Strongyloides stercoralis (Strongyloidiasis)
Authors: Michael Brown, BA (Oxon), BM BCh, MRCP, PhD, DTM&H, David I. Grove, M.D., DSc, FRACP, FRCPA, DTM&H
Parasitology
The life cycle of this parasitic nematode is complex. Filariform larvae in the soil penetrate exposed skin, pass by way of the blood stream to the lungs, break into the alveolar spaces, ascend the trachea, and are swallowed to their final habitat in the small intestine where the larvae develop into parthenogenetic female adult worms 2-5 mm long. Adult females produce eggs which hatch in the mucosa and bore through the epithelium into the faeces. At this stage, they can either develop into free-living adults which continue their life cycles indefinitely in the soil or they can develop directly via 3 moults into filariform larvae. These are usually passed in the faeces as well but they may re-invade the host, in the lower gastrointestinal tract or perianal skin, before evacuation. This “autoinfection” means that Strongyloides stercoralis is one of the few helminth species which can complete its life cycle and therefore multiply in humans.
Epidemiology
S. stercoralis infects an estimated 30-100 million people, with a distribution throughout tropical and subtropical areas. Prevalence in rural areas of Sub-Saharan Africa, South-east Asia, Central & South America can reach 20%; a lower level of active transmission persists in temperate regions such as Southern Europe and the Southern States of the US. In highly endemic areas infection intensities peak in childhood and then plateau or decline. Because of the chronicity of infection, prevalence remains high in immigrants from endemic areas, with prevalence of 30-80% in SE Asian immigrants screened in North America (30). High prevalence have been observed among British former 2nd World War Far East prisoners of war who were screened 30 or more years after exposure (20).
Clinical Manifestations
Many patients with uncomplicated strongyloidiasis are asymptomatic. Larva currens, a creeping, pruritic eruption on the skin, and abdominal (particularly epigastric) pain are the commonest clinical manifestations. Diarrhea, pruritis ani, and eosinophilic pneumonia may also occur. More severe manifestations are seen in chronically infected subjects who become immunosuppressed due to corticosteroids, hematological malignancy or retroviral infections (particularly HTLV-1). In these circumstances high levels of autoinfection occur which may lead to a severe “hyperinfection” syndrome characterized by severe colitis and occasionally bowel obstruction. Dissemination to other organs may follow, causing haemorrhagic pneumonia and gram-negative bacteraemia. It should be considered as an underlying cause in patients with gram-negative meningitis. Disseminated strongyloidiasis has a high mortality rate. Peripheral eosinophilia is common in chronic strongyloidiasis (and may be the only manifestation of infection) but if often absent in patients with hyperinfection or disseminated strongyloidiasis.
Laboratory Diagnosis
Strongyloides should be considered in any patient with chronic gastrointestinal symptoms and eosinophilia. The diagnosis is most commonly made by finding the characteristic larvae in stool. This can be difficult because the number of organisms are relatively few; for example, only 50 larvae per day in Strongyloides vs. 200,000 eggs per day for Ascaris. The sensitivity of diagnosis for a single stool may be only 30%. Therefore, a large quantity of stool (24 hour sample) and multiple stool specimens should be submitted for maximal sensitivity. The specimen should be concentrated in the clinical microbiology laboratory to increase the yield of microscopic examination. Culture techniques such as charcoal culture, Baermann and Harada-Mori methods are used in parasitological laboratories to allow larval development and multiplication in incubated stool samples. A more sensitive technique involves culturing the stool on to bacteriologic agar to discern a pattern of tracks from isolated bacteria clinging to the mobile larvae.
In patients with hyperinfection or disseminated infection, the larvae can be recovered from duodenal aspiration via endoscopy. In patients with pulmonary infiltrates and eosinophilia, the larvae can be recovered from sputum and bronchoalveolar lavage sputum.
The limited diagnostic yield of stool microscopy for chronic strongyloides, and complexity of culture techniques, has led to the development of serological diagnosis. Traditionally, Strongyloides-specific IgG ELISAs employ crude antigens recovered from S. ratti or human S. stercoralis infections, and have demonstrated high seroprevalence in some exposed populations (30, 52). More recently, recombinant antigens have been developed which may be more widely available (57). Cross-reactions with other nematode species may limit the specificity of serology, and there is conflicting evidence whether positive serology reflects previous as well as patent infection (10, 22, 34, 54, 56).
Pathogenesis
Adult worms live in the small intestinal epithelial layer. The peculiar biological behavior of Strongyloides stercoralis with its capacity to autoinfect is of critical importance in determining a successful outcome in the treatment of strongyloidiasis and it provides the potential for massive, overwhelming strongyloidiasis to supervene (25).
The pathogenesis of hyperinfection in the context of immunosuppression is not well understood. The popular concept is that immunosuppression makes the host more “permissive” to invasive disease, though there is little evidence in support of this. Evidence from animal models exploring the effect of host immunity on Strongyloides development provides an alternative hypothesis (31). This is that host immunosuppression favours the direct development of larvae to infective filariform larvae with resulting hyper-infection and dissemination.
The observed association between immunosuppression and disseminated strongyloidiasis has led to the hypothesis that this disseminated strongyloidiasis would be a ‘marker infection’ for HIV / AIDS. However, this prediction has not been fulfilled (37). A recent study of Strongyloides development in HIV-infected adults found that advanced HIV disease was associated with less direct development and therefore, potentially, protection against hyperinfection (70). The relationship between the occurrence of disseminated strongyloidiasis in individuals immunosuppressed by, for example, corticosteroid administration and the absence of disseminated infections in those immunosuppressed by HIV / AIDS likely reflect differences in the immune deficits generated in these conditions. HIV infection, for example, may be associated with preservation of type 2 immune responses (which may be antihelminthic) whereas HTLV-I infection, and iatrogenic immunosuppression, compromise these immune responses. There may also be direct ecdysteroid effects of corticosteroids onStrongyloides development (19).
SUSCEPTIBILITY IN VITRO AND IN VIVO
Benzimidazole anthelmintics and ivermectin are the most commonly used agents for the treatment of strongyloidiasis. The modes of action of these drugs are poorly understood. A number of mechanisms have been described with regard to the action of benzimidazole agents on nematodes. The first of these is probably the most important. These drugs bind to the beta subunit of microtubule protein in the cytoplasm and prevent the assembly of microtubules (35). This leads to degenerative changes in the cells of the tegument and the gut which in turn leads to impaired digestion and absorption of nutrients. Some benzimidazoles also affect lipid membranes leading to bioenergetic disruption resulting from transmembrane proton discharge. Some benzimidazoles but not others interfere with glucose metabolism or glucose transport resulting the in the depletion of glycogen stores. Resistance to some benzimidazoles has been well-documented in some animal populations with some parasites but whether or not acquired resistance has developed in human strongyloidiasis is unknown. A number of mechanisms have been suggested for the anthelmintic activity of ivermectin. This drug interferes with transmission at the neuromuscular junctions and results in muscular paralysis including pharyngeal pumping (41).
A very limited amount of information is available concerning in vitro activity of benzimidazoles. More information is available from animal models, but since S. stercoralis will not complete its development in mice and rats, much attention has been paid to the related parasites, S. ratti and, to a lesser degree, S. venezuelensis. Unfortunately, neither of these latter two parasites is capable of autoinfection. The human parasite, S. stercoralis, will complete its development in dogs and in gerbils; a limited amount of information is available from studies of strongyloidiasis in these animals.
In Vitro Susceptibility
The effects of the benzimidazole agents, thiabendazole, mebendazole and cambendazole and albendazole on various stages of the environmental component of the life cycle ofS. ratti have been reported (26, 27). Whereas S. stercoralis is found in stools in the form of first-stage larvae, S. ratti appears in faeces as eggs. None of these drugs inhibited the hatching of larvae from S. ratti eggs. When first-stage larvae are incubated in faeces in the laboratory, they moult twice after one and two days, respectively to become second-stage then third-stage larvae. All four drugs inhibited both of these moults. In addition, cambendazole but not thiabendazole or mebendazole impaired the viability (defined on the basis of structural integrity and movement) of first-, second-and third-stage larvae. Third-stage larvae are the infective form of the parasite. When such larvae were incubated with anthelmintics prior to being exposed to mice, cambendazole completely prevented the development of infection in exposed mice whereas thiabendazole, mebendazole and albendazole did not inhibit it at all. When parasitic adult S. ratti worms were removed from the intestines of mice and exposed to thiabendazole, mebendazole and cambendazole, there was no effect at all on viability in vitro.
Similar experiments were done with S. stercoralis. All four drugs completely prevented the transformation of first-stage larvae into infective third-stage larvae. It was possible to evaluate the infectivity of these latter larvae by examining mouse muscle after percutaneous infection with these parasites. Thiabendazole, mebendazole and albendazole had no effect on infectivity but cambendazole inhibited it completely. When parasitic adult S. stercoralis was removed from the intestines of dogs and exposed to the thiabendazole, mebendazole and cambendazole, there was no effect at all on viability.
These results suggest that cambendazole acts in a different manner to the other three drugs. They all inhibit moulting of worms but only cambendazole seems to impair the viability of worms in the periods between moults and to inhibit the infectivity of third-stage larvae.
The efficacy of thiabendazole and albendazole was compared with aqueous methanol extracts of various Jamaican plants in the ability to kill infective larvae of Strongyloides stercoralis (58). The inactivation for 50% of larvae was 35 and 74 hours for albendazole and thiabendazole, respectively. This compared with less than one hour for Mimosa pudica, 2 hours for love weed (Cuscuta americana), 9.5 hours for breadfruit (Artocarpus altilis) and 20 hours for chicken weed (Salvia serotina). It is possible that these plants contain an active agent.
In Vivo Susceptibility (Animal Models)
The effects of the various benzimidazole agents on various stages of the in vivo component of the life cycle of S. ratti infections of mice and rats and S. stercoralis infections of dogs have been reported. Ivermectin has been investigated in S. ratti infections of mice, S. venezuelensis infections of rats and S. stercoralis infections of dogs and gerbils. Cyclosporine has been studied in rats infected with S. ratti and dogs infected with S. stercoralis. S. stercoralis completes its development in dogs and this provides the best model for investigating the precise effects of anthelmintic agents on the various stages of the life cycle. Furthermore, the intensity of infection and severity of disease is greatly increased in immunosuppressed dogs.
Thiabendazole
In mice, thiabendazole had no effect on S. ratti infective larvae in the skin or migrating through the lungs nor did it prevent their maturation into adult worms in the intestine. It did not expel adult worms from the gut but reduced their fecundity and therefore the numbers of eggs excreted in the faeces by 80-90%. Thiabendazole had no effect on the infectivity of S. stercoralis larvae as assessed by the involvement of the muscles of mice (21). The activity of thiabendazole has also been studied in rats infected with S. ratti; it was inactive against larvae in the tissue phase but was reported as being completely effective in the intestinal phase (44). However, only stools were tested for larvae and the intestines were not examined for the presence of adult worms or larvae. In a different study, a single dose of thiabendazole reduced the number of eggs in the stools by approximately 50% (2). Thiabendazole did not abrogate the development of patent infection with S. stercoralis when administered at the same time as infection to immunocompetent dogs. Nor did it eradicate the parasites when given after the onset of established infection (i.e. when larvae appeared in the stools) in immunosuppressed dogs (28).
Mebendazole
In mice, mebendazole had no effect on the numbers of S. ratti larvae in the skin of mice but prevented most larvae from reaching the gut and developing into adult worms. In contrast to thiabendazole, mebendazole eliminated adult worms from the gut. Mebendazole had no effect on the infectivity of S. stercoralis larvae as assessed by the involvement of the muscles of mice (21). Mebendazole did not abrogate the development of patent infection with S. stercoralis when administered at the same time as infection to immunocompetent dogs. Nor did it eradicate the parasites when given after the onset of established infection (i.e. when larvae appeared in the stools) in immunosuppressed dogs (28).
Cambendazole
In mice, cambendazole had no effect on the numbers of S. ratti larvae in the skin of mice but prevented most larvae from reaching the gut and developing into adult worms. In contrast to thiabendazole, cambendazole eliminated adult worms from the gut. Cambendazole was 100-1,000 times more active than mebendazole. Cambendazole was the most effective of all the benzimidazoles and completely eliminated S. stercoralis infective larvae from the muscles of mice (21). Cambendazole abrogated the development of patent infection with S. stercoralis when administered at the same time as infection to immunocompetent dogs. It did not eradicate the parasites when given after the onset of established infection (i.e. larvae appearing in the stools) although, in contrast to thiabendazole and mebendazole, worm burdens were greatly reduced in immunosuppressed dogs treated with cambendazole (28).
Albendazole
In mice, albendazole had a dose-dependent inhibitory effect on S. ratti migratory larvae in the tissues and their development into adult worms. In contrast to thiabendazole, albendazole eliminated adult worms from the gut. Albendazole had a dose-dependent inhibitory effect on S. stercoralis in the muscles of mice (26). The activity of albendazole has also been studied in rats infected with S. ratti; it was inactive against larvae in the tissue phase but was reported as being completely effective in the intestinal phase (44). However, only stools were tested for larvae and the intestines were not examined for the presence of adult worms or larvae. Albendazole treatment for three days given at the same time as infection completely prevented the development of patent infections with S. stercoralis in immunocompetent dogs. When albendazole was given in a dose of 100 mg daily to immunosuppressed dogs with patent infections, larvae disappeared from the stools transiently. When the dogs intestines were examined 7 weeks after cessation of treatment, small numbers of adult worms and rhabditiform larvae were found (26).
Ivermectin
In studies of mice, ivermectin markedly reduced larval migration of S. ratti, prevented their development into adult worms in the gut and eradicated established adult worms from the intestinal tract. Furthermore, ivermectin eradicated S. stercoralis larvae from the muscles of mice (23). Rats were infected with S. venezuelensis and treated with a single oral dose of either a human or veterinary preparation of ivermectin (200 μg/kg); 73-84% of tissue larvae and 59-98% of intestinal adult worms were cleared (1). Similar results were seen when ivermectin was given by injection (12). Two dogs naturally infected with S. stercoralis were given a single dose of ivermectin (200 μg/kg); larvae disappeared from the faeces within a week but one dog had a recrudescence of infection and required a second course of treatment. Three dogs were infected experimentally and immunosuppressed with corticosteroids. A single dose of 800 μg/kg was ineffective in removing infective larvae from the sites of infection but no parasites were found in the intestines (39). A single intraperitoneal injection of ivermectin given in the usual human dose (200 μg/kg) to Mongolian gerbils (= jirds; Meriones unguiculatus) infected with S. stercoralis had no effect on the number of adult worms in the bowel or on their fecundity. When five times this dose was used, all worms were eradicated from the bowel (64).
Cyclosporin
Cyclosporin, an immunosuppressant agent used to prevent rejection in organ transplantation, was serendipitously discovered to have an anti-Strongyloides effect when it was used to immunosuppressed dogs infected with S. stercoralis. Instead, the infection was eradicated. Subsequent studies showed that cyclosporin eradicated S. ratti from rats (61). A later study reported that a single dose given to rats reduced the number of larvae in the stools by approximately 50% (2).
Conclusions
These studies suggest that cambendazole is the most active of the benzimidazole anthelmintics. It is the only one that kills first-, second-and third-stage larvae in vitro and prevents the development of patent infections by infective larvae exposed to drug in vitro. With regard to S. ratti, cambendazole is the most effective on a weight-for weight basis against larvae migrating through the tissues and in eliminating adult worms from the gut. Cambendazole and ivermectin were the only drugs which eradicated S. stercoralis larvae from the muscles of mice and the former drug produced the greatest reduction in worm burden in dogs infected with S. stercoralis. However, the availability of cambendazole is limited. Of the alternative drugs available, experimental studies indicate that albendazole and ivermectin are the next most effective and have similar efficacy.
ANTIPARASITIC THERAPY
The peculiar biological behavior of Strongyloides stercoralis is of critical importance in determining a successful outcome in the treatment of strongyloidiasis (25). The aim of treatment in most worm infections is to simply reduce the number of worms to the point at which the low intensity of infection is unlikely to cause disease. This is relatively easy to achieve in most intestinal nematode infections such as hookworm and roundworm as these parasites cannot reproduce within the human body. Recurrent infection with such parasites requires exposure to more larvae in the environment in the case of hookworm or the ingestion of eggs in food in the case of Ascaris. This simple approach is not applicable in strongyloidiasis as, in contrast to other worm infections, these parasites have the capacity to replicate within the human host. Consequently, unless all worms are eradicated by treatment, those few remaining will multiply and build up the worm burden again. The problem is compounded by the fact that the diagnosis is often difficult to make in the first place because larvae are sometimes very sparse in the stools. It is therefore often impossible to be certain that all the worms have been eradicated. In some immunosuppressed patients in whom infection cannot be cleared, it is necessary to offer repeated courses of treatment.
S. stercoralis is relatively resistant to anthelmintic therapy when compared with other nematodes. Anti-Strongyloides activity is seen in two major classes of drugs, benzimidazoles and avermectins. Several benzimidazoles and one avermectin are available for human use. In addition, cyclosporin may have some effectiveness in strongyloidiasis. Unfortunately, the treatment of strongyloidiasis is often problematic.
Drug of Choice
Ivermectin: Ivermectin is the drug of choice for initial therapy of all forms of strongyloidiasis. It is available in many countries and is probably the most effective drug. Unfortunately, a single course of treatment cannot always be relied upon to eradicate infection. Ivermectin is administered orally in the dosage indicated in Table 1.
Ivermectin was first used for the treatment of human strongyloidiasis in 1989. It is relatively well absorbed with a bioavailability of 50% after oral administration. A proportion of ivermectin is metabolized. The half-life of ivermectin is about 10 hours while that of the metabolites is about three days. Almost all of the drug, whether unchanged or a metabolite, is excreted in the faeces. Ivermectin is generally well tolerated. One or two percent of patients may complain of gastrointestinal symptoms or a rash. The product information provided by the manufacturer indicates that the safety of the drug has not been established in pregnancy. However, this is discussed in more detail in Section III. B. 5; ivermectin should not be used unless the mother’s life is at risk. It is excreted in breast milk so should be used with caution in nursing mothers. Ivermectin is available as 3mg tablets. It is generally recommended that it be given in a single dose of 200 μg/kg, or on two consecutive days.
There have now been a number of series investigating the efficacy of ivermectin in the treatment of strongyloidiasis. Reported cure rates vary between 67% and 100% (Table 2). In direct comparisons, it has been shown that ivermectin is more effective and better tolerated than thiabendazole and albendazole (16, 18, 40, 51, 65, 67). It must be remembered that follow-up undertaken shortly after administration of ivermectin may overestimate efficacy as the parasite may require a long time to build up worm numbers to a detectable level. The limited sensitivity of faecal analysis is underlined in the study of Toma and colleagues (67); those authors found that pyrvinium pamoate had an apparent cure rate of 23% even though this drug probably has no activity against S. stercoralis.
Special Situations
Failure to Respond to Treatment in Uncomplicated Strongyloidiasis
If patients fail to respond to a single course of therapy, then repeated courses of treatment with ivermectinshould be offered. It infection still cannot be eradicated, then one of the alternative agents can be tried (Table 1).
Attempted Eradication of Infection in Immunosuppressed Patients Without Disseminated Infection
Every attempt should be made to eradicate infection in patients who have recently had or are about to have an organ transplant or are given corticosteroids for some other indication but have not yet developed disseminated infection. Similar regimens can be tried for these patients as well as for patients with HTLV-I infection who are prone to hyperinfection (Table 1).
Initial Therapy of Disseminated Strongyloidiasis in Immunosuppressed Patients
Overwhelming strongyloidiasis occurs in patients with impaired defenses, particularly those who are receiving corticosteroid therapy. Most reported cases have been seen in patients with renal transplantation or lymphoma. Disseminated strongyloidiasis is sometimes seen in patients with AIDS but not as frequently as was originally predicted. Complications of disseminated infection include pneumonia and respiratory failure, intestinal obstruction, Gram-negative septicaemia and meningitis. All of these aspects may need attention. As with other forms of strongyloidiasis, ivermectin is the preferred initial therapy of overwhelming strongyloidiasis. The response to treatment with ivermectin in overwhelming strongyloidiasis is variable. Two patients with AIDS who were given a single dose of ivermectin relapsed whereas sustained clinical and parasitological responses were seen in seven such patients who were given four courses of the drug over 16 days (68). On the other hand, a patient with hypogammaglobulinaemia could not be cured despite repeated courses of treatment over 14 months (5). Some patients with disseminated strongyloidiasis have intestinal obstruction and cannot tolerate oral administration. Hyperinfection has been controlled in several patients who failed to respond to or could not tolerate oral therapy by subcutaneous administration of veterinary preparations of ivermectin (14, 42, 59, 69). Another patient has been treated successfully with rectal ivermectin (66). The favorable response to repeated doses of ivermectin reported in a small series by Torres and colleagues suggests that repeated courses or a long course of treatment may be required to eradicate infection in some patients (68).
Patients with disseminated strongyloidiasis and intestinal obstruction are difficult to treat. Such patients usually need suction and drainage as well as intravenous fluids. It may not be possible to administer anthelmintics orally in such situations. The options lie between subcutaneous injection of a veterinary preparation of ivermectin as discussed above, and rectal administration of thiabendazole as will be discussed later. Bacterial superinfections need treatment with appropriate antibiotics, usually those that cover coliforms. Many regimens would be appropriate until a specific organism is identified and its antibiotic susceptibilities determined; these include parenteral administration of a broad-spectrum penicillin, third-generation cephalosporin or quinolone together with an aminoglycoside. If possible, the intensity or immunosuppressive therapy should be reduced. Theoretically, changing the regimen to include cyclosporin might be helpful, in the hope that that agent will have some anti-Strongyloides effect (63). In desperate circumstances, cessation of immunosuppressive therapy may be the only means of saving a patient’s life, even if means losing a renal graft. Despite intensive measures, many patients with severe, complicated strongyloidiasis still die.
Suppression Therapy in Immunosuppressed Patients
In some immunosuppressed patients, it is impossible to eradicate infection. This may occur either before the onset of disseminated infection or be apparent as persistent faecal excretion of larvae after an initial response to treatment of a patient with overwhelming infection. In such circumstances, the best that can be hoped for is to keep the worm burden to a minimum by repeated administration of an anthelmintic. An appropriate initial regimen to try would be to give a single dose ofivermectin each month (Table 1).
Pregnancy
The manufacturers advise caution in the treatment of strongyloidiasis with ivermectin and benzimidazole agents including albendazole and mebendazole. All of these drugs are said to have embryotoxic, fetotoxic, mutagenic and teratogenic potential (7). However, recent information indicates that hundreds of pregnant women have been given ivermectin without ill-effect (11, 15). It appears that ivermectin does not easily cross the placenta (11). Similarly, WHO recommendations attest to the safety of single dose albendazole in pregnancy and so three-day courses are probably similarly safe (60). A decision on whether or not to treat a pregnant woman with strongyloidiasis should be made upon a balancing of the risks to the mother of not treating her with the small potential for damage to the unborn child. In women with uncomplicated strongyloidiasis, it is generally reasonable to defer treatment until after birth. However, if the mother is seriously ill with disseminated strongyloidiasis, then treatment in mandatory. In such circumstances, ivermectin is the drug of choice as it is more effective than albendazole in this life-threatening situation.
Alternative Therapy
The agent that is chosen to treat strongyloidiasis will depend upon availability which may vary from country to country, effectiveness, toxicity, cost and in some cases, governmental subsidy. In some countries such as Australia, ivermectin is subsidized, but only for a single course. Cambendazole is another extremely effective drug for the treatment of strongyloidiasis but its availability is limited to Brazil and possibly some other countries in South America. Albendazole is relatively reliable but needs to be given in repeated doses. Mebendazole is ineffective unless it is given for a three-week course which is cumbersome. Thiabendazole is the least effective and most toxic.
When ivermectin is unavailable, albendazole is the second choice although cambendazole may be a better option in those countries in which it is available (Table 1). Thiabendazole ought only to be used if the other drugs cannot be used, either because of limitation of supply or because of their costliness. If patients fail to respond to a single course of therapy, then a prolonged course with either mebendazole or albendazole should be offered. A similar regimen is appropriate for immunosuppressed patients with disseminated strongyloidiasis in whom an attempt is being made to eradicate infection.
Albendazole
Albendazole is the benzimidazole agent most recently introduced into clinical practice and was first described in the treatment of strongyloidiasis in 1981. Albendazole is thought to be poorly absorbed although there is probably more absorption than with mebendazole; most of the drug is therefore active against intestinal forms of the parasite. Albendazole that is absorbed is metabolized rapidly into albendazole sulphoxide and this is probably active against tissue phases of the parasite. The half-life of the metabolite is about 8 hours; it is eliminated principally in the bile. Because of its poor absorption, albendazole has negligible toxicity when given in the doses used for strongyloidiasis. It has a remarkable safety record in several hundred million patient exposures over a 20 year period (33). It appears to be safe in pregnancy in single dose, and so a risk-benefit assessment could favour its use for symptomatic strongyloidiasis. Contraindications are the same as for thiabendazole. Albendazole is generally available as 200 and 400 mg tablets and as a suspension of 100 mg/ml. It is usually given in a dose of 400mg once or twice daily irrespective of weight for persons two years of age and over for 3 days.
Analysis of the first 23 trials reported on the effectiveness of albendazole disclosed conflicting results (24). The doses varied but the claimed cure rates varied from 28% to 100%. Subsequent studies of treatment for several days have indicated cure rates of between 38% and 80% (4, 13, 29, 40, 43, 50, 67, 51, 65). Some authors have suggested that albendazole should be the treatment of choice for strongyloidiasis but there is considerable disagreement with the titles of papers ranging from albendazole is effective treatment for chronic strongyloidiasis to the weak performance of albendazole in the treatment of strongyloidiasis (4).
A single repetition of the standard course after either one or two weeks made little difference to the cure rate (13, 50). Since albendazole has a greater larvicidal activity than mebendazole in experimental animals, it is likely that courses of treatment prolonged for three to four weeks are likely to be more effective. Unfortunately, no trials have yet been reported which examine this possibility.
Cambendazole
Cambendazole was first used for the treatment of human strongyloidiasis in 1976. It is thought to be well absorbed from the gut and has a half-life of about 12 hours. Little is known about the pharmacokinetics of the drug. Limited studies in humans have found negligible severe toxicity although some patients complained of dizziness or diarrhea (3, 8). Unfortunately, several fatal idiosyncratic reactions occurred in cattle and this led to its withdrawal in most parts of the world (32, 38). It is not licensed for human use in the United States of America but is available in Brazil. It should not be used in pregnancy. Contraindications are the same as for thiabendazole. Cambendazole is available as cambendazol (uci-farma, Sao Paulo, Brazil) 180 mg tablets. The currently recommended therapy is a single dose of 5 mg/kg for immunocompetent patients.
Analysis of 8 trials undertaken in South America between 1976 and 1983 revealed cure rates of between 83% and 100% (24). A total of 380 patients were studied with an average cure rate of 94%. The dose of cambendazole given varied from 2.5 to 25 mg/kg. However, these patients were only followed up for several weeks and it is possible that a longer follow-up may have disclosed a higher relapse rate.
Although there have been no formal trials, cambendazole has been used with success in a patient with AIDS and disseminated strongyloidiasis (36). Repeated treatment for 12 months with thiabendazole failed to cure the patient. The patient was then given cambendazole 360 mg daily for 10 days then a single dose of 360 mg every two weeks. The patient died four months later from Pseudomonas aeruginosa pneumonia but no parasites were even seen in multiple faecal and sputum specimens, including broncho-alveolar lavage fluid obtained at bronchoscopy.
Mebendazole
Mebendazole was the second benzimidazole introduced for human use in 1973. It is poorly absorbed and most of the drug is active against intestinal forms of the parasite. The mebendazole that is absorbed is decarboxylated in the liver and has a half-life of about 6 hours. 90% of the orally administered drug is excreted unchanged in the faeces while the remaining 10% is excreted in the urine as the metabolite. Because of its poor absorption, mebendazole has negligible toxicity when given in the doses used for strongyloidiasis. It should not be used in pregnancy. Contraindications are the same as for thiabendazole. Mebendazole is generally available as 100 mg tablets and as a suspension of 100 mg/ml. It is usually given in a dose of 100 mg twice daily irrespective of weight for persons two years of age and over.
Mebendazole has had a disappointing record when given for only three days. Analysis of 9 trials suggested a cure rate of approximately 50% but this is probably an overestimate (24). In a comparative, trial, Beus found that mebendazole given for 5 days was only as half as effective as thiabendazole given for 2 days (6). There have, however, been suggestions that treatment for prolonged periods or repeated courses of mebendazole may be more effective. Wilson and Kaufman gave mebendazole in a dose of 1.5 g daily for 14 days and apparently cured a patient with an infected intestinal blind loop who had failed to respond to thiabendazole therapy (71). Mravak and colleagues gave mebendazole for three days to two patients without effect but cured them when 500 mg was given daily for 20 consecutive days (45). Shikiya and colleagues studied various regimens (62). They claimed a 94% cure rate when mebendazole was given in a dose of 100 mg twice daily for 28 days to 16 patients; and 87% cure rate when given in the same dose for 5 days then the course repeated at weeks 1, 3 and 4 in 31 patients; and a 96% cure rate when given to 48 patients in a similar regimen except that each course was of only 4 days duration. The efficacy of these regimens was confirmed when patients were re-examined between 8 months and two years later but approximately half of the patients had abnormal liver function tests. The replication time for S. stercoralis to complete its development from first-stage larva to a gravid adult worm is between two and three weeks. It is likely that sequential therapy over several weeks is effective by eliminating adult worms in the gut initially and then removing those that develop over the next two to three weeks from autoinfecting larvae that migrate from the tissues back to the bowel.
Thiabendazole
Thiabendazole was the first benzimidazole introduced for the treatment of strongyloidiasis and has now been in use for forty years. It is rapidly absorbed from the gut then almost all the drug is metabolized in the liver by hydroxylation then conjugated to form inactive glucuronate and sulphonate esters. The half-life varies between one and two hours. More than 90% of the drug is excreted in the urine within 24 hours. It is probably widely distributed in the tissues and at least some drug enters the cerebrospinal fluid. Side-effects are common. Two-thirds of patients complain of nausea, which may be quite severe. Many patients will complain of malaise, dizziness, anorexia, smelly urine or a variety of neuropsychiatric disturbances. There may also be abdominal pain, vomiting, headache, facial flushing and pruritus. Thiabendazole is best avoided during pregnancy, especially in the first trimester, as there have been reports of teratogenic effects in experimental animals given benzimidazole agents early in pregnancy. It should not be given if there has been a prior hypersensitivity reaction to a benzimidazole agent. Thiabendazole is generally available as 500 mg tablets and as a suspension of 100 mg/ml. It is usually given in a dose of 25 mg/kg for 3 days.
Over 20 trials of the effectiveness of thiabendazole in uncomplicated strongyloidiasis were reported in the first decade after its introduction (24). The results seemed impressive with apparent efficacies ranging from 60-100%. However, they need to be viewed with some circumspection as it is extremely difficult to be certain that infection has been eradicated and that there are no worms remaining to multiply and cause persistent disease as a result of autoinfection. Further, many studies were carried out in endemic areas where reinfection was also possible. There are relatively few studies that have followed patients for a prolonged period in order to see whether there are any relapses. It is probable that infection persists in up to 20-30% of treated patients (17, 22, 49, 53). It has been suggested that cure rates may be improved by recurrent courses of treatment but the side-effect rate was high and nearly half of the patients dropped out in one trial (53).
The value of thiabendazole in patients with overwhelming strongyloidiasis due to disseminated infection has not been studied systematically in large series because of the sporadic occurrence of such infections. When those reports that were available were collated, it became apparent that of those patients who survived for at least three days after the initiation of thiabendazole therapy, more than one half of the patients failed to respond completely to treatment and many died (24). Some patients required repeated courses of treatment to suppress the infection. There is no intravenous preparation of thiabendazole but rectal administration of thiabendazole suspension (1.5g in 15 ml) as a retention enema once daily for 14 days was successful in one patient (9).
ADJUNCTIVE THERAPY
Appropriate antibiotic therapy should be given for complicating bacterial infections in patients with disseminated strongyloidiasis.
ENDPOINTS FOR MONITORING THERAPY
It is often difficult to be certain whether a person treated for uncomplicated strongyloidiasis has been cured. A minority of patients have pathognomonic clinical features such as larva currens or typical crops of wheals around the waist and on the buttocks. If these disappear, it is highly suggestive that the patient is cured. This conclusion is supported if no parasites can be identified in the faeces. It is much more difficult to be certain whether a patient without typical symptoms has been cured. The finding of larvae in the stools after treatment clearly indicates failure. On the other hand, absence of larvae does not guarantee cure as the numbers present may be too small to detect. A number of methods have been described to examine faeces including direct examination of wet preparations, various concentration techniques and several culture methods to generate infective larvae. There is not much to choose between them in terms of sensitivity but employment of more than one method on the same specimen will increase the chances of detecting a positive infection. Similarly, the more samples that are examined, the more probable is it that infection will be found.
As stated earlier, unless all worms are eradicated, those remaining multiply and infection persists. Some but not all patients have an eosinophilia; if this declines following treatment, it is encouraging but again does not necessarily indicate cure. The same problem applies to measurement of serum levels of specific antibodies against S. stercoralis. High titres may be maintained for months or years in some patients despite eradication of parasites. Conversely, in other patients, the titres fall after months or years if the worm burden is reduced markedly (10, 22, 34, 54, 56). If titres fall and stay low after several years, that is strong evidence of cure, particularly if supported by failure to find parasites in faeces.
It is often simpler to assess the immediate effectiveness of treatment in patients with disseminated strongyloidiasis as the worm burden is enormously greater and it is much easier to find parasites in faeces and body fluids. First-stage larvae should be sought in faeces and third-stage larvae should be sought in sputum or endotracheal aspirates. Persistently positive specimens indicate failure of treatment. Those patients in whom larvae apparently disappear should be followed carefully with repeated examination of clinical specimens for the presence of parasites.
VACCINES
There are no vaccines available for the prevention of strongyloidiasis.
PREVENTION
Reservoir hosts including dogs and primates maintain the life cycle of Strongyloides and outbreaks have occurred in animal handlers. Custodial institutions caring for mentally retarded patients have also been sites for outbreaks in the past. Patients with hyperinfection are infectious (filariform larvae in stool, urine, sputum, tracheal aspirate) and barrier precautions should be observed to avoid skin contact. Culture techniques in the laboratory which generate infective filariform larvae are potentially a source of infection also.
Antiparasitic Agent Prophylaxis
Prophylaxis is not generally applicable with regard to the primary acquisition of Strongyloides infection. However, eradication of infection as ”prophylaxis” against development of disseminated infection is recommended in some groups, such as people due to receive immunosuppression. A case can be made for serological screening of all such patients, from endemic areas, with eosinophilia, although the absence of eosinophilia in at least 25% infected subjects will limit the effectiveness of this approach. Health economics analyses in the US have explored the benefit of presumptive antihelmintic treatment of all new arrivals from endemic countries, to reduce the burden of disseminated infection (46, 47). The cost-effectiveness of eradicating Strongyloides in this group is limited by the relative expense of ivermectin and lower efficacy of albendazole.
There are some patients in whom infection cannot be eradicated. Such patients are generally immunosuppressed, commonly with corticosteroids. Corticosteroids certainly suppress the immune system which facilitates replication of increasing numbers of worms. It has also been postulated that these drugs have an ecdysteroid effect that enhances moulting and replication (19). When the worm burden increases beyond a certain level then severe, complicated strongyloidiasis may supervene. The only alternative is to keep the lid on the worm burden by reducing the numbers of larvae circulating in the tissues and adult worms producing eggs in the bowel. This is done be repeated administration of anthelmintics for one day each month as suggested in Table 1.
COMMENTS
Strongyloidiasis is one of the more difficult nematode infections to treat. The parasite is intrinsically resistant to many of the commonly used anthelmintics. This problem is compounded by the necessity to eradicate infection because of its biological property, unusual among worms, of being able to replicate within the human host. Furthermore, the diagnosis is often difficult to make. Many trials of agents for the treatment of strongyloidiasis have made an assessment of cure only weeks after administration; these are likely to overestimate cure rates and should be viewed with caution.
REFERENCES
1. Amato Neto V, Carignani FL, Matsubara L, Braz LM. [The treatment of rats experimentally infected with Strongyloides venezuelensis by orally administered ivermectin.] Revista da Sociedade Brasileira de Medicina Tropical (Rio de Janeiro) 1997; 30: 481-484. In Portuguese [PubMed]
2. Armson A, Cunningham GA, Grubb WB, Mendis AH. Murine strongyloidiasis: the effects of cyclosporin A and thiabendazole administered singly and in combination. International Journal for Parasitology 1995; 25: 533-535. [PubMed]
3. Baranski MC, Da Silva AF, Kotaka PI, Gomes NR, Giavannoni M, Telles JE. Tratamento da estrongiloidiase humana com nove anti-helmintico, o cambendazole. Revista da Sociedade Brasileira de Medicina Tropical (Rio de Janeiro) 1997; 30: 481-484. In Portuguese. Also in English translation, Treatment of human strongyloidiasis with cambendazole, a new anthelmintic. Double Blind study. Materia Medica Polona 1983; 15: 36-38. [PubMed]
4. Archibald LK, Beeching NJ, Gill GV, Bailey JW, Bell DR. Albendazole is effective treatment for chronic strongyloidiasis. Quarterly Journal of Medicine 1993 86: 191-193. [PubMed]
5. Ashraf M, Gue CL, Baddour LM. Case report: strongyloidiasis refractory to treatment with ivermectin. American Journal of Medical Science 1996; 311: 178-179. [PubMed]
6. Beus A. Poredbeno ispitivanje tiabendazol I mebendazola u strongiloidozi. [Comparative study of thiabendazole and mebendazole in strongyloidiasis.] Lijec Vjesn 1989; 111: 98-101. In Serbo-Croatian (Roman) [PubMed]
7. Bicalho SA, Leao OJ, Pena Q. Cambendazole in the treatment of human strongyloidiasis. American Journal of Tropical Medicine and Hygiene 1983; 1181-1183.[PubMed]
8. Boken DJ, Leoni PA, Preheim LC. Treatment of Strongyloides stercoralis hyperinfection syndrome with thiabendazole administered per rectum. Clinical Infectious Diseases 1993; 1: 123-126.[PubMed]
9. Bialek R, Knobloch J. [Parasitic infections in pregnancy and congenital parasitoses. II. Helminth infections.] Zeitschrift fur Geburtshilfe Neonatologie 1999; 203: 128-133. In German[PubMed]
10. Boscolo M, Gobbo M, Mantovani W, Degani M, Anselmi M, Monteiro GB, Marocco S, Angheben A, Mistretta M, Santacatterina M, Tais S, Bisoffi Z. Evaluation of an indirect immunofluorescence assay for strongyloidiasis as a tool for diagnosis and follow-up. Clin Vaccine Immunol 2007;14(2):129-33. [PubMed]
11. Brown KR. Changes in the use profile of Mectizan: 1987-1997. Annals of Tropical Medicine and Parasitology 1998; 92 Suppl 1: S61-64. [PubMed]
12. Campos R, Pinto PL, Amato Neto V, Matsubara L, Miyamoto A, de Carvalho SA, Takiguti CK, Moreira AA. [Treatment of experimental infection by Strongyloides venezuelensis in rats with the use of injectable ivermectin and levamisole.] Revista do Instituto de Medicina Tropical de Sao Paulo 1989; 31: 48-52. In Portuguese [PubMed]
13. Chanthavanich P, Nontasut P, Prarinyanuparp V, Sa-Nguankiat S. Repeated doses of albendazole against strongyloidiasis in Thai children. Southeast Asian Journal of Tropical Medicine and Public Health 1989; 20: 221-226. [PubMed]
14. Chiodini PL, Reid AJ, Wiselka MJ, Firmin R, Foweraker J. Parenteral ivermectin in Strongyloides hyperinfection. Lancet 2000; 355: 43-44. [PubMed]
15. Chippaux JP, Gardon-Wendel N, Gardon J, Ernould JC. Absence of any adverse effect of inadvertent ivermectin treatment during pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993; 87: 318. [PubMed]
16. Datry A, Hilmarsdottir I, Mayorga-Sagastume R, Lyagoubi M, Gaxzotte P, Biligui S, Chodakewitz J, Neu D, Danis M, Gentilini M. Treatment of Strongyloides stercoralis infection with ivermectin compared with albendazole: results of an open study of 60 cases. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994; 88: 344-345. [PubMed]
17. Domart A, Gentilini M, Therizol M, Carbon C. Traitement de la strongyloidose par le thiabendazole. (A propos de 100 cas). Societe Medicale des Hopitaux de Paris 1967; 118: 1047-1050. [PubMed]
18. Gann PH, Neva FA, Gam AA. A randomized trial of single- and two-dose ivermectin versus thiabendazole for treatment of strongyloidiasis. Journal of Infectious Diseases 1994; 169: 1076-1079. [PubMed]
19. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clinical Microbiological Reviews 1992; 5: 345-355. [PubMed]
20. Gill GV, Welch E, Bailey JW, Bell DR, Beeching NJ. Chronic Strongyloides stercoralis infection in former British Far East prisoners of war. Qjm 2004;97(12):789- 95. [PubMed]
21. Grove DI. Strongyloides ratti and S. stercoralis: the effects of thiabendazole, mebendazole and cambendazole in infected mice. American Journal of Tropical Medicine and Hygiene 1982a; 31: 469-476. [PubMed]
22. Grove DI. Treatment of strongyloidiasis with thiabendazole: an analysis of toxicity and effectiveness. Transactions of the Royal Society of Tropical Medicine and Hygiene 1982b; 76: 114-118. [PubMed]
23. Grove DI. Effects of 22,23-dihydroavermectin B1 on Strongyloides ratti and S. stercoralis infections in mice. Annals of Tropical Medicine and Parasitology 1983; 77: 405-410. [PubMed]
24. Grove DI. Treatment. In: Grove DI ed, Strongyloidiasis: a major roundworm infection of man, London: Taylor & Francis, 1989: 199-231. [PubMed]
25. Grove DI. Human strongyloidiasis. Advances in Parasitology 1996; 38: 251-309. [PubMed]
26. Grove DI, Lumsden J, Northern C. Efficacy of albendazole against Strongyloides ratti and S. stercoralis in vitro, in mice, and in normal and immunosuppressed dogs. Journal of Antimicrobial Chemotherapy 1998; 21: 75-84. [PubMed]
27. Grove DI, Northern C. Strongyloides ratti and S. stercoralis: effects of cambendazole, thiabendazole and mebendazole in vitro. Revista do Instituto de Medicina Tropical de Sao Paulo 1986; 28: 97-103. [PubMed]
28. Grove DI, Northern C. The effects of thiabendazole, mebendazole and cambendazole in normal and immunosuppressed dogs infected with a human strain of Strongyloides stercoralis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988; 82: 146-149. [PubMed]
29. Gryschek RC, Amato Neto V, Matsubara L, Campos R. Fraco desempenho do albendazol no tratamento da estrongiloidiase. [The weak performance of albendazole in the treatment of strongyloidiasis.] Revista da Sociedade Brasileira de Medicina Tropical (Rio de Janeiro) 1992; 25: 205-206. [PubMed]
30. Gyorkos TW, Genta RM, Viens P, MacLean JD. Seroepidemiology of Strongyloides infection in the Southeast Asian refugee population in Canada. Am J Epidemiol 1990;132(2):257-64. [PubMed]
31. Harvey SC, Gemmill AW, Read AF, Viney ME. The control of morph development in the parasitic nematode Strongyloides ratti. Proc Biol Sci 2000;267(1457):2057-63. Igra-Siegman Y, Kapila R, Sen P, Kaminski ZC, Louria DB. Syndrome of hyperinfection with Strongyloides stercoralis. Rev Infect Dis 1981;3(3):397-407. [PubMed]
32. Hogg RA. Death after cambendazole dosing. Veterinary Record 1978;103:477. [PubMed]
33. Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology 2000; Suppl: S113-132. [PubMed]
34. Kobayashi J, Sato Y, Toma H, Takara M, Shiroma Y. Application of enzyme immunosassay for postchemotherapy evaluation of human strongyloidiasis. Parasitology 1994;18:19-23.[PubMed]
35. Kohler P. The biochemical basis of anthelmintic action and resistance. International Journal for Parasitology 2001; 336-345. [PubMed]
36. Levi GC, Kallas EG, Ramos Moreira Leite K. Disseminated Strongyloides stercoralis infection in an AIDS patient: the role of suppressive therapy. Brazilian Journal of Infectious Diseases 1997; 1: 49-53. [PubMed]
37. Lucas SB. Missing infections in AIDS. Trans R Soc Trop Med Hyg 1990;84 Suppl 1:34- 8. [PubMed]
38. Main DC, Vass DE. Cambendazole toxicity in calves. Australian Veterinary Journal 1980; 56: 237-238. [PubMed]
39. Mansfield LS, Schad GA. Ivermectin treatment of naturally acquired and experimentally induced Strongyloides stercoralis infections in dogs. Journal of the American Veterinary Medical Association 1992; 201: 726-730. [PubMed]
40. Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, Hatz C. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. American Journal of Tropical Medicine and Hygiene 1996; 55: 477-481. [PubMed]
41. Martin RJ. Modes of action of anthelmintic drugs. Veterinary Journal 1997; 154: 11-34. [PubMed]
42. Marty FM, Lowry CM, Rodriguez M, Milner DA, Pieciak WS, Sinha A, Fleckenstien L, Baden LR. Treatment of human disseminated strongyloidiasis with a parenteral veterinary formulation of ivermectin. Clin Infect Dis 2005;41(1):e5-8. [PubMed]
43. Mojon M, Nielsen PB. Treatment of Strongyloides stercoralis with albendazole. A cure rate of 86 per cent. Zentralblatt fur Bakteriologie, Mikrobiologie und Hygiene 1987; 263: 619-624. [PubMed]
44. Mojon M, Saura C, Roojee N, Tran Manh Sung R. Albendazole and thiabendazole in murine strongyloidiasis. Journal of Antimicrobial Therapy 1987; 19: 79-85. [PubMed]
45. Mravak S, Schopp W, Bienzle U. Treatment of strongyloidiasis with mebendazole. Acta Tropica 1983; 40: 93-94. [[PubMed]
46. Muennig P, Pallin D, Challah C, Khan K. The cost-effectiveness of ivermectin vs. albendazole in the presumptive treatment of strongyloidiasis in immigrants to the United States. Epidemiol Infect 2004;132(6):1055-63. [PubMed]
47. Muennig P, Pallin D, Sell RL, Chan MS. The cost effectiveness of strategies for the treatment of intestinal parasites in immigrants. N Engl J Med 1999;340(10):773-9. [PubMed]
48. Naquira C, Jimenez G, Guerra JG, Bernal R, Nalin DR, Neu D, Aziz M. Ivermectin for human strongyloidiasis and other intestinal helminths. American Journal of Tropical Medicine and Hygiene 1989; 40: 304-309.[PubMed]
49. Nauenberg W, Edelman MH, Spingarn CL. Observations on the treatment of strongyloidiasis with thiabendazole in New York City. Mount Sinai Journal of Medicine 1970; 37: 607-611. [PubMed]
50. Niimura S, Hirata T, Zaha O, Nakamura H, Kouchi A, Uehara T, Uechi H, Ohshiro J, Shikiya K, Kinjo F. [Clinical study of albendazole therapy for strongyloidiasis.] Kansenshogaku Zasshi 1992; 66: 1231-1235. In Japanese. [PubMed]
51. Nontasut P, Muennoo C, Sanguankiat S, Fongsri S, Vichit A. Prevalence of strongyloides in Northern Thailand and treatment with ivermectin vs albendazole. Southeast Asian J Trop Med Public Health 2005;36(2):442-4. [PubMed]
52. Nutman TB, Ottesen EA, Ieng S, Samuels J, Kimball E, Lutkoski M, Zierdt WS, Gam A, Neva FA. Eosinophilia in Southeast Asian refugees: evaluation at a referral center. J Infect Dis 1987;155(2):309-13. [PubMed]
53. Oyakawa T, Kuniyoshi T, Arakaki T, Higashionna A, Shikiya K, Sakugawa H, Kadena K, Kitsukawa K, Kinjo F, Saito A. [New trial with thiabendazole for treatment of human strongyloidiasis.] Kansenshogaku Zasshi 1991; 65: 304-310. In Japanese. [PubMed]
54. Page WA, Dempsey K, McCarthy JS. Utility of serological follow-up of chronic strongyloidiasis after anthelminthic chemotherapy. Trans R Soc Trop Med Hyg 2006;100(11):1056-62.[PubMed]
55. Palau LA, Pankey GA. Strongyloides hyperinfection in a renal transplant recipient receiving cyclosporine: possible Strongyloides stercoralis transmission by kidney transplant. American Journal of Tropical Medicine and Hygiene 1997; 57: 413-415. [PubMed]
56. Pelletier LL Jr, Baker CB, Gam AA, Nutman TN, Neva FA. Diagnosis and evaluation of treatment of chronic strongyloidiasis in ex-prisoners of war. Journal of Infectious Diseases 1988; 157: 573-576. [PubMed]
57. Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A Luciferase Immunoprecipitation Systems Assay Enhances the Sensitivity and Specificity of Diagnosis of Strongyloides stercoralis Infection. J Infect Dis 2008;198(3):444-451. [PubMed]
58. Robinson RD, Williams LA, Lindo JF, Terry SI, Mansignh A. Inactivation of Strongyloides stercoralis filariform larvae in vitro by six Jamaican plant extracts and three commercial anthelmintics. West Indian Medical Journal 1990; 39: 213-217. [PubMed]
59. Salluh JI, Feres GA, Velasco E, Holanda GS, Toscano L, Soares M. Successful use of parenteral ivermectin in an immunosuppressed patient with disseminated strongyloidiasis and septic shock. Intensive Care Med 2005;31(9):1292. [PubMed]
60. Savioli L, Crompton DW, Neira M. Use of anthelminthic drugs during pregnancy. Am J Obstet Gynecol 2003;188(1):5-6. [PubMed]
61. Schad GA. Cyclosporine may eliminate the threat of overwhelming strongyloidiasis in immunosuppressed patients. Journal of Infectious Diseases 1986; 153: 178. [PubMed]
62. Shikiya K, Zaha O, Niimura S, Ikema M, Nakamura H, Nakayoshi T, Uechi H, Kinjo F, Saito A, Ohwan T. [Long term eradication rate of mebendazole therapy for strongyloidiasis.] Kansenshogaku Zasshi 1992; 66: 354-359. In Japanese.[PubMed]
63. Shikiya K, Zaha O, Niimura S, Uehara T, Ohshiro J, Kinjo F, Saito A, Asato R. [Clinical study of ivermectin against 125 strongyloidiasis patients.] Kansenshogaku Zasshi 1994; 68: 13-20. In Japanese. [PubMed]
64. Sithithaworn P, Fujimaki Y, Mitsui Y, Prasanthong R, Yutanawiboonchai W, Aoki Y. Efficacy of ivermectin against Strongyloides stercoralis infection in jirds (Meriones unguiculatus), Experimental Parasitology 1998; 89: 205-212. [PubMed]
65. Suputtamongkol Y, Kungpanichkul N, Silpasakorn S, Beeching NJ. Efficacy and safety of a single-dose veterinary preparation of ivermectin versus 7-day high-dose albendazole for chronic strongyloidiasis. Int J Antimicrob Agents 2008;31(1):46-9. [PubMed]
66. Tarr PE, Miele PS, Peregoy KS, Smith MA, Neva FA, Lucey DR. Case report: Rectal adminstration of ivermectin to a patient with Strongyloides hyperinfection syndrome. Am J Trop Med Hyg 2003;68(4):453-5. [PubMed]
67. Toma H, Sato Y, Shiroma Y, Kobayashi J, Shimabukruro I, Takara M. Comparative studies on the efficacy of three anthelmintics on treatment of human strongyloidiasis in Okinawa, Japan. Southeast Asian Journal of Tropical Medicine and Public Health 2000; 31: 147-151.[PubMed]
68. Torres JR, Isturiz R, Murillo J, Guzman M, Contreras R. Efficacy of ivermectin in the treatment of strongyloidiasis complicating AIDS. Clinical Infectious Diseases 1993; 17: 900-902.[PubMed]
69. Turner SA, Maclean JD, Fleckenstein L, Greenaway C. Parenteral administration of ivermectin in a patient with disseminated strongyloidiasis. Am J Trop Med Hyg 2005;73(5):911-4.[PubMed]
70. Viney ME, Brown M, Omoding NE, Bailey JW, Gardner MP, Roberts E, Morgan D, Elliott AM, Whitworth JA. Why does HIV infection not lead to disseminated strongyloidiasis? J Infect Dis 2004;190(12):2175-80. [PubMed]
71. Wilson KH, Kauffman CA. Persistent Strongyloides stercoralis infection in a blind loop of bowel. Successful treatment with mebendazole. Archives of Internal Medicine 1983; 77: 425.[PubMed]
Table 1. Approaches to Anthelmintic Therapy In Patients With Uncomplicated and Complicated Strongyloidiasis or who fail to respond to Initial Therapy.
All Drugs are Administered Orally except where indicated. Anthelmintics are listed in Order of Preference. Dosing regimens are based on trials and case reports referenced in the main text.
| Initial regimens for uncomplicated strongyloidiasis |
|---|
· Ivermectin 200 mg/kg once or on two consecutive days · Albendazole 400 mg twice daily for 3 days · Cambendazole 5 mg/kg once · Thiabendazole 25 mg/kg twice daily for 3 days |
| Regimens for eradication of S. stercoralis in patients with uncomplicated strongyloidiasis who fail to respond to the initial regimen or for the attempted eradication of S. stercoralis in immunosuppressed patients who do not have disseminated disease |
· Ivermectin 200 mg/kg weekly for 4 weeks · Albendazole 400 mg daily for 3 weeks · Mebendazole 100 mg daily for 3 weeks |
| Regimens for the initial treatment of severe, complicated strongyloidiasis in immunosuppressed patients |
· Ivermectin 200 mg/kg twice weekly for 2 weeks, then weekly for 4 weeks, then monthly for 3 months · Albendazole 400 mg twice daily for 14 days · Cambendazole 5 mg/kg daily for 10-20 days · Thiabendazole 25 mg/kg twice daily for 20 days |
| Regimens for the initial treatment of severe, complicated strongyloidiasis in patients unable to take oral therapy |
· subcutaneous veterinary preparations of ivermectin (eg Panomec® or Ivomec®) 200 mg/kg twice weekly then switch to standard oral route when possible · rectal ivermectin 200 mg/kg in a retention enema (eg 30mls Ora-Plus® or Keltrol) daily for 7 days . rectal thiabendazole 1.5g in 15ml retention enema daily for 14 days |
| Regimens for containment of strongyloidiasis in immunosuppressed patients in whom infection cannot be eradicated |
· Ivermectin 200 mg/kg for one day each month · Albendazole 400 mg for one day each month · Thiabendazole 25 mg/kg twice a day for one day each month |
Table 2. Recent Studies of The Efficacy of Ivermectin In Uncomplicated Strongyloidiasis.
| References | Regimen | Number of patients | Time till evaluation | Cure rate (%) |
|---|---|---|---|---|
ivermectin 50-200 mg/kg once or twice on consecutive days |
101 |
1 month |
67-100 |
|
|
|
ivermectin 6 mg twice, 2 weeks apart |
125 |
? |
86 |
i. ivermectin 200 mg/kg once |
15 17 17 |
6 months |
100 100 94 |
|
i. ivermectin 150- 200 mg/kg once |
24 29 |
3 months |
83 38 |
|
i. ivermectin 200 mg/kg once |
152 149 |
3 weeks |
83 45 |
|
i. ivermectin 6 mg once |
67 84 60 |
12 months |
97 77 23 |
|
i. ivermectin 200mg/kg once ii. albendazole 400mg twice daily for 5 days |
78 33 |
1 month |
99 77 |
|
i. veterinary ivermectin 200mg/kg once ii. albendazole 400mg twice daily for 7 days |
21 21 |
median 19 days median 13 days |
76 38 |
Figure 1. Typical rash of larva currens in a Caucasian traveller.
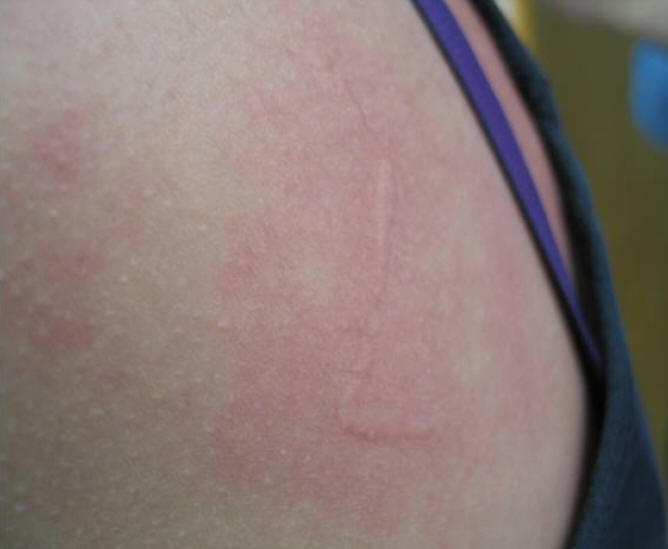
Photograph courtesy of Hospital for Tropical Diseases, London, UK
Figure 2: Rhabditiform larva of Strongyloides stercoralis.
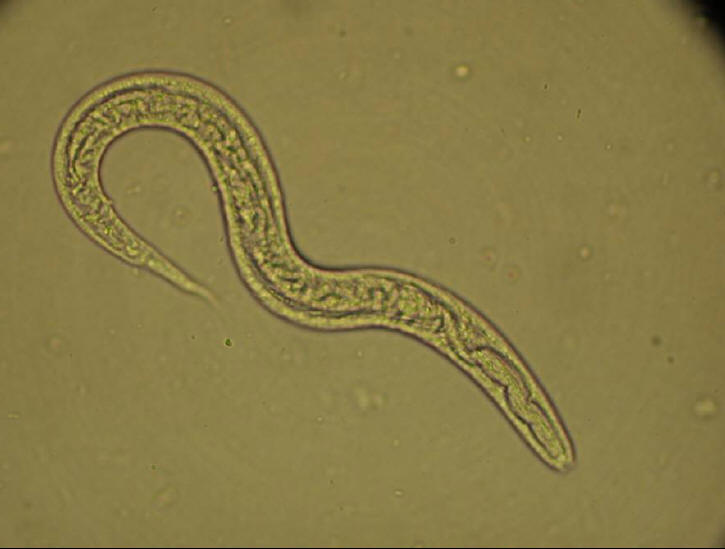
Photograph courtesy of Hospital for Tropical Diseases, London, UK
Figure 3: Duodenal biopsy in a patient with Strongyloides hyperinfection The larvae are seen within the lumen of the crypts. The intestinal mucosa, which is densely inflamed, shows the presence of an adult worm, larvae and eggs.
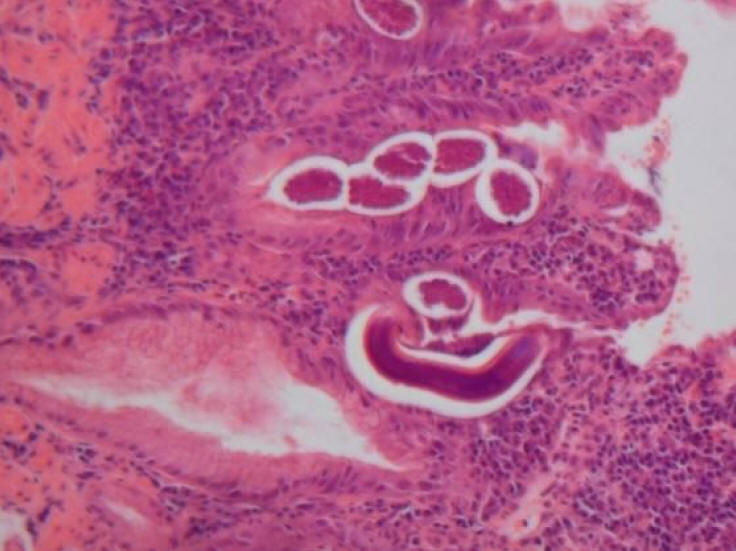
Photograph courtesy of Hospital for Tropical Diseases, London, UK
What's New?
Marcos LA, et al. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102(4):314-8. Epub 2008 Mar 5.
Machado ER, et al. Parasitological and immunological diagnosis of Strongyloides stercoralis in patients with gastrointestinal cancer. Scand J Infect Dis. 2007;6:1-5 [Epub ahead of print]
GUIDED MEDLINE SEARCH FOR
Review articles
Lichtenberger P, Doblecki-Lewis S. Strongyloidiasis in Transplant Recipients
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
History
Yeh J. Historical Overview of Strongyloides stercoralis. 2008
