laboratory Standards Institute (CLSI) disk diffusion testing. The results are shown on Table 1. In general, these organisms appear less susceptible to fluconazole than Candida spp. as the resistance rates were around 10%, depending on the species. However, they remain susceptible to voriconazole. Unfortunately, fluconazole-resistant isolates of these yeasts also exhibit decreased susceptibility to voriconazole (Table 2).
Regarding the other antifungal agents, in vitro susceptibility studies by the CLSI methods are limited and include small numbers of isolates (65, 81,5). As it is shown on (Table 1),Trichosporon spp exhibit variable (but elevated) MICs for amphotericin B and moderate susceptibility to fluconazole and itraconazole. Amphotericin B lacks fungicidal activity againstTrichosporon spp (36). At least 3 studies found non-T. asahii isolates to be more susceptible thanT. asahii isolates to amphotericin B, fluconazole, and itraconazole. Trichosporon spp exhibit an intrinsic resistance to echinocandins, as evidenced by the high MICs reported so far (Table 3), and the reports of breakthrough infections among patients receiving these antifungals (53,27). Breakthrough infection has been reported in a patient receiving posaconazole (73). B. capitatuswith the exception of echinocandins exhibit susceptibility to most antifungals, especially to amphotericin B, itraconazole, posaconazole, and voriconazole (Table 3), although isolates with decreased susceptibility to these agents have been observed.
Combination Drugs
In a murine model of disseminated trichosporonosis, micafungin in combination with AMB showed a synergistic effect and demonstrated a higher degree of efficacy in prolonging survival and reducing the kidney fungal burden than either agent alone (82). In a similar study, the activity of the combination of amphotericin B deoxycholate plus fluconazole appeared to be superior to that of either agent alone (3). On the contrary, in a murine model of blastoschizomycosis, amphotericin B combined with micafungin, flucytosine or voriconazole was not more effective than fluconazole (83).
ANTIMICROBIAL THERAPY
Drug of Choice
The treatment of white piedra is difficult because the skin is colonized and serves as a resistant reservoir of the pathogen; therefore there is a high relapse rate and possibly re-infection due to contaminated clothes. The American Academy of Dermatology Guidelines Committee suggested in 1995 complete removal of the infected hair by shaving and application of topical antifungals, including ciclopirox, selenium sulphide 1–2.5% lotion, 6% precipitated sulphur in petroleum, chlorhexidine solution, Castellani paint, pyrithione zinc, 2–10% glutaraldehyde, imidazoles, and amphotericin B lotion (17). The need for shaving the infected hair is not universally accepted (37). The duration of treatment is until disappearance of all nodules and negative cultures.
Shaving and topical remedies may work for genital white piedra, but it is difficult as therapeutic option for the white piedra of the scalp hair, because shaving of the scalp hair is often not acceptable for cosmetic reasons, especially by young women who are mainly affected. Recent clinical studies suggested that an oral azole, such as itraconazole (100mg once a day), for 3 weeks to 1 month, in combination with topical azole antifungal shampoos for 2-3 months is an effective treatment without the need for scalp shaving (37).
Disseminated trichosporonosis, especially in a neutropenic patient should be treated with voriconazole, which should be considered as drug of choice based on the existing limited data (25,67). Fluconazole, and itraconazole should be considered as second line antifungals, based on elevated MICs and moderate susceptibility (Tables 1 and 2). Amphotericin B is an alternative, although breakthrough infections and resistance problems have been reported (99,4). It should be pointed out that echinocandins are not effective in trichosporonosis, as breakthrough infections have been described in patients receiving these antifungals (53,27).
B. capitatus infections should be treated with amphotericin B, since in 2 recent series of leukemic patients this antifungal showed clinical and microbiological efficacy (25,48). The suggested regimen is liposomal amphotericin B (5 mg/kg/day), IV. It should be noted however that in published series up to 36% of infections occurred as breakthrough infection in patients receiving amphotericin B or fluconazole as treatment or prophylaxis (25,48).
Special Infections (e.g., endocarditis, meningitis, etc.)
The treatment of Trichosporon endocarditis should be aggressive, because of the high mortality rate, and include surgical valve replacement and lengthy medical therapy with voriconazole (70).
A case of Blastoschizomyces capitatus meningitis occurring in an allogeneic bone marrow recipient on steroid and cyclosporine therapy for chronic graft-versus-host disease was treated successfully with an 11-month course of oral fluconazole (24).
Alternative Therapy
In patients who cannot be treated with voriconazole because of hepatotoxicity or other side effects amphotericin B, fluconazole, itraconazole and posaconazole might be alternative antifungals, although treatment failures and breakthrough infections have been reported (4,73). In another report from an ICU two patients infected with multi-drug resistant strains of Trichosporonwere cured with liposomal amphotericin B (106). Echinocandins are not a therapeutic option.
In patients with B. capitatus infections who cannot tolerate intravenous amphotericin B, the alternative options include voriconazole, and posaconazole (10,22), and combination of high dose fluconazole plus amphotericin B (25,48).
Combination Therapy
Successful treatment of disseminated trichosporonosis in a neutropenic patients using lipid complex amphotericin B (5 mg /kg/day) plus 5-fluorocytosine (100 mg kg/day) has been reported (101). In a report from a burn center combination of high dose fluconazole (800 mg QD, IV) with amphotericin B deoxycholate (0.8mg/kg/day, IV) resulted in microbiological cure, but eventually the patient died from bacterial infections (106).
ADJUNCTIVE THERAPY
Catheter removal and administration of colony stimulating factors might be of help (16) in neutropenic patients with disseminated infections with Trichosporon or B. capitatus.
ENDPOINTS FOR MONITORING THERAPY
Medical treatment of trichosporonosis in a neutropenic patient should be continued until the resolution of both neutropenia and fever, resolution of imaging findings and negative cultures.
VACCINES
No vaccines are available.
REFERENCES
1. Abdala E, Lopes RI, Chaves CN, Heins-Vaccari EM, Shikanai-Yasuda MA. Trichosporon asahii fatal infection in a non-neutropenic patient after orthotopic liver transplantation. Transpl Infect Dis. 2005;7:162-5 [PubMed]
2. Ahmad S, Al-Mahmeed M, Khan ZU. Characterization of Trichosporon species isolated from clinical specimens in Kuwait. J Med Microbiol. 2005;54:639-46. [PubMed]
3. Anaissie EJ, Hachem R, Karyotakis NC, Gokaslan A, Dignani MC, Stephens LC, Tin-U CK. Comparative efficacies of amphotericin B, triazoles, and combination of both as experimental therapy for murine trichosporonosis. Antimicrob Agents Chemother. 1994;38:2541-4. [PubMed]
4. Antachopoulos C, Papakonstantinou E, Dotis J, Bibashi E, Tamiolaki M, Koliouskas D, Roilides E. Fungemia due to Trichosporon asahii in a neutropenic child refractory to amphotericin B: clearance with voriconazole. J Pediatr Hematol Oncol. 2005;27:283-5. [PubMed]
5. Arikan S, Hasçelik G. Comparison of NCCLS microdilution method and Etest in antifungal susceptibility testing of clinical Trichosporon asahii isolates. Diagn Microbiol Infect Dis. 2002;43:107-11. [PubMed]
6. Avram A, Buot G, Binet O, Gracia AM, Cesarini JP. Clinical and mycological study of 11 cases of genitopubic trichosporosis nodosa (white piedra). Ann Dermatol Venereol. 1987; 114: 819-27. [PubMed]
7. Barchiesi F, Morbiducci V, Ancarani F, et al. Trichosporon beigelii fungaemia in an AIDS patient. AIDS 1993; 7:139. [PubMed]
8. Beigel H. 1865. Cited by Langeron M. In: Darier J, editor. Nouvelle Practique Dermatologique. Paris: Masson and Cie; 1936. p. 377.
9. Bhansali S, Karanes C, Palutke W, Crane L, Kiel R, Ratanatharathorn V. Successful treatment of disseminated Trichosporon beigelii (cutaneum) infection with associated splenic involvement. Cancer. 1986;58:1630-2. [PubMed]
10. Bouza E, Munoz P. Invasive infections caused by Blastoschizomyces capitatus andScedosporium spp. Clin Microbiol Infect 2004; 10 Suppl 1:76. [PubMed]
11. Davies F, Logan S, Johnson E, Klein JL. Sternal wound infection by Trichosporon inkin following cardiac surgery. J Clin Microbiol. 2006;44:2657-9. [PubMed]
12. de Almeida HL Jr, Rivitti EA, Jaeger RG. White piedra: ultrastructure and a new microecological aspect. Mycoses 1990; 33: 491–97. [PubMed]
13. de Hoog GS, Smith MT, Gueho E. A revision of the genus Geotrichum and its teleomorphs. Stud. Mycol. 1986;29:1-131.
14. Di Bonaventura G, Pompilio A, Picciani C, Iezzi M, D'Antonio D, Piccolomini R. Biofilm formation by the emerging fungal pathogen Trichosporon asahii: development, architecture, and antifungal resistance. Antimicrob Agents Chemother. 2006; 50:3269-76. [PubMed]
15. Diddens HA, Lodder J. Umgrenzung der Gattung Trichosporon und systematische Behandlung ihrer Arten, 1942. p. 408-471. In Die Hefesammlung des "Centraalbureau vor Schimmelculltures". Beitrage zu einer Monographie der Heferarten, vol. 2. Die anakosporogenen Hefen. N.V. Noor-Hollandsche Uitgevers Maatschappij, Amsterdam.
16. Dignani MC, Rex JH, Chan KW, Dow G, deMagalhaes-Silverman M, Maddox A, Walsh T, Anaissie E. Immunomodulation with interferon-gamma and colony-stimulating factors for refractory fungal infections in patients with leukemia. Cancer. 2005; 104:199-204. [PubMed]
17. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: piedra—guidelines/outcomes committee. J Am Acad Dermatol 1996;34:122-4. [PubMed]
18. Ellner K, McBride ME, Rosen T, Berman, D. Prevalence of Trichosporon beigelii:Colonization of normal perigenital skin. J Med Vet Mycol 1991; 29:99. [PubMed]
19. Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evol.Microbiol. 2000;50: 1351–1371. [PubMed]
20. Finkelstein R, Singer P, Lefler E. Catheter-related fungemia caused by Trichosporon beigeliiin non-neutropenic patients. Am J Med 1989; 86:133. [PubMed]
21. Fisher DJ, Christy C, Spafford P, Maniscalco WM, Hardy DJ, Graman PS. Neonatal Trichosporon beigelii infection: report of a cluster of cases in a neonatal intensive care unit. Pediatr Infect Dis J. 1993;12:149-55 [PubMed]
22. Gadea I, Cuenca-Estrella M, Prieto E, Diaz-Guerra TM, Garcia-Cia JI, Mellado E, Tomas JF, Rodriguez-Tudela JL. Genotyping and antifungal susceptibility profile of Dipodascus capitatus isolates causing disseminated infection in seven hematological patients of a tertiary hospital. J Clin Microbiol. 2004;42:1832-6. [PubMed]
23. Giacchino M, Chiapello N, Bezzio S, Fagioli F, Saracco P, Alfarano A, Martini V, Cimino G, Martino P, Girmenia C. Aspergillus galactomannan enzyme-linked immunosorbent assay cross-reactivity caused by invasive Geotrichum capitatum. J Clin Microbiol. 2006;44:3432-4. [PubMed]
24. Girmenia C, Micozzi A, Venditti M, Meloni G, Iori AP, Bastianello S, Martino P. Fluconazole treatment of Blastoschizomyces capitatus meningitis in an allogeneic bone marrow recipient. Eur J Clin Microbiol Infect Dis. 1991;10:752-6. [PubMed]
25. Girmenia C, Pagano L, Martino B, et al. Invasive infections caused by Trichosporon speciesand Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J Clin Microbiol 2005; 43:1818.[PubMed]
26. Gokaslan A, Anaissie E. A novel murine model of disseminated trichosporonosis. Infect Immun. 1992;60:3339-44. [PubMed]
27. Goodman D, Pamer E, Jakubowski A, Morris C, Sepkowitz K. Breakthrough trichosporonosis in a bone marrow transplant recipient receiving caspofungin acetate. Clin Infect Dis. 2002;35:E35-6 [PubMed]
28. Guarro J, Gené J, Stchigel AM. Developments in fungal taxonomy. Clin Microbiol Rev. 1999;12:454-500. [PubMed]
29. Guého E, Smith, MT, de Hoog, GS, et al. Contributions to a revision of the genus Trichosporon. Antonie Van Leeuwenhoek 1992; 61:289. [PubMed]
30. Guého E, Improvisi L, de Hoog GS, Dupont B. Trichosporon on humans: a practical account. Mycoses 1994; 37: 3–10. [PubMed]
31. Hara S, Yokote T, Oka S, Akioka T, Kobayashi K, Hirata Y, Miyoshi T, Tsuji M, Hanafusa T. Endophthalmitis due to Trichosporon beigelii in acute leukemia. Int J Hematol. 2007;85:415-7.[PubMed]
32. Hajjeh RA, Blumberg HM. Bloodstream infection due to Trichosporon beigelii in a burn patient: case report and review of therapy. Clin Infect Dis. 1995;20:913-6. [PubMed]
33. Haupt HM, Merz WG, Beschorner WE. Colonization and infection with Trichosporon species in the immunosuppressed host. J Infect Dis 1983; 147:199. [PubMed]
34. Kalter DC, Tschen JA, Cernoch PL, et al. Genital white piedra: epidemiology, microbiology, and therapy. J Am Acad Dermatol 1986; 14: 982–93. [PubMed]
35. Karashima R, Yamakami Y, Yamagata E, Tokimatsu I, Hiramatsu K, Nasu M. Increased release of glucuronoxylomannan antigen and induced phenotypic changes in Trichosporon asahiiby repeated passage in mice. J Med Microbiol. 2002;51:423-32 [PubMed]
36. Keay S, Denning DW, Stevens DA. Endocarditis due to Trichosporon beigelii: In vitro susceptibility of isolates and review. Rev Infect Dis 1991; 13:383. [PubMed]
37. Khandpur S, Reddy BS. Itraconazole therapy for white piedra affecting scalp hair. J Am Acad Dermatol. 2002;47:415-8. [PubMed]
38. Kiken DA, Sekaran A, Antaya RJ, Davis A, Imaeda S, Silverberg NB. White piedra in children. J Am Acad Dermatol. 2006;55:956-61 [PubMed]
39. Kontoyiannis DP, Torres HA, Chagua M, Hachem R, Tarrand JJ, Bodey GP, Raad II. Trichosporonosis in a tertiary care cancer center: risk factors, changing spectrum and determinants of outcome. Scand J Infect Dis. 2004;36:564-9 [PubMed]
40. Leaf HL, Simberkoff MS. Invasive trichosporonosis in a patient with acquired immunodeficiency syndrome. J Infect Dis 1989; 160:356. [PubMed]
41. Li HM, Du HT, Liu W, Wan Z, Li RY. Microbiological characteristics of medically important Trichosporon species. Mycopathologia. 2005 ;160:217-25 [PubMed]
42. Londero AT, Ramos CD, Fischman O. White piedra of unusual localization. Sabouraudia 1966; 5: 132–33. [PubMed]
43. Lo Passo C, Pernice I, Celeste A, Perdichizzi G, Todaro-Luck F. Transmission ofTrichosporon asahii oesophagitis by a contaminated endoscope. Mycoses. 2001;44:13-21[PubMed]
44. Lopes JO, Alves SH, Klock C, Oliveira LT, Dal Forno NR. Trichosporon inkin peritonitis during continuous ambulatory peritoneal dialysis with bibliography review. Mycopathologia 1997;139:15–18. [PubMed]
45. Lyman CA, Devi SJN, Nathanson J, Frasch CE, Pizzo PA, Walsh TJ. Detection and quantitation of the glucuronoxylomannan-like polysaccharide antigen from clinical and nonclinical isolates of Trichosporon beigelii and implications for pathogenicity. J Clin Microbiol 1995; 33: 126–130. [PubMed]
46. Lyman CA, Garrett KF, Pizzo PA, Walsh TJ. Response of human polymorphonuclear leukocytes and monocytes to Trichosporon beigelii: host defense against an emerging opportunistic pathogen. J Infect Dis. 1994;170:1557-65. [PubMed]
47. Martino P, Venditti M, Micozzi A, Morace G, Polonelli L, Mantovani MP, Petti MC, Burgio VL, Santini C, Serra P, Mandelli F. Blastoschizomyces capitatus: an emerging cause of invasive fungal disease in leukemia patients. Rev. Infect. Dis. 1990;12:570–582. [PubMed]
48. Martino R, Salavert M, Parody R, Tomás JF, de la Cámara R, Vázquez L, Jarque I, Prieto E, Sastre JL, Gadea I, Pemán J, Sierra J. Blastoschizomyces capitatus infection in patients with leukemia: report of 26 cases. Clin Infect Dis. 2004;38:335-41. [PubMed]
49. Martinez-Lacasa J, Maña J, Niubó R, Rufi G, Saez A, Fernández-Nogués F. Long-term survival of a patient with prosthetic valve endocarditis due to Trichosporon beigelii. Eur J Clin Microbiol Infect Dis. 1991;10:756-8 [PubMed]
50. Marty FM, Barouch DH, Coakley EP, Baden LR. Disseminated trichosporonosis caused byTrichosporon loubieri. J Clin Microbiol. 2003;41:5317-20. [PubMed]
51. Mathews MS, Prabhakar S. Chronic meningitis caused by Trichosporon beigelii in India. Mycoses. 1995;38:125-6. [PubMed]
52. McManus EJ, Jones JM. Detection of a Trichosporon beigelii antigen cross-reactive with Cryptococcus neoformans capsular polysaccharide in serum from a patient with disseminated Trichosporon infection. J Clin Microbiol. 1985;21:681-5 [PubMed]
53. Matsue K, Uryu H, Koseki M, Asada N, Takeuchi M. Breakthrough trichosporonosis in patients with hematologic malignancies receiving micafungin. Clin Infect Dis. 2006; 42:753-7.[PubMed]
54. Mekha N, Sugita T, Ikeda R, Nishikawa A, Poonwan N. Real-time PCR assay to detect DNA in sera for the diagnosis of deep-seated trichosporonosis. Microbiol Immunol. 2007;51:633-5.[PubMed]
55. Melcher GP, Reed KD, Rinaldi MG, Lee JW, Pizzo PA, Walsh TJ. Demonstration of a cell wall antigen cross-reacting with cryptococcal polysaccharide in experimental disseminated trichosporonosis. J Clin Microbiol. 1991;29:192-6 [PubMed]
56. Meguro-Hashimoto A, Takatoku M, Ohmine K, Toshima M, Mori M, Nagai T, Muroi K, Ozawa K. The usefulness of magnetic resonance imaging (MRI) for disseminated trichosporosis of the gastrocnemius muscles. J Infect. 2006;53:e135-8 [PubMed]
57. Miró O, Sacanella E, Nadal P, Lluch MM, Nicolás JM, Millá J, Urbano-Márquez A.Trichosporon beigelii fungemia and metastatic pneumonia in a trauma patient. Eur J Clin Microbiol Infect Dis. 1994;13:604-6. [PubMed]
58. Middelhoven WJ, Scorzetti G, Fell JW. Systematics of the anamorphic basidiomycetous yeast genus Trichosporon Behrend with the description of five novel species: Trichosporon vadense, T. smithiae, T. dehoogii, T. scarabaeorum and T. gamsii. Int J Syst Evol Microbiol 2004; 54 :975–986. [PubMed]
59. Mooty MY, Kanj SS, Obeid MY, Hassan GY, Araj GF. A case of Trichosporon beigeliiendocarditis. Eur J Clin Microbiol Infect Dis. 2001;20:139-42. [PubMed]
60. Nahass GT, Rosenberg SP, Leonardi CL, Penneys NS. Disseminated infection withTrichosporon beigelii. Report of a case and review of the cutaneous and histologic manifestations. Arch Dermatol. 1993;129:1020-3. [PubMed]
61. Nakajima M, Sugita T, Mikami Y. Granuloma associated with Trichosporon asahii infection in the lung: Unusual pathological findings and PCR detection of Trichosporon DNA. Med Mycol. 2007;1:1-4 [PubMed]
62. O'Gorman C, McMullan R, Webb CH, Bedi A. Trichosporon asahii. Blood-stream infection in a non-cancer patient receiving combination antifungal therapy. Ulster Med J. 2006;75:226-7.[PubMed]
63. Palungwachira P, Chongsathien S, Palungwachira P. White piedra. Australas J Dermatol 1991;32:75-9. [PubMed]
64. Patel SA, Borges MC, Batt MD, Rosenblate HJ. Trichosporon cholangitis associated with hyperbilirubinemia, and findings suggesting primary sclerosing cholangitis on endoscopic retrograde cholangiopancreatography. Am J Gastroenterol. 1990;85:84-7. [PubMed]
65. Paphitou NI, Ostrosky-Zeichner L, Paetznick VL, Rodriguez JR, Chen E, Rex JH. In vitro antifungal susceptibilities of Trichosporon species. Antimicrob Agents Chemother. 2002;46:1144-6. [PubMed]
66. Piérard GE, Read D, Piérard-Franchimont C, Lother Y, Rurangirwa A, Arrese Estrada J. Cutaneous manifestations in systemic trichosporonosis. Clin Exp Dermatol. 1992; 17:79-82.[PubMed]
67. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Meis JF, Gould IM, Fu W, Colombo AL, Rodriguez-Noriega E; Global Antifungal Surveillance Study. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2007;45:1735-45. [PubMed]
68. Polacheck I, Salkin IF, Kitzes-Cohen R, Raz R. Endocarditis caused by Blastoschizomyces capitatus and taxonomic review of the genus. J Clin Microbiol 1992; 30:2318. [PubMed]
69. Pryce TM, Palladino S, Kay ID, Coombs GW. Rapid identification of fungi by sequencing the ITS1 and ITS2 regions using an automated capillary electrophoresis system. Med Mycol. 2003; 41:369-81. [PubMed]
70. Ramos JM, Cuenca-Estrella M, Gutierrez F, Elia M, Rodriguez-Tudela JL. Clinical case of endocarditis due to Trichosporon inkin and antifungal susceptibility profile of the organism. J Clin Microbiol. 2004 ;42:2341-4 [PubMed]
71. Rastogi VL, Nirwan PS. Invasive trichosporonosis due to Trichosporon asahii in a non-immunocompromised host: a rare case report. Indian J Med Microbiol. 2007;25:59-61. [PubMed]
72. Reddy BT, Torres HA, Kontoyiannis DP. Breast implant infection caused by Trichosporon beigelii. Scand J Infect Dis. 2002;34:143-4. [PubMed]
73. Rieger C, Geiger S, Herold T, Nickenig C, Ostermann H. Breakthrough infection ofTrichosporon asahii during posaconazole treatment in a patient with acute myeloid leukaemia. Eur J Clin Microbiol Infect Dis. 2007;26:843-5 [PubMed]
74. Rodriguez-Tudela JL, Gomez-Lopez A, Alastruey-Izquierdo A, Mellado E, Bernal-Martinez L, Cuenca-Estrella M. Genotype distribution of clinical isolates of Trichosporon asahii based on sequencing of intergenic spacer 1. Diagn Microbiol Infect Dis. 2007;58:435-40. [PubMed]
75. Rose HD, Kurup VP. Colonization of hospitalized patients with yeast organisms. Sabouraudia 1977; 15:251. [PubMed]
76. Salkin IF, Gordon MA, Samsonoff WA, Rieder CL. Blastoschizomyces captitatus, a new combination. Mycotaxon 1985;22:373-380.
77. Sano M, Sugitani M, Ishige T, Homma T, Kikuchi K, Sunagawa K, Obana Y, Uehara Y, Kumasaka K, Uenogawa K, Kobayashi S, Hatta Y, Takeuchi J, Nemoto N. Supplemental utility of nested PCR for the pathological diagnosis of disseminated trichosporonosis. Virchows Arch. 2007;451:929-35. [PubMed]
78. Schaffner A, Davis CE, Schaffner T, Markert M, Douglas H, Braude AI. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986;78:511-24 [PubMed]
79. Schimpff SC, Young VM, Greene WH, Vermeulen GD, Moody MR, Wiernik PH. Origin of infection in acute nonlymphocytic leukemia. Significance of hospital acquisition of potential pathogens. Ann Intern Med. 1972;77:707-14. [PubMed]
80. Schwartz RA. Superficial fungal infections. Lancet. 2004 ;364:1173-82. [PubMed]
81. Serena C, Pastor FJ, Ortoneda M, Capilla J, Nolard N, Guarro J. In vitro antifungal susceptibilities of uncommon basidiomycetous yeasts. Antimicrob Agents Chemother. 2004 ;48:2724-6. [PubMed]
82. Serena C, Pastor FJ, Gilgado F, Mayayo E, Guarro J. Efficacy of micafungin in combination with other drugs in a murine model of disseminated trichosporonosis. Antimicrob Agents Chemother. 2005;49:497-502. [PubMed]
83. Serena C, Rodríguez MM, Mariné M, Pastor FJ, Guarro J. Combined therapies in a murine model of blastoschizomycosis. Antimicrob Agents Chemother. 2007; 51:2608-10. [PubMed]
84. Sheikh HA, Mahgoub S, Badi K. Postoperative endophthalmitis due to Trichosporon cutaneum. Br J Ophthalmol. 1974;58:591-4. [PubMed]
85. Singh N, Belen O, Léger MM, Campos JM. Cluster of Trichosporon mucoides in children associated with a faulty bronchoscope. Pediatr Infect Dis J. 2003;22:609-12. [PubMed]
86. Song HJ, Chung SL, Lee KS. Trichosporon inkin subcutaneous infection in a rheumatoid arthritis patient. Int J Dermatol. 2007;46:282-3. [PubMed]
87. Stenderup A, Schonheyder H, Ebbesen P, Melbye M. White piedra and Trichosporon beigeliicarriage in homosexual men. J Med Veter Mycol 1986;24:401-6. [PubMed]
88. Sugita T, Ikeda R, Nishikawa A. Analysis of Trichosporon isolates obtained from the houses of patients with summer-type hypersensitivity pneumonitis. J Clin Microbiol. 2004; 42:5467-71.[PubMed]
89. Sugita T, Nakajima M, Ikeda R, Matsushima T, Shinoda T. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol 2002; 40 ; 1826–1830. [PubMed]
90. Sugita T, Nishikawa A, Ichikawa T, Ikeda R, Shinoda T. Isolation of Trichosporon asahii from environmental materials. Med Mycol 2000;38: 27–30. [PubMed]
91. Surmont I, Vergauwen B, Marcelis L, Verbist L, Verhoef G, Boogaerts M. First report of chronic meningitis caused by Trichosporon beigelii. Eur J Clin Microbiol Infect Dis. 1990;9:226-9. [PubMed]
92. Swann EA, Taylor JW. Phylogenetic diversities of yeast producing basidiomycetes. Mycol. Res. 1995; 99:1205–1220.
93. Tashiro T, Nagai H, Kamberi P, Goto Y, Kikuchi H, Nasu M, Akizuki S. DisseminatedTrichosporon beigelii infection in patients with malignant diseases: immunohistochemical study and review. Eur J ClinMicrobiol Infect Dis. 1994; 13:218–224. [PubMed]
94. Tashiro T, Nagai H, Nagoaka H, Coto Y, Kamberi P, Nasu M. Trichosporon beigeliipneumonia in patients with hematologic malignancies. Chest 1995; 108:190-95. [PubMed]
95. Thérizol-Ferly M, Kombila M, Gomez de Diaz M, Duong TH, Richard-Lenoble D. White piedra and Trichosporon species in equatorial Africa, I: history and clinical aspects—an analysis of 449 superficial inguinal specimens. Mycoses 1994; 37: 249–53. [PubMed]
96. Torssander J, Carlsson B, Von Krogh G. Trichosporon beigelii: An increased occurrence in homosexual men. Mykosen 1985; 28:355. [PubMed]
97. Viscomi SG, Mortelé KJ, Cantisani V, Glickman J, Silverman SG. Fatal, complete splenic infarction and hepatic infection due to disseminated Trichosporon beigelii infection: CT findings. Abdom Imaging. 2004;29:228-30 [PubMed]
98. von Arx JA, Rodrigues de Miranda L, Smith MT, Yarrow D. The genera of the yeasts and the yeast-like fungi. Stud. Mycol. 1977;14:1-42.
99. Walsh TJ, Melcher GP, Lee JW, Pizzo PA. Infections due to Trichosporon species: New concepts in mycology, pathogenesis, diagnosis and treatment. Curr Top Med Mycol 1993; 5:79.[PubMed]
100. Walsh TJ, Melcher GP, Rinaldi MG, Lecciones J, McGough DA, Kelly P, Lee J, Callender D, Rubin M, Pizzo PA. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J Clin Microbiol. 1990;28:1616-22. [PubMed]
101. Walsh TJ, Newman KR, Moody M, Wharton RC, Wade JC. Trichosporonosis in patients with neoplastic disease. Medicine (Baltimore). 1986;65:268-79. [PubMed]
102. Watson KC, Kallichurum S. Brain abscess due to Trichosporon cutaneum. J Med Microbiol. 1970;3:191-3.[PubMed]
103. Wills TS, Degryse A, Lavina J, Sinnott JT. Blastoschizomyces capitatus pneumonia in an immunocompetent male. South Med J. 2004;97:702-4. [PubMed]
104. Yoshida K, Ando M, Sakata T, Araki S. Prevention of summer-type hypersensitivity pneumonitis: effect of elimination of Trichosporon cutaneum from the patients' homes. Arch Environ Health. 1989;44:317-22. [PubMed]
105. Wills TS, Degryse A, Lavina J, Sinnott JT. Blastoschizomyces capitatus pneumonia in an immunocompetent male. South Med J. 2004;97:702-4 [PubMed]
106. Wolf DG, Falk R, Hacham M, Theelen B, Boekhout T, Scorzetti G, Shapiro M, Block C, Salkin IF, Polacheck I. Multidrug-resistant Trichosporon asahii infection of nongranulocytopenic patients in three intensive care units. J Clin Microbiol. 2001;39:4420-5 [PubMed]
107. Yamagata E, Kamberi P, Yamakami Y, Hashimoto A, Nasu M. Experimental model of progressive disseminated trichosporonosis in mice with latent trichosporonemia. J Clin Microbiol. 2000;38:3260-6. [PubMed]
108. Youker SR, Andreozzi RJ, Appelbaum PC, Credito K, Miller JJ. White piedra: further evidence of a synergistic infection. J Am Acad Dermatol 2003;49:746-9 [PubMed]
TABLE 1. In vitro susceptibilities of Trichosporon spp. and Blastoschizomyces capitatus isolates to fluconazole and voriconazole as determined by CLSI disk diffusion testing a (67)
| Species | Fluconazole b | Voriconazole b | ||||
|---|---|---|---|---|---|---|
| No. of isolates tested | % S | % R | No. of isolates tested | % S | % R | |
Trichosporon spp.c |
443 |
84.7 |
9.0 |
422 |
95.0 |
2.4 |
T. beigelii/T. cutaneum |
125 |
77.6 |
12.0 |
123 |
83.7 |
12.2 |
T. mucoides |
51 |
94.1 |
0.0 |
51 |
100.0 |
0.0 |
T. asahii |
53 |
79.2 |
15.1 |
53 |
92.5 |
7.5 |
T. inkin |
13 |
92.3 |
7.7 |
13 |
100.0 |
0.0 |
T. ovoides |
3 |
100.0 |
0.0 |
3 |
100.0 |
0.0 |
Blastoschizomyces capitatus |
86 |
81.4 |
11.6 |
86 |
91.9 |
3.5 |
a: Isolates were obtained from 124 institutions. b: Fluconazole and voriconazole disk diffusion testing was performed in accordance with CLSI document M44-A. The interpretive breakpoints (zone diameters) were as follows: S, 19 mm (fluconazole) and 17 mm (voriconazole); R, 14 mm (fluconazole) and 13 mm (voriconazole). c. Trichosporon species not otherwise identified.
TABLE 2. In vitro susceptibilities of fluconazole-resistant isolates of Trichosporon spp. and Blastoschizomyces capitatus to voriconazole as determined by CLSI disk diffusion testing a (67)
| No. of isolates tested | % S | % SDD | % R | |
|---|---|---|---|---|
Trichosporon spp.b |
38 |
55.3 |
21.1 |
23.7 |
T. beigelii / T. cutaneum |
15 |
13.3 |
6.7 |
80.0 |
T. asahii |
8 |
50.0 |
0.0 |
50.0 |
T. inkin |
1 |
100.0 |
0.0 |
0.0 |
Blastoschizomyces capitatus |
10 |
60.0 |
10.0 |
30.0 |
a: Isolates were obtained from 124 institutions. The zone diameters for the voriconazole disk diffusion susceptibility categories were as follows: S, 17 mm; SDD, 14 to 16 mm; R, 13 mm. b: Trichosporon species not otherwise identified.
Table 3. In vitro susceptibilities of Trichosporon spp and B. capitatus to antifungal agents (5)
Species |
Antifungal agent |
No of isolates tested |
MIC (μg/ml) |
||
|---|---|---|---|---|---|
| Range | 50% | 90% | |||
T. asahii |
Amphotericin B |
43 |
1-8 |
4 |
4 |
|
Fluconazole |
43 |
0.25-16 |
2 |
8 |
|
Itraconazole |
43 |
0.06-4 |
.5 |
1 |
|
Ravuconazole |
10 |
0.25-0.5 |
0.25 |
0.5 |
|
Posaconazole |
24 |
0.06 - >16 |
0.12 |
N/A |
|
Voriconazole |
10 |
0.25-2 |
0.5 |
2 |
|
Micafungin |
10 |
>64 |
>64 |
>64 |
Non - T. asahii |
Amphotericin B |
15 |
0.6-1 |
0.25 |
N/A |
|
Fluconazole |
15 |
0.5-4 |
2 |
N/A |
|
Itraconazole |
15 |
0.03-0.5 |
0.012 |
N/A |
|
Ravuconazole |
15 |
0.03 - >16 |
0.5 |
N/A |
|
Posaconazole |
15 |
0.03-0.5 |
0.12 |
N/A |
|
Voriconazole |
15 |
0.0-0.25 |
0.06 |
N/A |
B. capitatus |
Amphotericin B |
23 |
0.06-0.25 |
0.12 |
0.12 |
|
Fluconazole |
23 |
1-32 |
8 |
8 |
|
Itraconazole |
23 |
0.03-0.5 |
0.12 |
0.25 |
|
Flucytocine |
23 |
0.12-16 |
0.12 |
4 |
|
Posaconazole |
25 |
N/A |
0.12 |
0.25 |
|
Voriconazole |
23 |
0.03-0.5 |
0.25 |
0.25 |
|
Caspofungin |
25 |
N/A |
16 |
>16 |
MIC: minimum inhibitory capacity, N/A: not available
Figure 1
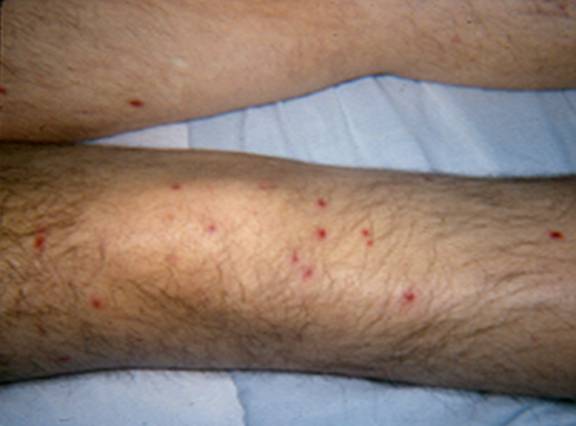
Figure 2
What's New
Chittick P, et al. Case of fatal Blastoschizomyces capitatus infection occurring in a patient receiving empiric micafungin therapy. Antimicrob Agents Chemother 2009;53:5306-5307.
GUIDED MEDLINE SEARCH FOR
Reviews
None.
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
History
None.
